Key Points
-
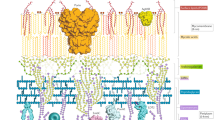
The cell wall of mycobacteria is composed of complex macromolecules, and its composition differs from many other bacterial species, owing to the presence of mycolic acid and arabinogalactan layers. Cell wall synthesis is highly plastic, which leads to the generation of single cells that have distinct surfaces.
-
Mycobacterial growth is distinct from that of other rod-shaped bacteria. Rather than elongating by inserting peptidoglycan into the lateral cell wall, mycobacteria grow from their poles and divide asymmetrically. Two models that describe the processes of polar growth and asymmetric cell division are discussed.
-
The macromolecular machineries that are involved in cell growth and division in mycobacteria are organized differently from those in other model organisms. Many of the enzymes that form these complexes are required for the normal growth of mycobacteria, especially during infection.
-
Mycobacteria regulate cell proliferation via atypical mechanisms, including protein processing, phosphorylation of unique targets and pupylation (which is analogous to eukaryotic ubiquitylation).
-
The mycobacterial cell cycle produces population heterogeneity, owing to asymmetric cell division and stochastic epigenetic changes. This variability could facilitate the survival of M. tuberculosis in disparate physiological niches in the human host and promote antibiotic tolerance.
-
Substantial cell wall remodelling, particularly of the peptidoglycan and mycolic acid layers, occurs during infection. These changes increase the inherent population heterogeneity and may also promote survival in the human host.
Abstract
Mycobacterium tuberculosis, which is the aetiological agent of tuberculosis, owes much of its success as a pathogen to its unique cell wall and unusual mechanism of growth, which facilitate its adaptation to the human host and could have a role in clinical latency. Asymmetric growth and division increase population heterogeneity, which may promote antibiotic tolerance and the fitness of single cells. In this Review, we describe the unusual mechanisms of mycobacterial growth, cell wall biogenesis and division, and discuss how these processes might affect the survival of M. tuberculosis in vivo and contribute to the persistence of infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
World Health Organization. Global tuberculosis report 2013 (WHO, 2013).
Das, B. et al. CD271+ bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci. Transl. Med. 5, 170ra13 (2013).
Ernst, J. D. The immunological life cycle of tuberculosis. Nature Rev. Immunol. 12, 581–591 (2012).
Philips, J. A. & Ernst, J. D. Tuberculosis pathogenesis and immunity. Annu. Rev. Pathol. Mech. Dis. 7, 353–384 (2012).
Barry, C. E. et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nature Rev. Microbiol. 7, 845–855 (2009).
Davis, J. M. & Ramakrishnan, L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136, 37–49 (2009).
Lin, P. L. et al. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nature Med. 20, 75–79 (2014).
Via, L. E. et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76, 2333–2340 (2008).
Mattila, J. T. et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J. Immunol. 191, 773–784 (2013).
Aldridge, B. B. et al. Asymmetry and aging of mycobacterial cells lead to variable growth and antibiotic susceptibility. Science 335, 100–104 (2012). This study presents a model for asymmetric mycobacterial polar growth and division and finds that the poles elongate at different rates throughout the cell cycle.
Joyce, G. et al. Cell division site placement and asymmetric growth in mycobacteria. PLoS ONE 7, e44582 (2012). This study identifies the irregularity of septal site selection in mycobacteria, underscoring additional population heterogeneity as a result of compartmentalization.
Singh, B. et al. Asymmetric growth and division in Mycobacterium spp.: compensatory mechanisms for non-medial septa. Mol. Microbiol. 88, 64–76 (2013).
Santi, I., Dhar, N., Bousbaine, D., Wakamoto, Y. & McKinney, J. D. Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria. Nature Commun. 4, 1–10 (2013). This study presents a model of mycobacterial growth and division and finds that asymmetric elongation of the cell poles only occurs after cytokinesis.
Seiler, P. et al. Cell-wall alterations as an attribute of Mycobacterium tuberculosis in latent infection. J. Infect. Dis. 188, 1326–1331 (2003).
