Key Points
-
Extracellular vesicle (EV) research in Gram-positive bacteria, mycobacteria and fungi was neglected until recently, owing to the presumption that vesicles could not traverse the thick cell walls found in these organisms.
-
EVs are now understood to be produced by all types of microorganism, including those with thick cell walls, and are biologically active.
-
EVs from bacteria, mycobacteria and fungi contain virulence factors, such as toxins, that are involved in pathogenesis and elicit strong host immune responses. For example, Cryptococcus neoformans EVs carry the capsular polysaccharide glucuronoxylomannan, which is an important virulence factor.
-
Interaction of EVs with the host is specific to the microorganism from which the EVs were produced and is based on the lipid content and cargo of the EVs.
-
Research into EVs produced by microorganisms with thick cell walls is a very young field. By learning how these microorganisms use EVs, we hope that researchers will gain insight into pathogenesis, therapeutics and vaccines.
Abstract
Extracellular vesicles (EVs) are produced by all domains of life. In Gram-negative bacteria, EVs are produced by the pinching off of the outer membrane; however, how EVs escape the thick cell walls of Gram-positive bacteria, mycobacteria and fungi is still unknown. Nonetheless, EVs have been described in a variety of cell-walled organisms, including Staphylococcus aureus, Mycobacterium tuberculosis and Cryptococcus neoformans. These EVs contain varied cargo, including nucleic acids, toxins, lipoproteins and enzymes, and have important roles in microbial physiology and pathogenesis. In this Review, we describe the current status of vesiculogenesis research in thick-walled microorganisms and discuss the cargo and functions associated with EVs in these species.
Similar content being viewed by others
Main
The secretion of proteins, molecules, polysaccharides and other factors is a vital process in all living organisms. In a variety of both prokaryotic and eukaryotic microorganisms, many of these cellular factors have been associated with extracellular vesicles (EVs), implying that vesicles may serve as an export system1,2,3,4,5,6,7,8,9,10,11,12. EVs consist of lipid-bilayers that form lumen-containing spheres ranging in size from 20 nm to 500 nm in diameter, and they are produced by eukaryotes, archaea and bacteria13. In the literature, EVs from Gram-positive bacteria and from mycobacteria are usually called membrane vesicles; for clarity, we refer to all extracellular vesicles as EVs, including those that have elsewhere been referred to as membrane vesicles. The existence of EVs across all three domains of the tree of life suggests that vesicular transport is a universal phenomenon.
Bacterial EVs were first reported in Escherichia coli in the 1960s, and the presence of fungal EVs was first proposed in 1973 (Refs 14,15,16,17,18). The existence of EVs produced by Gram-positive bacteria was not mentioned in the literature until 1990 (Ref. 19). In recent decades, research into EVs from Gram-negative bacteria increased substantially, but there was little to no EV-related research with Gram-positive bacteria, mycobacteria or fungi. EVs from Gram-negative bacteria originate from the outer membrane and are thus usually referred to as outer-membrane vesicles (OMVs)20 (Box 1). OMVs carry varied cargo, including virulence factors, adhesins, DNA, RNA, communication compounds, toxins, immunomodulatory factors and nutrient-scavenging factors. OMVs have been associated with cytotoxicity, the invasion of host cells, membrane fusion, the production of biofilms, and the transfer of viruses, DNA, receptors and antibiotic-resistance proteins21,22,23,24. These OMVs can therefore play a major part in microbial pathogenesis, and vesicles from Gram-negative microorganisms have recently been developed into therapeutic vaccines25.
Historically, the lack of interest in EVs in Gram-positive bacteria, mycobacteria and fungi relative to those in Gram-negative bacteria has primarily been due to the inference that the thick cell wall of Gram-positive bacteria, mycobacteria and fungi precluded their existence. OMVs from Gram-negative bacteria are released from the outer membrane by a pinching-off process, encapsulating components from the periplasmic space21,26,27,28,29,30,31 (Fig. 1a), and there is presumably no physical barrier to the release of these OMVs to the extracellular space. By contrast, Gram-positive bacteria lack an outer membrane but have a thick peptidoglycan cell wall outside of the cell membrane32 (Fig. 1b). In mycobacteria, peptidoglycan is covalently attached to arabinogalactan, which in turn is attached to mycolic acids. The upper segment of this cell wall associates with free lipids and is surrounded by an outermost capsule composed of polysaccharides, proteins and lipids33 (Fig. 1c). The architecture of Gram-positive bacteria and mycobacteria is analogous to that of fungi, which also have a thick wall outside of the cellular membrane. The fungal cell wall consists of semistriated layers of chitin, β-glucans and mannoproteins (also known as mannans)34 (Fig. 1d). Some fungi also contain melanin in their walls, although whether these molecules are permanent or transitory is unknown35. The presence of these thick cell walls hindered the search for EVs owing to the assumption that membrane-derived vesicles could not escape such large barriers.
a | The cell wall of Gram-negative bacteria consists of a thin layer of peptidoglycan in the periplasmic space between the inner and outer lipid membranes. The outer membrane contains lipopolysaccharides on its outer leaflet and facilitates non-vesicle-mediated transport through channels such as porins or specialized transporters. It is thought that vesicles from these organisms are produced by the pinching off of the outer membrane, resulting in outer-membrane vesicles (OMVs)26,28,29,30. The lack of an outer membrane, as well as the presence of a thick cell wall, in Gram-positive bacteria, mycobacteria and fungi led to a long-standing belief that these organisms did not produce extracellular vesicles. b | Gram-positive bacteria have a single lipid membrane surrounded by a cell wall composed of a thick layer of peptidoglycan and lipoteichoic acid, which is anchored to the cell membrane by diacylglycerol32. c | Cell walls of mycobacteria consist of thin layers of peptidoglycan and arabinogalactan, and a thick layer of mycolic acids33. Glycolipids and porins are also found in these cell walls, as is lipoarabinomannan, which is anchored to the cell membrane by diacylglycerol. This cell wall surrounds a single lipid membrane. d | A single plasma membrane is also present in fungi, surrounded by a cell wall consisting of various layers of the polysaccharides chitin, β-glucan and mannan (in the form of mannoproteins)34.
In 2007, EVs were isolated and characterized from biofilms of Mycobacterium ulcerans and from the fungal pathogen Cryptococcus neoformans, setting the stage for the study of vesiculogenesis in fungi, mycobacteria and Gram-positive bacteria36,37. Several studies have now purified EVs from cultured supernatants of cell-walled microorganisms, using physical separation protocols38 (Box 2). In this Review, we discuss those cell-walled microorganisms that have been found to produce EVs, the physiological properties of these EVs and hypotheses on the mechanisms of vesiculogenesis, and we outline the outstanding questions that are most pressing for the field to address.
Evidence for extracellular vesicles
In Gram-positive bacteria, mycobacteria and fungi, proteins secreted via specific pathways are important for cell-to-cell communication, killing of competitors, nutrient acquisition, detoxification of the external milieu and virulence39,40,41. However, surveys of the secretome often identify proteins lacking export signals or proteins otherwise predicted to be cell associated, and why this should be the case has remained an enigma. In the Gram-positive bacterium Bacillus subtilis, as many as half of those proteins observed to be secreted had not been predicted to be released into the extracellular space39,42. In mycobacteria, mass spectrometry analysis of the culture filtrate showed that 25% of secreted proteins do not contain a secretion signal, and 10–30% of the fungal secretome does not have a predicted secretion signal43,44,45.
