Abstract
Clinical trials of vaccines against Mycobacterium tuberculosis are well under way and results are starting to come in. Some of these results are not so encouraging, as exemplified by the latest Aeras-422 and MVA85A trials. Other than empirically determining whether a vaccine reduces the number of cases of active tuberculosis, which is a daunting prospect given the chronic nature of the disease, we have no way of assessing vaccine efficacy. Therefore, investigators seek to identify biomarkers that predict vaccine efficacy. Historically, focus has been on the production of interferon-γ by CD4+ T cells, but this has not been a useful correlate of vaccine-induced protection. In this Opinion article, we discuss recent advances in our understanding of the immune control of M. tuberculosis and how this knowledge could be used for vaccine design and evaluation.
Similar content being viewed by others
Main
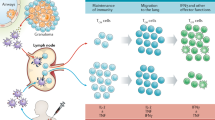
Tuberculosis (TB) is caused by the pathogenic bacterium Mycobacterium tuberculosis, which is transmitted between people via aerosol droplets that contain bacteria. The droplets are inhaled and deposited in distal lung alveoli1 (Fig. 1). M. tuberculosis is an intracellular bacterium and, although it can infect different cell types, alveolar macrophages are its 'favourite' niche. The initial stages of infection are characterized by innate immune responses that involve the recruitment of inflammatory cells to the lungs2; induction of an adaptive immune response occurs only later, after the dissemination of M. tuberculosis to draining lymph nodes3,4,5. In the lymph nodes, presentation of bacterial antigens by dendritic cells leads to priming and expansion of antigen-specific T cells, which differentiate from naïve T cells into effector T cells. The effector T cells then migrate to the infected lung and, in combination with other leukocytes, stimulate the formation of granulomas. Granulomas are organized structures that contain macrophages, lymphocytes and fibroblasts6. Within the granuloma, macrophages are activated — for example, by interferon-γ (IFNγ) secreted by CD4+ T cells (that is, TH1 cells) — which is thought to restrict the dispersal and replication of M. tuberculosis.
Infection is initiated by the inhalation of aerosol droplets that contain bacteria. The initial stages of infection are characterized by innate immune responses that involve the recruitment of inflammatory cells to the lung. Following bacterial dissemination to the draining lymph node, dendritic cell presentation of bacterial antigens leads to T cell priming and triggers an expansion of antigen-specific T cells, which are recruited to the lung. The recruitment of T cells, B cells, activated macrophages and other leukocytes leads to the establishment of granulomas, which can contain Mycobacterium tuberculosis. Most infected individuals will remain in a 'latent' state of infection, in which no clinical symptoms are present. A small percentage of these people will eventually progress and develop active disease, which can lead to the release of M. tuberculosis from granulomas that have eroded into the airways. When individuals with active tuberculosis (TB) cough, they can generate infectious droplets that transmit the infection.
Although the human immune system can control the infection, control does not invariably lead to sterilization. In fact, most people who are infected with M. tuberculosis are clinically asymptomatic, which is a state that is referred to as latent TB7. These latently infected people — who are estimated to make up one-third of the global population — represent an enormous reservoir of potential disease. Epidemiological studies find that 5–10% of people with latent TB will develop active disease sometime during their lives8. Individuals with active TB cough and generate infectious droplets that propagate the infection (Fig. 1).
An effective vaccine is needed to stop the ongoing pandemic. Bacille Calmette–Guérin (BCG), which is an attenuated form of Mycobacterium bovis, was introduced nearly a century ago as a vaccine against M. tuberculosis, but it has had little effect on eliminating TB. In part, this is because BCG efficacy against active pulmonary TB is extremely variable between populations and BCG-induced protection is significantly lower in the developing world9. Remarkable progress has been made in the development of new vaccine candidates and several are now in clinical trials (Box 1). Although there is some pessimism about whether a vaccine that averts infection can be developed, the general consensus is that a vaccine that prevents progression to active disease could reduce the prevalence of pulmonary TB and ultimately break the cycle of transmission.