Bhamidi, S. et al. A bioanalytical method to determine the cell wall composition of Mycobacterium tuberculosis grown in vivo. Anal. Biochem. 421, 240–249 (2012).
Rachman, H. et al. Unique transcriptome signature of Mycobacterium tuberculosis in pulmonary tuberculosis. Infect. Immun. 74, 1233–1242 (2006).
Jain, M. et al. Lipidomics reveals control of Mycobacterium tuberculosis virulence lipids via metabolic coupling. Proc. Natl Acad. Sci. USA 104, 5133–5138 (2007). This paper reports the discovery that the cell wall lipids of M. tuberculosis change in relation to the anabolism of host carbon sources.
Typas, A., Banzhaf, M., Gross, C. A. & Vollmer, W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nature Rev. Microbiol. 10, 123–136 (2012).
Meniche, X. et al. Sub-polar addition of new cell wall is directed by DivIVA in mycobacteria. Proc. Natl Acad. Sci. USA (in the press).
Wakamoto, Y. et al. Dynamic persistence of antibiotic-stressed mycobacteria. Science 339, 91–95 (2013). This study shows that, in addition to deterministic processes, stochastic processes generate population heterogeneity in mycobacteria and contribute to mycobacterial persistence.
Gee, C. L. et al. A phosphorylated pseudokinase complex controls cell wall synthesis in mycobacteria. Sci. Signal. 5, ra7 (2012). This study identifies MviN as an essential transporter of peptidoglycan subunits in mycobacteria and describes a unique phosphorylation network that regulates its activity.
Flärdh, K. Cell polarity and the control of apical growth in Streptomyces. Curr. Opin. Microbiol. 13, 758–765 (2010).
Hett, E. C. & Rubin, E. J. Bacterial growth and cell division: a mycobacterial perspective. Microbiol. Mol. Biol. Rev. 72, 126–156 (2008).
Kang, C.-M., Nyayapathy, S., Lee, J.-Y., Suh, J.-W. & Husson, R. N. Wag31, a homologue of the cell division protein DivIVA, regulates growth, morphology and polar cell wall synthesis in mycobacteria. Microbiology 154, 725–735 (2008).
Jani, C. et al. Regulation of polar peptidoglycan biosynthesis by Wag31 phosphorylation in mycobacteria. BMC Microbiol. 10, 327 (2010).
Plocinski, P. et al. Mycobacterium tuberculosis CwsA interacts with CrgA and Wag31, and the CrgA–CwsA complex is involved in peptidoglycan synthesis and cell shape determination. J. Bacteriol. 194, 6398–6409 (2012).
Plocinski, P. et al. Mycobacterium tuberculosis CwsA overproduction modulates cell division and cell wall synthesis. Tuberculosis 93, S21–S27 (2013).
Nguyen, L. et al. Antigen 84, an effector of pleiomorphism in Mycobacterium smegmatis. J. Bacteriol. 189, 7896–7910 (2007).
Hett, E. C., Chao, M. C. & Rubin, E. J. Interaction and modulation of two antagonistic cell wall enzymes of mycobacteria. PLoS Pathog. 6, e1001020 (2010).
Zhang, Y. J. et al. Tryptophan biosynthesis protects mycobacteria from CD4 T-cell-mediated killing. Cell 155, 1296–1308 (2013).
Dasgupta, A., Datta, P., Kundu, M. & Basu, J. The serine/threonine kinase PknB of Mycobacterium tuberculosis phosphorylates PBPA, a penicillin-binding protein required for cell division. Microbiology 152, 493–504 (2006).
Schoonmaker, M. K., Bishai, W. R. & Lamichhane, G. Nonclassical transpeptidases of Mycobacterium tuberculosis alter cell size, morphology, cytosolic matrix, protein localization, virulence and resistance to β-lactams. J. Bacteriol. 196, 1394–1402 (2014).
van der Ploeg, R. et al. Colocalization and interaction between elongasome and divisome during a preparative cell division phase in Escherichia coli. Mol. Microbiol. 87, 1074–1087 (2013).