In 1990, vesicle-like blebs were reported on the surface of Bacillus spp. but were not investigated further19. It was first hypothesized that fungi release EVs outside their cell membrane in the early 1970s, when freeze-etching electron microscopy studies of the opportunistic pathogen C. neoformans revealed structures located between the cell wall and plasma membrane. These structures, termed 'paramural bodies', resembled multivesicular bodies (MVBs) fusing with the plasma membrane to release intraluminal vesicles into the extracellular space. The authors speculated that paramural bodies “play a role in secreting cytoplasmic vesicles” analogous to that of MVBs18. However, the possibility that these vesicles would transverse the cell wall to reach the extracellular space does not appear to have been considered. Fungal EV production by membrane blebbing was again suggested by transmission electron microscopy (TEM) and scanning electron microscopy (SEM) of Candida albicans in 1990 (Ref. 46). These results were recently confirmed in three different strains of C. albicans, including clinical isolates47. Despite this growing body of data suggesting that vesicles could reach the extracellular milieu through the cell wall, the notion remained overlooked because the structure of the cell wall was still thought to be too rigid to be permeable to large structures such as EVs. Aside from the question of how EVs traverse the thick cell wall, these studies also raised the question of why organisms invest in a secretion mechanism that has a high energy cost. In addition to vesicular secretion, these organisms have canonical secretion systems — such as the endoplasmic reticulum–Golgi secretion pathway in fungi and the SecA or Tat secretion systems in bacteria — that secrete proteins containing predicted secretion signals. Of note, most of the genetic machinery involved in these canonical secretion systems has been elucidated. Conversely, very few genes have been shown to influence vesiculation in fungi or bacteria. To date, there are no studies comparing the contribution of EV production and other secretion systems to the overall energy cost of cellular export.
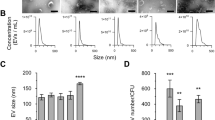
Extracellular vesicles from Gram-positive bacteria. In 2009, the protein composition of EVs produced by the Gram-positive bacterium Staphylococcus aureus was characterized by mass spectrometry. This large-scale study of EVs from a Gram-positive organism reported their size as ∼20–100 nm in diameter, which is comparable to EVs isolated from Gram-negative bacteria, and found that their contents included a variety of proteins that are important for survival and virulence3. Subsequently, EVs have been isolated from planktonic and biofilm cultures for a variety of Gram-positive bacteria, as well as, more rarely, in in vitro and in vivo studies3,4,5,6,7,8,9,10,11,48,49,50,51,52,53. Streptomyces coelicolor EVs were isolated from droplets that grew on sporulating lawns of the bacterium48, and EVs in the process of release from Listeria monocytogenes cells have been visualized with TEM (Box 2). Ultrastructural characterization (including TEM, negative-staining TEM and SEM) of EV preparations from B. subtilis revealed a heterogeneous EV population and correlated EV diameter with electron density, suggesting the existence of a potential cargo-sorting method based on EV size10. Interestingly, EVs produced by Staphylococcus spp., Streptococcus pneumoniae and L. monocytogenes seem to be much smaller than EVs from other Gram-positive bacteria studied, with the caveat that definitive conclusions of EV diameter would require comparisons using the same methodology3,8,9. EVs from these organisms range from 20 nm to 150 nm in diameter, whereas Bacillus spp., Clostridium perfringens and S. coelicolor EVs range from 20 nm to 400 nm in diameter4,10,11,48. This variability in the size of EVs suggests that although vesiculogenesis may be a universal phenomenon, organisms synthesize and regulate EVs in different ways.
Extracellular vesicles from mycobacteria. Mycobacteria also release EVs as a means to secrete a large, complex group of proteins and lipids into the extracellular milieu6,37,38,50,52,54. Vesicle-like blebs were observed on the surface of mycobacterial cells by TEM and SEM and were similar in size to purified EVs, consistent with the notion that these structures represent EVs in the process of release6 (Box 2). This study extended the phenomenon of EV production to the more medically important strains of mycobacteria, Mycobacterium tuberculosis and Mycobacterium bovis bacille Calmette–Guérin (BCG)6. The size distribution of EVs isolated from M. bovis BCG was similar to that of OMVs from Gram-negative bacteria and that of EVs from most Gram-positive bacteria, ranging from 50 nm to 300 nm in diameter. This study also showed that other mycobacterial strains, including non-pathogenic and fast-growing strains, also produced EVs, suggesting that extracellular vesiculogenesis is a conserved phenomenon in the Mycobacterium genus. A recent study of mycobacterial EV production under iron-limiting conditions indirectly supports the role for EVs in virulence. When M. tuberculosis faces iron limitation, a typical situation found within macrophages, the production of EVs enriched in siderophores increases, supporting the growth of siderophore-deficient bacteria52. This scenario indicates that EVs can contribute to cell-to-cell communication in M. tuberculosis and overcome iron limitation within the host.
Extracellular vesicles from fungi. Characterization of fungal EVs began in 2007, when TEM captured vesicles in the C. neoformans cell wall and EVs were recovered from culture supernatants36 (Box 2). Fungal production of EVs requires living cells, as neither heat-treated nor sodium azide-killed cultures produced EVs36,55. Although there is evidence that cellular apoptosis can be accompanied by the production of EVs56, the time at which fungal EVs were analysed corresponded to the logarithmic phase of growth, when presumably no apoptosis occurs.
Evidence that suggests a role for EVs in pathogenesis came from the identification of virulence factors as part of the EV cargo. However, virulence cannot be the sole function of EVs, as the non-pathogenic model yeast Saccharomyces cerevisiae produces EVs with 400 cargo proteins57. It is interesting that despite all the work carried out on secretion in yeast over several decades, EVs were characterized in the opportunistic pathogen C. neoformans before they were found in the yeast model organism S. cerevisiae. EVs have now been described in many fungi, and their EV proteomes have revealed a wide array of protein cargoes, including proteins that have a role in cell metabolism, signal transduction and virulence, as well as structural scaffold proteins and nuclear proteins2,12,57,58.
Release of extracellular vesicles
How are EVs released from the cell wall? Little is known about this important process, but support for three non-mutually exclusive hypotheses exists in the literature (Fig. 2). EVs may be forced through the wall by turgor pressure after release from the plasma membrane. In this case, EV release may be regulated by cell wall pore size or thickness1,59 (Fig. 2a). Alternatively, or in addition, cell wall-modifying enzymes released with EVs may facilitate a loosening of the wall to enable EV release (Fig. 2b); such enzymes have been found in both fungal and bacterial EV purifications2,3. Finally, EVs might transit through channels, and it is possible that the deformation of EVs allows their passage through pores that are narrower than the measured EV diameter; EVs might be guided to the channels by tubulin, which has been found in many fungal proteomics preparations, or other molecular cables1,59 (Fig. 2c). The higher permeability of cell walls in live fungal cells compared with those in dead cells might determine the size of EV that can be transported through the cell wall60. In addition, these differences between live and dead fungal cells may explain the lack of EV release by dead fungal cells.
Three hypotheses explain possible non-mutually exclusive mechanisms by which extracellular vesicles (EVs) traverse thick cell walls. a | EVs may be forced through the wall by turgor pressure after release from the plasma membrane. Pore size or cell wall thickness may regulate the size and ability of EVs to pass through the cell wall1,59. b | Cell wall-modifying enzymes released with EVs may 'loosen' the wall and increase pore size to facilitate EV release. Preparations of EVs from both fungi and Gram-positive bacteria include cell wall-modifying enzymes2,3. c | Protein channels or structural cables may guide EVs to the extracellular environment. Proteomic data show that many fungal EV preparations contain tubulin and/or actin, which are components of structural cables (not shown)1,59.
Data are mixed for support of the above hypotheses regarding EV transit through the fungal cell wall. The fungal cell wall is a dynamic organelle that surrounds the plasma membrane and is constantly remodelled according to cell cycle, metabolic and environmental conditions35,61,62,63. Pore size in the well-characterized S. cerevisiae cell wall varies from 50 nm to 500 nm and can increase to 400 nm under stress conditions64. This pore size is comparable to EV diameter, and the pore therefore represents a potential channel for EVs to traverse the cell wall in this species. In C. neoformans, the induction of melanization causes a decrease in cell wall pore size and is associated with the accumulation of vesicle-like structures between the plasma membrane and cell wall, which can be interpreted as a reduction in the porosity needed for EV export65,66. It is conceivable that remodelling of the cell wall to facilitate EV transit occurs in response to a secretion signal; alternatively, EV release may occur at natural 'break points' of the cell wall, such as at areas that undergo thinning during daughter cell budding67. EVs may stimulate remodelling by including wall-remodelling enzymes as their cargo: β-glucosidases and endochitinases have been identified in proteomic screens of both basidiomycetes and ascomycetes, raising the possibility that their enzymatic activities contribute to EV transit through the cell wall in these organisms2,36,57. Such a mechanism may be widespread, as EVs from the Gram-positive bacterium S. aureus carry peptidoglycan-degrading enzymes, such as Sle1, that can manipulate the thick Gram-positive peptidoglycan cell wall3. One possible explanation for the broad range of sizes of fungal EVs is that cytosol-derived EVs could swell when they are exposed to the lower osmolality of the extracellular environment (compared with that of the cytoplasm), causing them to burst and subsequently reseal. However, we lack definitive information on how EVs cross the cell wall of Gram-positive bacteria, mycobacteria and fungi, and this is an important area for further investigation.
In Gram-negative bacteria, genes affecting OMV production have been identified in E. coli using a screen for known OMV components and lipids68. Although vesiculogenesis in Gram-negative bacteria has been studied intensively, an OMV-null mutant has never been isolated; this raises the possibility that OMV formation is only partially under genetic regulation and is driven by physical and biochemical processes that are not attributable to single genes. Data about the genetic regulation of vesiculogenesis in Gram-positive bacteria, mycobacteria and fungi are lacking, with the exception of data concerning L. monocytogenes sigB (encoding RNA polymerase sigma factor σB) and M. tuberculosis vesiculogenesis and immune response regulator (virR; also known as rv0431), the roles of which in vesiculogenesis offer insight into the mechanism of this process8,50.
Evidence suggests that the transcription factor σB regulates some aspects of vesiculogenesis in L. monocytogenes8. σB regulates genes required for survival under cellular stress, as well as the expression of internalin B (InlB), which is important for bacterial invasion, and positive regulatory factor A (PrfA), which regulates the biosynthesis of the haemolytic toxin listeriolysin O (LLO)69,70. EVs isolated from a wild-type strain contained three times as much InlB as EVs from a ΔsigB mutant strain, whereas the expression of LLO associated with EVs remained the same in the two strains, indicating that σB can contribute to EV cargo regulation8. Fewer EVs were recovered from the ΔsigB mutant than from the wild-type strain, as measured by protein content. Although the quantification of EVs is often determined based on protein concentration, this method does not take into account the possibility that the same number of EVs could be produced, but each could be associated with less protein than in the wild type. EVs from the ΔsigB mutant strain also appeared deformed compared with EVs from a wild-type strain8. A global decrease in transcription may explain the protein quantity differences between these strains, but the variation in morphology indicates that σB, or proteins regulated by σB, may have a role in vesiculogenesis.
In a recent study, virR was identified as a regulator of immune modulation and EV formation in mycobacteria50. Disruption of virR augments cytokine production by mouse and human macrophages in response to the bacterium, and results in an attenuated phenotype in macrophages and mice50. However, virR deletion mutants have no growth defect in broth culture, which suggests a role for virR in M. tuberculosis virulence. virR seems to control the release of immunomodulatory factors, such as the lipoprotein LpqH, via EVs, as there is no evidence that virR deficiency globally enhances the non-EV secretory pathways mediated by SecA2 and Tat. However, only a limited number of proteins that are transported via the non-EV secretory pathway were tested (6 kDa early secretory antigenic target (Esat6), Ag85b, α-crystallin (HspX; also known as Acr), catalase–peroxidase (KatG; also known as CP) and β-lactamase (BlaC)), and it is possible that the virR mutation affects secretion of other non-EV substrates50. VirR is a cytoplasmic protein with a highly hydrophobic region, which suggests that it binds to the inner face of the cell membrane or to a protein with a hydrophobic surface. Co-immunoprecipitation studies led to the identification of LpqH, Rv1488 and Rv0383c as VirR binding partners. It is doubtful that VirR restricts the amount of LpqH in EVs simply by binding to LpqH, as the level of this lipoprotein was the same in cell wall and membrane fractions of wild-type M. tuberculosis, the virR-null mutant and the corresponding complemented strain50. As all of the VirR interacting partners are predicted membrane proteins, VirR might form a higher-order complex with LpqH, Rv1488 and Rv0383c that regulates EV formation at the membrane. Notably, the carboxyl terminus of VirR has a domain annotated as cell envelope-related or LytR family transcriptional regulator; as these regulators have important roles in peptidoglycan formation, cell wall formation and stress tolerance, these data suggest that VirR mediates similar functions.
It is noteworthy that progress in this field has been slowed by continuing debates as to whether the observed EVs were artefacts of lipid self-assembly in solution or were released from dead cells. The absence of null mutants fed into this controversy, because of the precedence of using null mutants as proof of existence for certain processes. However, the multiple reports that an active metabolism is required for EV production and that killed cells do not generate EVs weigh heavily against the argument that the observed EVs were not physiological. Taken together, the inability to recover null mutants for EV production and the ubiquity of EVs suggest that vesiculogenesis is an integral function of microbial cells and not under the control of single genes.
Cargo and functions of extracellular vesicles
EVs can carry a wide range of intraluminal cargo, including nucleic acids, polysaccharides and proteins (Fig. 3a). Although early studies reported that EVs from Gram-positive bacteria did not contain DNA, EVs from Streptococcus mutans and C. perfringens were subsequently reported to contain extracellular DNA (eDNA) and chromosomal DNA, respectively11,19,53. In C. perfringens, genes encoding α-toxin (also known as PLC) and the toxin perfringolysin O (PFO) were amplified from purified EVs. As this DNA was recovered from EV preparations treated with DNase I, it seems that nucleic acids in EVs are protected from exonucleases11. Recently, fungal EVs were shown to contain a variety of RNAs, including microRNA, mRNA and rRNA71,72. EVs isolated from the non-pathogenic species B. subtilis are enriched in lipoproteins and contain proteins important for survival, such as Sunl (also known as YolF), which confers self-immunity to sublancin, an antibiotic produced by the bacterium10,73. Antibiotics have also been associated with EVs, including actinorhodin, which was identified in EV-containing exudates that grow on the surface of S. coelicolor lawns; actinorhodin was purified along with many other proteins, such as bacterioferritin-binding proteins and TerB and TerD proteins, which are involved in iron metabolism and tellurite resistance, respectively. However, the proteomics was performed on fractions from the entire S. coelicolor exudate droplet rather than on purified EVs, so conclusions about EV-associated cargo must await further studies48.