Kysela, D. T., Brown, P. J. B., Huang, K. C. & Brun, Y. V. Biological consequences and advantages of asymmetric bacterial growth. Annu. Rev. Microbiol. 67, 417–435 (2013).
Adams, D. W. & Errington, J. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nature Rev. Microbiol. 7, 642–653 (2009).
Li, Y. et al. FtsZ protofilaments use a hinge-opening mechanism for constrictive force generation. Science 341, 392–395 (2013).
England, K., Crew, R. & Slayden, R. Mycobacterium tuberculosis septum site determining protein, Ssd encoded by rv3660c, promotes filamentation and elicits an alternative metabolic and dormancy stress response. BMC Microbiol. 11, 79 (2011).
Thakur, M. & Chakraborti, P. K. GTPase activity of mycobacterial FtsZ is impaired due to its transphosphorylation by the eukaryotic-type Ser/Thr kinase, PknA. J. Biol. Chem. 281, 40107–40113 (2006).
Dziedzic, R. et al. Mycobacterium tuberculosis ClpX interacts with FtsZ and interferes with FtsZ assembly. PLoS ONE 5, e11058 (2010).
Chauhan, A. et al. Interference of Mycobacterium tuberculosis cell division by Rv2719c, a cell wall hydrolase. Mol. Microbiol. 62, 132–147 (2006).
Sureka, K. et al. Novel role of phosphorylation-dependent interaction between FtsZ and FipA in mycobacterial cell division. PLoS ONE 5, e8590 (2010).
Datta, P. et al. Interaction between FtsW and penicillin-binding protein 3 (PBP3) directs PBP3 to mid-cell, controls cell septation and mediates the formation of a trimeric complex involving FtsZ, FtsW and PBP3 in mycobacteria. Mol. Microbiol. 62, 1655–1673 (2006).
Plocinski, P. et al. Characterization of CrgA, a new partner of the Mycobacterium tuberculosis peptidoglycan polymerization complexes. J. Bacteriol. 193, 3246–3256 (2011).
Zhang, Y. J. et al. Global assessment of genomic regions required for growth in Mycobacterium tuberculosis. PLoS Pathog. 8, e1002946 (2012).
Slayden, R. A. & Belisle, J. T. Morphological features and signature gene response elicited by inactivation of FtsI in Mycobacterium tuberculosis. J. Antimicrob. Chemother. 63, 451–457 (2009).
Mukherjee, P. et al. Novel role of Wag31 in protection of mycobacteria under oxidative stress. Mol. Microbiol. 73, 103–119 (2009).
Hett, E. C., Chao, M. C., Deng, L. L. & Rubin, E. J. A. Mycobacterial enzyme essential for cell division synergizes with resuscitation-promoting factor. PLoS Pathog. 4, e1000001 (2008).
Hett, E. C. et al. A partner for the resuscitation-promoting factors of Mycobacterium tuberculosis. Mol. Microbiol. 66, 658–668 (2007).
Deng, L. L. et al. Identification of a novel peptidoglycan hydrolase CwlM in Mycobacterium tuberculosis. Biochim. Biophys. Acta 1747, 57–66 (2005).
Machowski, E. E., Senzani, S., Ealand, C. & Kana, B. D. Comparative genomics for mycobacterial peptidoglycan remodelling enzymes reveals extensive genetic multiplicity. BMC Microbiol. 14, 75 (2014).
Mavrici, D. et al. Mycobacterium tuberculosis FtsX extracellular domain activates the peptidoglycan hydrolase, RipC. Proc. Natl Acad. Sci. USA 111, 8037–8042 (2014).
Avery, S. V. Microbial cell individuality and the underlying sources of heterogeneity. Nature Rev. Microbiol. 4, 577–587 (2006).