Extracellular vesicles (EVs) contain a varied array of factors, depending on the organism in question, and thus have several functions. a | Some microbial pathogens use EVs to transport virulence factors or to modulate the host immune response. Clostridium perfringens packages chromosomal DNA into EVs, including genes that encode toxins. EVs produced by mycobacteria contain ligands that are recognized by Toll-like receptors (TLRs) in the plasma membrane of host cells. For example, triacylated lipoproteins on the outer leaflet of Mycobacterium tuberculosis EVs are recognized by TLR1–TLR2 heterodimers. After TLR binding, the nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways are activated, leading to cytokine production. However, a prolonged activation of these pathways can lead to the repression of genes involved in antigen presentation. Bacillus anthracis concentrates anthrax toxins into EVs that are thought to deliver the intraluminal contents directly into the host cell cytoplasm by fusing with cholesterol microdomains in the host cell membrane. b | Subpopulations of EVs might be retained in the microbial cell wall to deliver materials that are required for cell wall synthesis and maintenance. In melanizing fungi, laccase is an important enzyme involved in the synthesis of the protective melanin polymer contained in the cell wall. Laccase has been identified in EVs produced by these fungi, and it has been proposed that these laccase-containing EVs are retained in the cell wall to enable in situ melanin synthesis.
Virulence factors associated with extracellular vesicles. EVs from cell-walled organisms are associated with various virulence factors that are important for drug resistance, immune system evasion, host cell invasion and pathogenesis. EV pellets purified from S. aureus are enriched in penicillin-binding proteins, which naturally bind to β-lactam antibiotics, and the membrane-associated global regulator MsrR, which has been implicated in methicillin resistance3,5,74. S. aureus EVs also contain superantigens, lipase and immunoglobulin G-binding proteins (such as protein A), which help the bacterium to evade the immune system; staphopain A, a protein that may be important for cellular invasion3,51,75; and α-haemolysins, which form cytotoxic pores and may induce apoptosis.
Proteomic surveys of EVs from C. neoformans, Histoplasma capsulatum and Paracoccidioides brasiliensis have identified virulence-associated protein cargo, including heat-shock proteins, superoxide dismutase and catalases; more sensitive biochemical assays with C. neoformans EVs identified cryptococcal laccase, an enzyme that is important for the synthesis of the cell wall melanin polymer that protects fungi from the immune response and radiation1,2,76,77,78,79. Fungal EV cargo also includes glycoconjugates, such as the highly immunogenic α-galactosyl epitope found on the P. brasiliensis cell wall, and glucuronoxylomannan (GXM), a major capsule polysaccharide component in C. neoformans36,58. The incorporation of these cell wall-associated enzymes and glycoconjugates suggests that one function of EVs is to deliver cell wall building materials to the exterior of the wall.
EVs might also act as vehicles for interspecies communication and the transfer of DNA or proteins conferring antibiotic resistance. For example, S. aureus EVs deliver the resistance protein β-lactamase (BlaZ) to ampicillin-sensitive Gram-negative and Gram-positive bacteria, which is consistent with a role for EVs in the spread of antibiotic resistance3,5,7.
Toxins associated with extracellular vesicles. Many toxins produced by microbial organisms are required for pathogenesis. Some of these toxins form pores in host membranes, resulting in membrane disruption or the entry of other virulence factors and leading to cytotoxicity, the suppression of immune responses or the initiation of apoptosis. For intracellular pathogens, toxins have been implicated in invasion and escape from host cells.
Toxins produced by Gram-positive bacteria have been associated with EVs, and in many cases, toxin-containing EVs are biologically active. As mentioned above, EVs purified from L. monocytogenes contain the virulence factors InlB and LLO8; both of these proteins are important for cellular invasion and escape from host vacuoles80,81, although the role of LLO- and InlB-associated EVs in pathogenesis still needs to be elucidated. In S. pneumoniae, EVs contain the toxin pneumolysin (Ply), a pore-forming cytolysin that lacks an export signal and is an important virulence factor for colonization and pathogenesis9,82. Cytotoxins lacking an export signal, such as the pore-forming toxin ClyA (also known as HlyE), have also been identified in OMVs from E. coli, suggesting that toxins are also exported by vesicle transport in Gram-negative bacteria. Vesicle-mediated transport explains how Ply, ClyA and other cytotoxins that do not encode an export signal can be exported from the cell to the extracellular space83.
In the anthrax-producing bacterium Bacillus anthracis, the components of anthrax toxin (lethal factor (LF), oedema factor (EF; also known as Cya) and protective antigen (PA)), as well as the cholesterol-dependent cytolysin anthrolysin (ALO), are found in the EV pellet of the bacterial cell culture after centrifugation, but not in the supernatant, suggesting that the overwhelming majority of toxin released from cells is encapsulated in EVs4,84,85 (Fig. 3a). By contrast, two major toxins of C. perfringens — necrotic enteritis toxin B (NetB), which forms membrane pores, and haemolytic α-toxin — were found only in supernatants and not in EV pellets, suggesting that not all toxins are secreted via EVs11,86,87.
In many cases, intact EVs from Gram-positive bacteria are more cytotoxic to cells than disrupted EVs or purified toxin alone. Intact EVs but not lysed EVs from S. aureus are cytotoxic to cells, and purified M. ulcerans EVs containing the toxin mycolactone are more cytotoxic to cells than purified toxin alone, providing further evidence that the capacity of toxin-associated EVs to mediate damage is an integral part of bacterial pathogenesis37,51. Fluorescently labelled mycolactone accumulates in the cytosol of host cells and does not compete with unlabelled mycolactone, suggesting that the toxin passively diffuses through the cell membrane rather than utilizing a membrane receptor88. This cytosolic location and action could be a result of EV–membrane fusion and the subsequent release of mycolactone into the host cytoplasm. Additional evidence that EVs fuse with host cell membranes comes from the delivery of the S. aureus toxins protein A and α-toxin into host cells via cholesterol microdomains5,51 (Fig. 3a).
Host response to microbial extracellular vesicles. EVs can directly influence the immune system to suppress antifungal responses. Mouse macrophages stimulated with C. neoformans EVs secrete nitric oxide and the cytokines tumour necrosis factor (TNF), transforming growth factor-β (TGFβ) and interleukin-10 (IL-10)89. EVs containing GXM, a known immunomodulator, skew the response profile towards a T helper 2 cell (TH2 cell) bias, with higher concentrations of TGFβ and IL-10 than TNF90,91. However, EVs from an acapsular mutant do not incorporate GXM as cargo and skew the host response toward a TH1 cell profile, with increased TNF and nitric oxide production91.
EVs are biologically active and can cause disease without the presence of live cells. Purified EVs from S. aureus upregulated pro-inflammatory mediators in vitro and in vivo, and elicited a TH17-type response and increased production of IgE, causing atopic dermatitis-like inflammation on tape-stripped mouse skin49. C. perfringens EVs were not toxic to macrophages, probably owing to the absence of PFO toxin in these EVs, but did elicit the production of inflammatory cytokines such as TNF, IL-6 and granulocyte colony-stimulating factor (GCSF) in in vitro experiments11.
Proteomic analysis of M. tuberculosis and M. bovis BCG EVs showed that they were enriched in proteins associated with virulence, including the lipoproteins LpqH, LppX, LprA and PstS1, which are a well-known group of Toll-like receptor 2 (TLR2) ligands that interfere with the antigen presentation process in dendritic cells and macrophages6,92 (Fig. 3a). Of note, EVs from the non-pathogenic strain Mycobacterium smegmatis showed no enrichment in lipoproteins. As expected from their protein and lipid cargo, mycobacterial EVs elicit a profound TLR2-dependent inflammatory response in vitro and in vivo (Fig. 3a). Remarkably, when EV-treated mice were challenged with M. tuberculosis H37Rv, they showed an increase in granulomatous inflammation in the lungs, as well as an increase in the bacterial loads in both lungs and the spleen6.
Mice immunized with EVs often elicit an immune response specific to EV components. Sera from mice immunized with B. anthracis and S. pneumoniae EVs react with toxin components and produce EV-specific antibodies, respectively4,9. Interestingly, human serum produces a stronger western blot signal when reacting to S. pneumoniae EV fractions than when reacting to whole bacterial cells, indicating that EVs might have a vital role in the host–pathogen interaction9. In a combined mouse in vivo and human in vitro study, EV administration before C. neoformans challenge resulted in an increase in cryptococcal crossing of the blood–brain barrier and an accumulation of cryptococcus-derived EVs in fungal brain lesions, suggesting a new role for EVs in fungal pathogenesis93. The incorporation of cell wall-associated fungal antigens such as laccase and α-galactosyl-containing glycoconjugates into EVs suggests that EVs distract the immune system by delivering infection-related substances to sites distal to the site of infection, thus acting as molecular decoys that draw immune cells away36,58.
Some of the polar lipids associated with mycobacterial EVs, such as phosphatidylinositol dimannoside (PIM2) and phosphatidylinositol hexamannoside (PIM6), are ligands of TLR2 (Ref. 94). In mycobacteria, EVs induced a TLR2-dependent inflammatory response in macrophages in both in vitro and in vivo infections and after direct administration in mouse lungs6. The inflammatory response to mycobacterial EVs was detrimental in mice when combined with an aerosol challenge using a virulent strain of M. tuberculosis, producing a 'Koch phenomenon' that suggests a role for EVs in M. tuberculosis pathogenesis. Remarkably, no increase in pathogenesis was observed when aerosolized M. tuberculosis infection was combined with the infusion of M. smegmatis EVs, suggesting that specific components of the M. tuberculosis EVs were responsible for eliciting the deleterious immune response6.
EVs not only elicit an immune response when administered to mice, but in many cases also protect against disease. Mice immunized with purified EVs from B. anthracis, S. pneumoniae and M. tuberculosis lived longer than control mice and, in some cases, were protected from disease, indicating the potential for the utilization of EVs in vaccine development4,9,54. The injection of C. albicans EVs into the model invertebrate Galleria mellonella before infection with the yeast itself increased host survival, suggesting that some fungal EVs also elicit protective host responses47. EVs from C. perfringens, however, were not protective in mice, possibly because the major toxins NetB and α-toxin do not seem to form part of the EV cargo11.
Extracellular vesicles associated with biofilms. Biofilms are thought to be the primary state in which many microbial organisms exist in the environment, and biofilm formation poses major problems in clinical settings. Biofilms protect microbial cultures from disruption, from removal from surfaces and from antimicrobial molecules, and thus are the source of many nosocomial infections95. The role of EVs in biofilm formation has been investigated in B. subtilis, M. ulcerans, S. mutans and C. neoformans10,37,53,96. In B. subtilis biofilms, EVs were present in the matrix and were visualized protruding from biofilm-embedded cells10. In M. ulcerans biofilms, EVs were restricted to the extracellular matrix surrounding the outer portion of the cellular community, and were not distributed throughout the entire intricate biofilm structure37. eDNA, which acts as a structural component of biofilms, was associated with EVs isolated from S. mutans planktonic cultures. The presence of eDNA-containing EVs in planktonic cultures suggests that EVs play a part in biofilm production, bacterial colonization and subsequent resistance to removal techniques53. Staphylococcus spp. are a leading cause of biofilm-related nosocomial infections, and although data have yet to be published for biofilms formed by S. aureus, it is likely that EVs are an important factor in such biofilm matrices as well. L. monocytogenes EVs isolated from planktonic cultures contained protein components of biofilms, but EVs have yet to be isolated directly from biofilms for this species8.
Extracellular vesicles and fungal melanization. Although many EVs contain virulence factors or enzymes destined for delivery outside the cell, a certain proportion of EVs may be deliberately retained in the cell wall. Lipid analysis of P. brasiliensis shows that cell wall extracts and purified EVs have a similar composition, which may be indicative of EVs either taking up permanent residence in the cell wall or transiently passing through the wall to be released extracellularly59. Fungal EVs carry laccase, an enzyme traditionally associated with depositing melanin in the cell wall97. A subpopulation of laccase-containing EVs may be directed for retention in the cell wall through an unknown molecular delivery system (Fig. 3b). EV involvement in C. albicans melanization is supported by the description of rounded, electron-dense blebs with EV-like dimensions budding from the melanized cell during cell wall melanization98. Future studies on cell wall transit will have to consider possible EV subpopulations destined for cell wall retention versus those destined for extracellular release.
Outlook
There is now conclusive evidence from many laboratories that microorganisms with thick cell walls, such as Gram-positive bacteria, mycobacteria and fungi, do produce EVs (comprehensive information on EV research can be found on the community web portal, EVpedia99,100,101,102). These vesicles are associated with important virulence factors and have been shown to be biologically active, causing host cell death, eliciting immune responses and, in some cases, conferring disease protection. Collectively, these findings directly implicate EVs in the pathogenesis of many infectious diseases. The existence of EVs produced by microorganisms with thick cell walls is now generally accepted, but their ability to cross the thick cell wall raises fascinating questions regarding the cellular mechanisms responsible for this phenomenon. As the EV field is young, many of these important questions remain unanswered. Although the study of EV production in Gram-positive bacteria, mycobacteria and fungi has intensified, the mechanism of vesiculogenesis in these cell-walled organisms remains poorly understood, and how the release of vesicular contents is regulated is also an important question. Future work may elucidate the temporal control of the production and/or disruption of EVs (Box 3).
The study of vesiculogenesis in Gram-negative bacteria has already resulted in vaccines derived from detergent-extracted OMVs (dOMVs), induced natural blebbing OMVs (nOMVs), OMV proteoliposomes and lipidic nanovesicles25. Vaccines from OMVs have been licensed for Neisseria meningitidis serogroup B, and OMV formulations against other Gram-negative bacteria are currently being pursued103,104. EVs isolated from pathogenic Gram-positive bacteria, such as B. anthracis and S. pneumonia, and from pathogenic mycobacteria, such as M. tuberculosis, elicit an immune response that in some cases offers disease protection, and these findings show that there is a potential for vaccines to be developed from EVs from Gram-positive bacteria4,9,38.
Although the genetic regulation of EV production, release and pathogenesis is an important topic, perhaps the most challenging unsolved question in the field is the mechanism by which EVs are able to traverse the Gram-positive, mycobacterial and fungal cell walls. Current knowledge in the field is mainly limited to basic biological studies that characterize EVs but lack mechanistic insights; however, these studies are an important foundation for future molecular and immunological research. As EV production is a phenomenon that affects all aspects of microbial physiology, ranging from biofilm formation to secretion and pathogenesis, we hope that this Review will stimulate more work on vesiculogenesis in microorganisms with thick cell walls and will encourage more investigators to look for EVs in their favourite Gram-positive bacteria, mycobacteria and fungi.