Sharma, K. et al. Transcriptional control of the mycobacterial embCAB operon by PknH through a regulatory protein, EmbR, in vivo. J. Bacteriol. 188, 2936–2944 (2006).
Salzman, V. et al. Transcriptional regulation of lipid homeostasis in mycobacteria. Mol. Microbiol. 78, 64–77 (2010).
Mondino, S., Gago, G. & Gramajo, H. Transcriptional regulation of fatty acid biosynthesis in mycobacteria. Mol. Microbiol. 89, 372–387 (2013).
Rajagopalan, M. et al. Mycobacterium tuberculosis origin of replication and the promoter for immunodominant secreted antigen 85B are the targets of MtrA, the essential response regulator. J. Biol. Chem. 285, 15816–15827 (2010).
Plocinska, R. et al. Septal localization of the Mycobacterium tuberculosis MtrB sensor kinase promotes MtrA regulon expression. J. Biol. Chem. 287, 23887–23899 (2012).
Rohde, K. H., Abramovitch, R. B. & Russell, D. G. Mycobacterium tuberculosis invasion of macrophages: linking bacterial gene expression to environmental cues. Cell Host Microbe 2, 352–364 (2007).
Kang, C.-M. et al. The Mycobacterium tuberculosis serine/threonine kinases PknA and PknB: substrate identification and regulation of cell shape. Genes Dev. 19, 1692–1704 (2005). This paper describes the essential role of the serine/threonine kinases PknA and PknB in the regulation of cell morphogenesis in mycobacteria.
Prisic, S. et al. Extensive phosphorylation with overlapping specificity by Mycobacterium tuberculosis serine/threonine protein kinases. Proc. Natl Acad. Sci. USA 107, 7521–7526 (2010).
Molle, V. et al. Phosphorylation of InhA inhibits mycolic acid biosynthesis and growth of Mycobacterium tuberculosis. Mol. Microbiol. 78, 1591–1605 (2010).
Veyron-Churlet, R., Zanella-Cleon, I., Cohen-Gonsaud, M., Molle, V. & Kremer, L. Phosphorylation of the Mycobacterium tuberculosis β-ketoacyl-acyl carrier protein reductase MabA regulates mycolic acid biosynthesis. J. Biol. Chem. 285, 12714–12725 (2010).
Corrales, R. M. et al. Phosphorylation of mycobacterial PcaA inhibits mycolic acid cyclopropanation: consequences for intracellular survival and phagosome maturation block. J. Biol. Chem. 287, 26187–26199 (2012).
Molle, V. & Kremer, L. Division and cell envelope regulation by Ser/Thr phosphorylation: Mycobacterium shows the way. Mol. Microbiol. 75, 1064–1077 (2010).
Chao, M. C. et al. Protein complexes and proteolytic activation of the cell wall hydrolase RipA regulate septal resolution in mycobacteria. PLoS Pathog. 9, e1003197 (2013).
Ruggiero, A. et al. Structure and functional regulation of RipA, a mycobacterial enzyme essential for daughter cell separation. Structure 18, 1184–1190 (2010).
Pearce, M. J., Mintseris, J., Ferryera, J., Gygi, S. P. & Darwin, K. H. Ubiquitin-like protein involved in the proteasome pathway of Mycobacterium tuberculosis. Science 322, 1101–1104 (2008). This paper describes the discovery of a prokaryotic version of the eukaryotic ubiquitin system, which is known as pupylation. Pupylation seems to be analogous, but not homologous, to ubiquitylation, as not all pupylated proteins are targeted to the proteasome.
Festa, R. A. et al. Prokayrotic ubiquitin-like protein (Pup) proteome of Mycobacterium tuberculosis. PLoS ONE 5, e8589 (2010).
Burns, K. E. et al. “Depupylation” of prokaryotic ubiquitin-like protein from mycobacterial proteasome substrates. Mol. Cell 39, 821–827 (2010).
Cambier, C. J. et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 505, 218–222 (2014).