References
Rodrigues, M. L. et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 7, 58–67 (2008).
Albuquerque, P. C. et al. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell. Microbiol. 10, 1695–1710 (2008).
Lee, E. et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 9, 5425–5436 (2009). This article is the first extensive proteomic study of EVs produced by Gram-positive bacteria.
Rivera, J. et al. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl Acad. Sci. 107, 19002–19007 (2010).
Gurung, M. et al. Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS ONE 6, e27958 (2011).
Prados-Rosales, R. et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J. Clin. Invest. 121, 1471–1483 (2011). This report is the first to describe the production of EVs in the two most medically important species of mycobacteria, M. tuberculosis and M. bovis BCG, stating that EVs from pathogenic strains of mycobacteria concentrate lipoproteins and modulate the immune response in a TLR2-dependent manner.
Lee, J. et al. Staphylococcus aureus extracellular vesicles carry biologically active β-lactamase. Antimicrob. Agents Chemother. 57, 2589–2595 (2013).
Lee, J. H. et al. Transcription factor σB plays an important role in the production of extracellular membrane-derived vesicles in Listeria monocytogenes. PLoS ONE 8, e73196 (2013).
Olaya-Abril, A. et al. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J. Proteomics 106, 46–60 (2014).
Brown, L., Kessler, A., Cabezas-Sanchez, P., Luque-Garcia, J. L. & Casadevall, A. Extracellular vesicles produced by the Gram-positive bacterium Bacillus subtilis are disrupted by the lipopeptide surfactin. Mol. Microbiol. 93, 183–198 (2014).
Jiang, Y., Kong, Q., Roland, K. L. & Curtiss, R. Membrane vesicles of Clostridium perfringens type A strains induce innate and adaptive immunity. Int. J. Med. Microbiol. 304, 431–443 (2014).
Rodrigues, M. L., Nakayasu, E. S., Almeida, I. C. & Nimrichter, L. The impact of proteomics on the understanding of functions and biogenesis of fungal extracellular vesicles. J. Proteomics 97, 177–186 (2014).
Deatherage, B. L. & Cookson, B. T. Membrane vesicle release in bacteria, eukaryotes and archaea: a conserved yet underappreciated aspect of microbial life. Infect. Immun. 80, 1948–1957 (2012). This review extensively covers EV biogenesis and function in bacteria, eukaryotes and archaea.
Bishop, D. G. & Work, E. An extracellular glycolipid produced by Escherichia coli grown under lysine-limiting conditions. Biochem. J. 96, 567–576 (1965).
Knox, K. W., Vesk, M. & Work, E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J. Bacteriol. 92, 1206–1217 (1966).
Work, E., Knox, K. W. & Vesk, M. The chemistry and electron microscopy of an extracellular lipopolysaccharide from Escherichia coli. Ann. N. Y. Acad. Sci. 133, 438–449 (1966). This paper reports some of the first TEM images of what have since been identified as OMVs from Gram-negative bacteria.
Knox, K. W., Cullen, J. & Work, E. An extracellular lipopolysaccharide–phospholipid–protein complex produced by Escherichia coli grown under lysine-limiting conditions. Biochem. J. 103, 192–201 (1967).
Takeo, K., Uesaka, I., Uehira, K. & Nishiura, M. Fine structure of Cryptococcus neoformans grown in vitro as observed by freeze-etching. J. Bacteriol. 113, 1442–1448 (1973).
Dorward, D. W. & Garon, C. F. DNA is packaged within membrane-derived vesicles of Gram-negative but not Gram-positive bacteria. Appl. Environ. Microbiol. 56, 1960–1962 (1990).
Scwechheimer, C. & Kuehn, M. Outer membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. http://dx.doi.org/10.1038/nrmicro3525, (2015).
Kuehn, M. J. & Kesty, N. C. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19, 2645–2655 (2005).
Schooling, S. R. & Beveridge, T. J. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188, 5945–5957 (2006).
Gyorgy, B. et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 68, 2667–2688 (2011).
Andaloussi, S. E. L., Mager, I., Breakefield, X. O. & Wood, M. J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12, 347–357 (2013).
Acevedo, R. et al. Bacterial outer membrane vesicles and vaccine applications. Front. Immunol. 5, 121 (2014).
Beveridge, T. J. Structures of Gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181, 4725–4733 (1999).
Ellis, T. N. & Kuehn, M. J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74, 81–94 (2010).
Kulp, A. & Kuehn, M. J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64, 163–184 (2010).
Costerton, J. W., Ingram, J. M. & Cheng, K. J. Structure and function of the cell envelope of Gram-negative bacteria. Bacteriol. Rev. 38, 87–110 (1974).
Schertzer, J. W. & Whiteley, M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. mBio 3, e00297-11 (2012).
Kulkarni, H. M. & Jagannadham, M. V. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiology 160, 2109–2121 (2014).
Shockman, G. D. & Barrett, J. F. Structure, function, and assembly of cell walls of Gram-positive bacteria. Annu. Rev. Microbiol. 37, 501–527 (1983).
Brennan, P. J. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 83, 91–97 (2003).
Bowman, S. M. & Free, S. J. The structure and synthesis of the fungal cell wall. Bioessays 28, 799–808 (2006).
Free, S. J. Fungal cell wall organization and biosynthesis. Adv. Genet. 81, 33–82 (2013).
Rodrigues, M. L. et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot. Cell 6, 48–59 (2007). This study definitively identifies and characterizes EVs from fungi.
Marsollier, L. et al. Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLoS Pathog. 3, e62 (2007). This work associates the toxin mycolactone with EVs and is the first research into EVs in mycobacteria.
Prados-Rosales, R., Brown, L., Casadevall, A., Montalvo-Quirós, S. & Luque-Garcia, J. L. Isolation and identification of membrane vesicle-associated proteins in Gram-positive bacteria and mycobacteria. MethodsX 1, 124–129 (2014).
Tjalsma, H. et al. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68, 207–233 (2004).
Ligon, L. S., Hayden, J. D. & Braunstein, M. The ins and outs of Mycobacterium tuberculosis protein export. Tuberculosis 92, 121–132 (2012).
Delic, M. et al. The secretory pathway: exploring yeast diversity. FEMS Microbiol. Rev. 37, 872–914 (2013).
Tjalsma, H., Bolhuis, A., Jongbloed, J. D., Bron, S. & van Dijl, J. M. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 64, 515–547 (2000).
Gomez, M., Johnson, S. & Gennaro, M. L. Identification of secreted proteins of Mycobacterium tuberculosis by a bioinformatic approach. Infect. Immun. 68, 2323–2327 (2000).
Vallejo, M. C. et al. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. J. Proteome Res. 11, 1676–1685 (2012).
Sorgo, A. G., Heilmann, C. J., Brul, S., de Koster, C. G. & Klis, F. M. Beyond the wall: Candida albicans secret(e)s to survive. FEMS Microbiol. Lett. 338, 10–17 (2013).
Anderson, J., Mihalik, R. & Soll, D. R. Ultrastructure and antigenicity of the unique cell wall pimple of the Candida opaque phenotype. J. Bacteriol. 172, 224–235 (1990).
Vargas, G. et al. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell. Microbiol. 17, 389–407 (2015).
Schrempf, H., Koebsch, I., Walter, S., Engelhardt, H. & Meschke, H. Extracellular Streptomyces vesicles: amphorae for survival and defence. Microb. Biotechnol. 4, 286–299 (2011).