Farhat, M. R. et al. Genomic analysis identifies targets of convergent positive selection in drug-resistant Mycobacterium tuberculosis. Nature Genet. 45, 1183–1189 (2013).
Kumar, P. et al. Meropenem inhibits D,D-carboxypeptidase activity in Mycobacterium tuberculosis. Mol. Microbiol. 86, 367–381 (2012).
Mahapatra, S., Scherman, H., Brennan, P. J. & Crick, D. C. N. Glycolylation of the nucleotide precursors of peptidoglycan biosynthesis of Mycobacterium spp. is altered by drug treatment. J. Bacteriol. 187, 2341–2347 (2005).
Skovierova, H. et al. Biosynthetic origin of the galactosamine substituent of arabinogalactan in Mycobacterium tuberculosis. J. Biol. Chem. 285, 41348–41355 (2010).
Lavollay, M. et al. The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by L,D-transpeptidation. J. Bacteriol. 190, 4360–4366 (2008). This paper describes the discovery that non-traditional 3–3 crosslinks are prevalent in mycobacterial peptidoglycan — a feature that is distinct from the peptidoglycan of other model organisms.
Barry, C. E., Crick, D. C. & McNeil, M. R. Targeting the formation of the cell wall core of M. tuberculosis. Infect. Disord. Drug Targets 7, 182–202 (2007).
Takayama, K., Wang, C. & Besra, G. S. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 18, 81–101 (2005).
Barkan, D., Hedhli, D., Yan, H. G., Huygen, K. & Glickman, M. S. Mycobacterium tuberculosis lacking all mycolic acid cyclopropanation is viable but highly attenuated and hyperinflammatory in mice. Infect. Immun. 80, 1958–1968 (2012).
Vander Beken, S. et al. Molecular structure of the Mycobacterium tuberculosis virulence factor, mycolic acid, determines the elicited inflammatory pattern. Eur. J. Immunol. 41, 450–460 (2010).
Dubnau, E. et al. Oxygenated mycolic acids are necessary for virulence of Mycobacterium tuberculosis in mice. Mol. Microbiol. 36, 630–637 (2000).
Griffin, J. E. et al. Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem. Biol. 19, 218–227 (2012).
Lee, W., VanderVen, B. C., Fahey, R. J. & Russell, D. G. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J. Biol. Chem. 288, 6788–6800 (2013).
Pandey, A. K. & Sassetti, C. M. Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl Acad. Sci. USA 105, 4376–4380 (2008).
Gupta, R. et al. The Mycobacterium tuberculosis protein LdtMt2 is a nonclassical transpeptidase required for virulence and resistance to amoxicillin. Nature Med. 16, 466–469 (2010). This study shows that the non-traditional 3–3 peptide crosslinks that are formed by LdtB are required for bacterial survival during chronic infection and for survival of amoxicillin treatment in a mouse model of TB.
Saxena, A. Srivastava, V. Srivastava, R. & Srivastava, B. S. Identification of genes of Mycobacterium tuberculosis upregulated during anaerobic persistence by fluorescence and kanamycin resistance selection. Tuberculosis 88, 518–525 (2008).
Vandal, O. H. et al. Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J. Bacteriol. 191, 625–631 (2009).
Patru, M.-M. & Pavelka, M. S. A. Role for the class A penicillin-binding protein PonA2 in the survival of Mycobacterium smegmatis under conditions of nonreplication. J. Bacteriol. 192, 3043–3054 (2010).
Keer, J., Smeulders, M. J., Gray, K. M. & Williams, H. D. Mutants of Mycobacterium smegmatis impaired in stationary-phase survival. Microbiology 146, 2209–2217 (2000).
Dutta, N. K. et al. Genetic requirements for the survival of tubercle bacilli in primates. J. Infect. Dis. 201, 1743–1752 (2010).
Griffin, J. E. et al. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 7, e1002251 (2011).
Rengarajan, J., Bloom, B. R. & Rubin, E. J. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc. Natl Acad. Sci. USA 102, 8327–8332 (2005).
Sassetti, C. M. & Rubin, E. J. Genetic requirements for mycobacterial survival during infection. Proc. Natl Acad. Sci. USA 100, 12989–12994 (2003).
Eoh, H. & Rhee, K. Y. Multifunctional essentiality of succinate metabolism in adaptation to hypoxia in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 110, 6554–6559 (2013).
Gill, W. P. et al. A replication clock for Mycobacterium tuberculosis. Nature Med. 15, 211–214 (2009).
Ford, C. B. et al. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nature Genet. 43, 482–486 (2011).
Chao, M. C. & Rubin, E. J. Letting sleeping dos lie: does dormancy play a role in tuberculosis? Annu. Rev. Microbiol. 64, 293–311 (2010).
Beste, D. J. V. et al. The genetic requirements for fast and slow growth in mycobacteria. PLoS ONE 4, e5349 (2009).
Cunningham, A. F. & Spreadbury, C. L. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton α-crystallin homolog. J. Bacteriol. 180, 801–808 (1998).
Galagan, J. E. et al. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature 499, 178–183 (2013).
Bhatt, A. et al. Deletion of kasB in Mycobacterium tuberculosis causes loss of acid-fastness and subclinical latent tuberculosis in immunocompetent mice. Proc. Natl Acad. Sci. USA 104, 5157–5162 (2007).
Balaban, N. Q. Bacterial persistence as a phenotypic switch. Science 305, 1622–1625 (2004).
Lenaerts, A. J. et al. Location of persisting mycobacteria in a guinea pig model of tuberculosis revealed by R207910. Antimicrob. Agents Chemother. 51, 3338–3345 (2007).
Baek, S.-H., Li, A. H. & Sassetti, C. M. Metabolic regulation of mycobacterial growth and antibiotic sensitivity. PLoS Biol. 9, e1001065 (2011).
Ojha, A. K. et al. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol. Microbiol. 69, 164–174 (2008).
Kaur, D., Guerin, M. E., Skovierová, H., Brennan, P. J. & Jackson, M. Chapter 2: biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis. Adv. Appl. Microbiol. 69, 23–78 (2009).
Bansal-Mutalik, R. & Nikaido, H. Mycobacterial outer membrane is a lipid bilayer and the inner membrane is unusually rich in diacyl phosphatidylinositol dimannosides. Proc. Natl Acad. Sci. USA 111, 4958–4963 (2014).
Sani, M. et al. Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins. PLoS Pathog. 6, e1000794 (2010).
Zumla, A., Nahid, P. & Cole, S. T. Advances in the development of new tuberculosis drugs and treatment regimens. Nature Rev. Drug Discov. 12, 388–404 (2013).
Hancock, I. C., Carman, S., Besra, G. S., Brennan, P. J. & Waite, E. Ligation of arabinogalactan to peptidoglycan in the cell wall of Mycobacterium smegmatis requires concomitant synthesis of the two wall polymers. Microbiology 148, 3059–3067 (2002).
Raymond, J. B., Mahapatra, S., Crick, D. C. & Pavelka, M. S. Identification of the namH gene, encoding the hydroxylase responsible for the N-glycolylation of the mycobacterial peptidoglycan. J. Biol. Chem. 280, 326–333 (2005).
Mahapatra, S. et al. Mycobacterial lipid II is composed of a complex mixture of modified muramyl and peptide moieties linked to decaprenyl phosphate. J. Bacteriol. 187, 2747–2757 (2005).
Girardin, S. E. et al. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 278, 41702–41708 (2003).
Coulombe, F. et al. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J. Exp. Med. 206, 1709–1716 (2009).
Hansen, J. M. et al. N-glycolylated peptidoglycan contributes to the immunogenicity but not pathogenicity of Mycobacterium tuberculosis. J. Infect. Dis. 209, 1045–1054 (2014).
Makarov, V. et al. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science 324, 801–804 (2009).