Hong, S. W. et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy 66, 351–359 (2011). This investigation shows that S. aureus EVs are biologically active and cause atopic dermatitis.
Rath, P. et al. Genetic regulation of vesiculogenesis and immunomodulation in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 110, E4790–E4797 (2013). This article describes the first gene shown to regulate vesiculogenesis in M. tuberculosis (virR ); a mutant deficient in VirR overproduces EVs and is attenuated in virulence, suggesting that vesiculogenesis is important for Mycobacterium spp. pathogenesis.
Thay, B., Wai, S. N. & Oscarsson, J. Staphylococcus aureus α-toxin-dependent induction of host cell death by membrane-derived vesicles. PLoS ONE 8, e54661 (2013).
Prados-Rosales, R. et al. Role for Mycobacterium tuberculosis membrane vesicles in iron acquisition. J. Bacteriol. 196, 1250–1256 (2014).
Liao, S. et al. Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J. Bacteriol. 196, 2355–2366 (2014).
Prados-Rosales, R. et al. Mycobacterial membrane vesicles administered systemically in mice induce a protective immune response to surface compartments of Mycobacterium tuberculosis. mBio 5, e01921-14 (2014).
Wolf, J. M., Rivera, J. & Casadevall, A. Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles. Cell. Microbiol. 14, 762–773 (2012). This study shows that EVs from Gram-positive bacteria and fungi can be disrupted by albumin.
Hristov, M., Erl, W., Linder, S. & Weber, P. C. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 104, 2761–2766 (2004).
Oliveira, D. L. et al. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS ONE 5, e11113 (2010).
Vallejo, M. C. et al. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic α-galactosyl epitopes. Eukaryot. Cell 10, 343–351 (2011).
Vallejo, M. C. et al. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PLoS ONE 7, e39463 (2012).
Klis, F. M., de Koster, C. G. & Brul, S. Cell wall-related bionumbers and bioestimates of Saccharomyces cerevisiae and Candida albicans. Eukaryot. Cell 13, 2–9 (2014).
Doering, T. L. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu. Rev. Microbiol. 63, 223–247 (2009).
Gow, N. A. & Hube, B. Importance of the Candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 15, 406–412 (2012).
Levin, D. E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189, 1145–1175 (2011).
de Souza Pereira, R. & Geibel, J. Direct observation of oxidative stress on the cell wall of Saccharomyces cerevisiae strains with atomic force microscopy. Mol. Cell. Biochem. 201, 17–24 (1999).
Jacobson, E. S. & Ikeda, R. Effect of melanization upon porosity of the cryptococcal cell wall. Med. Mycol. 43, 327–333 (2005).
Wolf, J., Espadas-Moreno, J., Luque-Garcia, J. L. & Casadevall, A. Interaction of Cryptococcus neoformans extracellular vesicles with the cell wall. Eukaryot. Cell 13, 1484–1493 (2014).
Kopecka, M., Yamaguchi, M., Gabriel, M., Takeo, K. & Svoboda, A. Morphological transitions during the cell division cycle of Cryptococcus neoformans as revealed by transmission electron microscopy of ultrathin sections and freeze-substitution. Scripta Med. 73, 369–380 (2000).
McBroom, A. J., Johnson, A. P., Vemulapalli, S. & Kuehn, M. J. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 188, 5385–5392 (2006).
Ollinger, J., Bowen, B., Wiedmann, M., Boor, K. J. & Bergholz, T. M. Listeria monocytogenes σB modulates PrfA-mediated virulence factor expression. Infect. Immun. 77, 2113–2124 (2009).
Parida, S. K. et al. Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol. Microbiol. 28, 81–93 (1998).
Nicola, A. M., Frases, S. & Casadevall, A. Lipophilic dye staining of Cryptococcus neoformans extracellular vesicles and capsule. Eukaryot. Cell 8, 1373–1380 (2009).
da Silva, R. P. et al. Extracellular vesicle-mediated export of fungal RNA. Sci. Rep. 5, 7763 (2015).
Dubois, J. Y. et al. Immunity to the bacteriocin sublancin 168 Is determined by the SunI (YolF) protein of Bacillus subtilis. Antimicrob. Agents Chemother. 53, 651–661 (2009).
Rossi, J., Bischoff, M., Wada, A. & Berger-Bachi, B. MsrR, a putative cell envelope-associated element involved in Staphylococcus aureus sarA attenuation. Antimicrob. Agents Chemother. 47, 2558–2564 (2003).
Bantel, H. et al. α-toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 155, 637–648 (2001).
Casadevall, A., Rosas, A. L. & Nosanchuk, J. D. Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol. 3, 354–358 (2000).
Zhu, X. & Williamson, P. R. Role of laccase in the biology and virulence of Cryptococcus neoformans. FEMS Yeast Res. 5, 1–10 (2004).
Eisenman, H. C. et al. Cryptococcus neoformans laccase catalyses melanin synthesis from both D- and L-DOPA. Microbiology 153, 3954–3962 (2007).
Khajo, A. et al. Protection of melanized Cryptococcus neoformans from lethal dose γ irradiation involves changes in melanin's chemical structure and paramagnetism. PLoS ONE 6, e25092 (2011).
Portnoy, D. A., Jacks, P. S. & Hinrichs, D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167, 1459–1471 (1988).
Cossart, P. et al. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57, 3629–3636 (1989).
Hirst, R. A. et al. Streptococcus pneumoniae deficient in pneumolysin or autolysin has reduced virulence in meningitis. J. Infect. Dis. 197, 744–751 (2008).
Wai, S. N. et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115, 25–35 (2003).
Smith, H., Keppie, J. & Stanley, J. L. The chemical basis of the virulence of Bacillus anthracis. V. The specific toxin produced by B. anthracis in vivo. Br. J. Exp. Pathol. 36, 460–472 (1955).
Mosser, E. M. & Rest, R. F. The Bacillus anthracis cholesterol-dependent cytolysin, Anthrolysin O, kills human neutrophils, monocytes and macrophages. BMC Microbiol. 6, 56 (2006).
Macfarlane, M. G. & Knight, B. C. The biochemistry of bacterial toxins: the lecithinase activity of Cl. welchii toxins. Biochem. J. 35, 884–902 (1941).
Keyburn, A., Bannam, T., Moore, R. & Rood, J. NetB, a pore-forming toxin from necrotic enteritis strains of Clostridium perfringens. Toxins 2, 1913–1927 (2010).
Snyder, D. S. & Small, P. L. Uptake and cellular actions of mycolactone, a virulence determinant for Mycobacterium ulcerans. Microb. Pathog. 34, 91–101 (2003).
Oliveira, D. L. et al. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect. Immun. 78, 1601–1609 (2010).
Monari, C. et al. Cryptococcus neoformans capsular glucuronoxylomannan induces expression of fas ligand in macrophages. J. Immunol. 174, 3461–3468 (2005).
Monari, C. et al. Glucuronoxylomannan, a microbial compound, regulates expression of costimulatory molecules and production of cytokines in macrophages. J. Infect. Dis. 191, 127–137 (2005).
Drage, M. G. et al. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell. Immunol. 258, 29–37 (2009).
Huang, S. H. et al. Cryptococcus neoformans-derived microvesicles enhance the pathogenesis of fungal brain infection. PLoS ONE 7, e48570 (2012).
Bansal, K. et al. PIM2 Induced COX-2 and MMP-9 expression in macrophages requires PI3K and Notch1 signaling. PLoS ONE 4, e4911 (2009).