Crick, D. C., Mahapatra, S. & Brennan, P. J. Biosynthesis of the arabinogalactan–peptidoglycan complex of Mycobacterium tuberculosis. Glycobiology 11, 107R–;118R (2001).
Skovierova, H. et al. AftD, a novel essential arabinofuranosyltransferase from mycobacteria. Glycobiology 19, 1235–1247 (2009).
Layre, E. et al. A comparative lipidomics platform for chemotaxonomic analysis of Mycobacterium tuberculosis. Chem. Biol. 18, 1537–1549 (2011).
Sacco, E. et al. The missing piece of the type II fatty acid synthase system from Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 104, 14628–14633 (2007).
Lea-Smith, D. J. et al. The reductase that catalyzes mycolic motif synthesis is required for efficient attachment of mycolic acids to arabinogalactan. J. Biol. Chem. 282, 11000–11008 (2007).
Leger, M. et al. The dual function of the Mycobacterium tuberculosis FadD32 required for mycolic acid biosynthesis. Chem. Biol. 16, 510–519 (2009).
Grzegorzewicz, A. E. et al. Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nature Chem. Biol. 8, 334–341 (2012).
Pacheco, S. A., Hsu, F. F., Powers, K. M. & Purdy, G. E. MmpL11 protein transports mycolic acid-containing lipids to the mycobacterial cell wall and contributes to biofilm formation in Mycobacterium smegmatis. J. Biol. Chem. 288, 24213–24222 (2013).
Varela, C. et al. MmpL genes are associated with mycolic acid metabolism in mycobacteria and corynebacteria. Chem. Biol. 19, 498–506 (2012).
Carel, C. et al. Mycobacterium tuberculosis proteins involved in mycolic acid synthesis and transport localize dynamically to the old growing pole and septum. PLoS ONE 9, e97148 (2014).
Scheffers, D.-J. & Pinho, M. G. Bacterial cell wall synthesis: new insights from localization studies. Microbiol. Mol. Biol. Rev. 69, 585–607 (2005).
Singh, S. K., SaiSree, L., Amrutha, R. N. & Reddy, M. Three redundant murein endopeptidases catalyse an essential cleavage step in peptidoglycan synthesis of Escherichia coli K12. Mol. Microbiol. 86, 1036–1051 (2012).
Meisner, J. et al. FtsEX is required for CwlO peptidoglycan hydrolase activity during cell wall elongation in Bacillus subtilis. Mol. Microbiol. 89, 1069–1083 (2013).
Kawai, Y., Daniel, R. A. & Errington, J. Regulation of cell wall morphogenesis in Bacillus subtilis by recruitment of PBP1 to the MreB helix. Mol. Microbiol. 71, 1131–1144 (2009).
Garner, E. C. et al. Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in B. subtilis. Science 333, 222–225 (2011).
Fenton, A. K. & Gerdes, K. Direct interaction of FtsZ and MreB is required for septum synthesis and cell division in Escherichia coli. EMBO J. 32, 1953–1965 (2013).
Errington, J., Daniel, R. A. & Scheffers, D. J. Cytokinesis in bacteria. Microbiol. Mol. Biol. Rev. 67, 52–65 (2003).
Smith, T. J., Blackman, S. A. & Foster, S. J. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiol. 146, 249–262 (2000).
de Boer, P. A. J. Advances in understanding E. coli cell fission. Curr. Opin. Microbiol. 13, 730–737 (2010).
Acknowledgements
The authors thank S. Dove, C. Sassetti and S. Fortune for discussion of the ideas presented in this Review and C. Boutte, M. Chao and N. Peters for critical reading of the manuscript. The authors thank J. McKinney and anonymous referees for excellent suggestions and improvements and A. Goranov for discussion of cell growth models. Support was provided by the US National Institutes of Health (NIH) (grant number U01-GM094568 to E.J.R.) and the US National Science Foundation (NSF) Graduate Research Fellowship (grant number DGE-1144152 to K.J.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Supplementary information
Supplementary information S1 (table)
Main cell elongation and division proteins (PDF 201 kb)
Glossary
- Granuloma
-
An organized cellular structure that forms owing to the immune response to an invading pathogen. An array of immune and fibrotic cells 'wall-off' the invading pathogen in an attempt to limit its spread within the host.
- Latency
-
An asymptomatic disease state in which the bacterial burden is low or undetectable.
- Non-traditional 3–3 peptide crosslinks
-
Peptide bonds between the third residues (meso-diaminopimelic acid (mDAP) in mycobacteria) of two peptide tails in peptidoglycan; a traditional crosslink is a peptide bond between the third and fourth residues (between DAP and D-Ala in mycobacteria) of two peptide tails.
- Cytokinesis
-
The process in which a plasma membrane forms in the mid-cell region of a mother cell during cell division.
- Septum
-
A layer of peptidoglycan that is formed at approximately mid-cell (in mycobacteria) that generates a physical barrier between nascent daughter cells.
- Microfluidics
-
A technology in which small fabricated chambers are used to isolate and image single cells.
- Microcolonies
-
Groups of progeny cells that are generated from the growth and division of single cells.
- Periplasm
-
The extracellular space between the plasma membrane and the outer membrane of Gram-negative bacteria. The mycolic acid layer of mycobacteria functions analogously to an outer membrane, and thus, the space between the plasma membrane and the mycolic acid layer is known as the periplasm in mycobacteria.
- Actinobacteria
-
A phylum of bacteria to which mycobacteria belong. This phylum also includes species such as Streptomyces coelicolor and Corynebacterium glutamicum.
- Phosphomimetic
-
An amino acid substitution, typically by an aspartic acid or glutamic acid, at the site of a phosphorylated residue in a protein, which mimics constitutive phosphorylation.
- MinCDE system
-
A protein system in Escherichia coli that regulates placement of the division septum in the cell. MinCDE oscillate from pole-to-pole and establish a concentration gradient to prevent Z-ring formation at the cell poles.
- SOS response
-
A response to DNA damage in which bacteria halt cell cycle progression and induce DNA repair mechanisms in an effort to repair the chromosome before continuing division.
- FASI and FASII complexes
-
(Fatty acid synthase I and II complexes). Complexes that are involved in mycolic acid synthesis. FASI, which is composed of the protein Fas, synthesizes the initial C20–C26 α-branch of mycolic acids. This branch is joined to a C60–C90 meromycolate branch that is synthesized by the FASII complex, which is composed of MabA, HadABC, InhA, KasAB and an unidentified isomerase.
- Pupylation
-
A system that typically targets proteins for proteasomal degradation by the addition of a small prokaryotic ubiquitin-like protein (Pup). Pupylation directs some, but not all, of its targets to the proteasome, which suggests that pupylation may have additional roles in regulating protein activity.
- Chemostat
-
A closed system for bacterial growth that is used to control the bacterial replication rate and/or metabolism by the use of chemically defined media.
Rights and permissions
About this article
Cite this article
Kieser, K., Rubin, E. How sisters grow apart: mycobacterial growth and division. Nat Rev Microbiol 12, 550–562 (2014). https://doi.org/10.1038/nrmicro3299
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3299
This article is cited by
-
Structure-based drug design and characterization of novel pyrazine hydrazinylidene derivatives with a benzenesulfonate scaffold as noncovalent inhibitors of DprE1 tor tuberculosis treatment
Molecular Diversity (2024)
-
In vivo imaging of MmpL transporters reveals distinct subcellular locations for export of mycolic acids and non-essential trehalose polyphleates in the mycobacterial outer membrane
Scientific Reports (2023)
-
An Arg/Ala-rich helix in the N-terminal region of M. tuberculosis FtsQ is a potential membrane anchor of the Z-ring
Communications Biology (2023)
-
Eukaryotic-like gephyrin and cognate membrane receptor coordinate corynebacterial cell division and polar elongation
Nature Microbiology (2023)
-
Types and functions of heterogeneity in mycobacteria
Nature Reviews Microbiology (2022)