Bryers, J. D. Medical biofilms. Biotechnol. Bioeng. 100, 1–18 (2008).
Robertson, E. J., Wolf, J. M. & Casadevall, A. EDTA inhibits biofilm formation, extracellular vesicular secretion, and shedding of the capsular polysaccharide glucuronoxylomannan by Cryptococcus neoformans. Appl. Environ. Microbiol. 78, 7977–7984 (2012).
Eisenman, H. C., Frases, S., Nicola, A. M., Rodrigues, M. L. & Casadevall, A. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology 155, 3860–3867 (2009).
Walker, C. A. et al. Melanin externalization in Candida albicans depends on cell wall chitin structures. Eukaryot. Cell 9, 1329–1342 (2010).
Choi, D. S., Kim, D. K., Kim, Y. K. & Gho, Y. S. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 13, 1554–1571 (2013).
Kim, D. K. et al. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell. Vesicles 2, 20384 (2013).
Choi, D. S., Kim, D. K., Kim, Y. K. & Gho, Y. S. Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom. Rev. 34, 474–490 (2014).
Kim, D. K. et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics 31, 933–939 (2015).
Fernandez, S. et al. A proteoliposome formulation derived from Bordetella pertussis induces protection in two murine challenge models. BMC Immunol. 14, S8 (2013).
Holst, J. et al. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27 (Suppl. 2), B3–B12 (2009).
Kadurugamuwa, J. L. & Beveridge, T. J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 177, 3998–4008 (1995).
Kadurugamuwa, J. L. & Beveridge, T. J. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J. Bacteriol. 178, 2767–2774 (1996).
Mashburn-Warren, L. M. & Whiteley, M. Special delivery: vesicle trafficking in prokaryotes. Mol. Microbiol. 61, 839–846 (2006).
Kolling, G. L. & Matthews, K. R. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl. Environ. Microbiol. 65, 1843–1848 (1999).
Mashburn, L. M. & Whiteley, M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437, 422–425 (2005).
Bomberger, J. M. et al. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5, e1000382 (2009).
Bernheimer, A. W. & Avigad, L. S. Nature and properties of a cytolytic agent produced by Bacillus subtilis. J. Gen. Microbiol. 61, 361–369 (1970).
Heerklotz, H. & Seelig, J. Detergent-like action of the antibiotic peptide surfactin on lipid membranes. Biophys. J. 81, 1547–1554 (2001).
Carrillo, C., Teruel, J. A., Aranda, F. J. & Ortiz, A. Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochim. Biophys. Acta 1611, 91–97 (2003).
Nakano, M. M., Marahiel, M. A. & Zuber, P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J. Bacteriol. 170, 5662–5668 (1988).
Quadri, L. E. et al. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry 170, 1585–1595 (1998).
Acknowledgements
A.C. was supported by the US National Institutes of Health (awards 5R01HL059842, 5R01AI033774, 5R37AI033142 and 5R01AI052733). R.P.-R. was supported in part by the US National Institutes of Health (award 1R21AI115091).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Virulence factors
-
Products that are important for infection by and survival of a pathogen. These factors can include adhesins, DNA, toxins and other molecules.
- Biofilms
-
Surface-dwelling bacterial cultures that are highly resistant to disruption and removal. Biofilms can consist of a single species or a community of multiple microorganisms.
- Cell wall
-
The rigid structure that surrounds a microorganism and has a role in cell shape and homeostasis.
- Melanin
-
A pigmented molecule derived from laccase-assisted oxidation of dihydroxy phenol compounds. Melanin can protect cells against oxidative damage.
- Multivesicular bodies
-
(MVBs). A subset of endosomes that contain membrane-bound intraluminal vesicles that originate by budding into the MVB. Typically, MVBs fuse with the cell membrane to release the intraluminal vesicles into the extracellular space.
- B-band lipopolysaccharide
-
(B-band LPS). LPS that is highly charged at neutral pH owing to the presence of a large number of phosphate groups and long O side chains, in contrast to Aband LPS.
- Siderophores
-
Iron-scavenging molecules produced by microorganisms. Some microorganisms can also steal siderophores from other microorganisms in order to obtain iron.
- Basidiomycetes
-
Filamentous fungi that reproduce sexually via the formation of specialized cells called basidia, which bear external spores called basidiospores. Some basidiomycetes can also reproduce asexually.
- Ascomycetes
-
Fungi that, when reproducing sexually, form a structure called an ascus in which the spores are formed. Some ascomycetes can also reproduce asexually.
- Extracellular DNA
-
(eDNA). DNA that is present in the extracellular milieu and might function in intercellular communication. eDNA can also be a structural component of biofilms and neutrophil extracellular traps.
- Penicillin-binding proteins
-
Proteins that are essential for bacterial cell wall biogenesis and also have the capacity to bind to penicillin.
- Glucuronoxylomannan
-
(GXM). A polysaccharide that is produced by the pathogenic fungus Cryptococcus neoformans and is a major component of the cellular capsule.
- Cytolysin
-
A toxin with the ability to lyse cells.
- Mycolactone
-
A macrolide toxin that is produced by a group of mycobacteria and causes Buruli ulcers in humans. It is required for virulence, is cytotoxic and blocks the translocation of immune proteins into the endoplasmic reticulum as a mechanism of immunosuppression.
- Cholesterol microdomains
-
Lipid domains in the cellular lipid bilayer that are enriched in cholesterol.
- T helper 2 cell
-
(TH2 cells). A subset of CD4+ T cells that is of paramount importance for host defence against extracellular pathogens. TH2 cells secrete the cytokines interleukin-4 (IL-4), IL-5, IL-6, IL-9, IL-10 and IL-13, leading to strong antibody responses.
- TH1 cell
-
A subset of CD4+ effector T cells that is required for host defence against intracellular viral and bacterial pathogens. TH1 cells secrete cytokines such as interferon-γ (IFNγ), interleukin-2 (IL-2), IL-10 and lymphotoxin, promoting macrophage activation, nitric oxide production and cytotoxic T lymphocyte proliferation.
- Granulomatous inflammation
-
The aggregation of mononuclear inflammatory cells, which can be accompanied by the infiltration of other leukocytes or by necrosis.
- Koch phenomenon
-
A rapid inflammatory response that develops to a reinfection with Mycobacterium tuberculosis and that is marked by necrotic lesions. The response is caused by hypersensitivity to products of the tubercle bacillus.
- Proteoliposomes
-
Synthetic liposomes with proteins embedded into the lipid bilayer.
- Lipidic nanovesicles
-
Nanoscale lipid-bilayer spheres or liposomes.
Rights and permissions
About this article
Cite this article
Brown, L., Wolf, J., Prados-Rosales, R. et al. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 13, 620–630 (2015). https://doi.org/10.1038/nrmicro3480
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3480
This article is cited by
-
Prokaryotic microvesicles Ortholog of eukaryotic extracellular vesicles in biomedical fields
Cell Communication and Signaling (2024)
-
Emerging role of microbiota derived outer membrane vesicles to preventive, therapeutic and diagnostic proposes
Infectious Agents and Cancer (2023)
-
Bacillus cereus extracellular vesicles act as shuttles for biologically active multicomponent enterotoxins
Cell Communication and Signaling (2023)
-
Emerging role of bacterial outer membrane vesicle in gastrointestinal tract
Gut Pathogens (2023)
-
F. prausnitzii-derived extracellular vesicles attenuate experimental colitis by regulating intestinal homeostasis in mice
Microbial Cell Factories (2023)