Most antiviral vaccines that have proven to be effective are based on antibody-mediated immunity. As is the case for many intracellular bacteria, M. tuberculosis is able to avoid most of the antibacterial effects that are mediated by antibodies by living and growing inside macrophages. Thus, on the basis of the substantial experimental foundation that T cell immunity is required to control primary M. tuberculosis infection, the consensus among vaccine specialists is that vaccine-induced T cell-mediated immunity will be required to prevent clinical TB. However, despite considerable advances in defining how the immune system responds to M. tuberculosis, our understanding of protective immunity following infection (that is, natural immunity) is incomplete. Furthermore, little is known about the mechanisms of vaccine-induced immunity and whether it differs from natural immunity, and studies to answer these questions have not kept pace with the speed with which new vaccines are entering clinical trials. It is unknown which immunological parameters or biomarkers predict who will control the infection and who will develop clinical disease, both in the setting of natural immunity and in the setting of vaccine-induced immunity. Such knowledge would revolutionize our approach to the surveillance, control and treatment of TB and would greatly accelerate vaccine design and evaluation. However, identifying biomarkers of vaccine protection is difficult; until there is a successful vaccine that induces protective immunity, how can such a biomarker be identified? As it stands, any success or failure of TB vaccines will mostly be empiric and difficult to predict.
In this Opinion article, we discuss immune defences against M. tuberculosis infection. T cells predominantly mediate protective immunity, and recent results begin to clarify how different T cell subsets and functions restrict bacterial growth. Finally, we discuss how one might use knowledge about these different mechanisms to develop new vaccine strategies for the prevention of TB.
The 'central dogma' of protective immunity
Establishing the importance of IFNγ. During the past four decades, the predominant paradigm in both basic and clinical research has been that the production of IFNγ by CD4+ T cells is the major driver of immunity to TB. Research in the 1970s found that T cells, and not antibodies, are required for host resistance to TB and established the mouse as a useful model of tuberculosis10. The T cell hypothesis was further refined in the 1980s with the identification of CD4+ T cells that produce IFNγ (that is, TH1 cells) as the dominant T cell subset that participates in the immune response to M. tuberculosis11,12. The use of knockout mice in the 1990s established a crucial role for CD4+ T cells, with additional roles for CD8+ T cells, invariant natural killer T (iNKT) cells and γδ T cells13,14. The discovery that AIDS — a condition that is often associated with TB — was caused by HIV, which is a virus that infects and kills CD4+ T cells, supported a key role for CD4+ T cells in immunity against M. tuberculosis in people15.
A central role for IFNγ — a cytokine that is involved in the response against viruses and intracellular bacteria — in antimycobacterial immunity is based on the extreme susceptibility of mice that lack IFNγ16,17. IFNγ activates macrophages to kill intracellular bacteria by activating downstream antimicrobial effector pathways, including inducible nitric oxide synthase (iNOS), IFNγ-inducible GTPases, phagosomal maturation and acidification, autophagy and vitamin D receptor signalling18,19,20,21,22,23. Genetic studies confirm a role for IFNγ in people: families with mutations in the interleukin-12 (IL-12)–IFNγ–signal transducer and activator of transcription 1 (STAT1) axis develop disseminated infections caused by BCG and non-tuberculosis mycobacteria (NTM) species. This inherited susceptibility, which is called Mendelian susceptibility to mycobacterial disease (MSMD), reveals the crucial nature of this signalling pathway, which was first described in mice16,17,24,25.
These discoveries helped to define the 'central dogma' of TB immunity, namely, that the production of IFNγ by T cells activates macrophages to kill intracellular M. tuberculosis (Fig. 2a). Indeed, detection of IFNγ produced by T cells is the most widely used method for monitoring immune responses following infection or vaccination.
a | The 'central dogma' of protective immunity to tuberculosis (TB) is that CD4+ T cells produce interferon-γ (IFNγ) (T helper 1 (TH1) cells), which synergizes with tumour necrosis factor (TNF; produced by the T cell or the macrophage), and together these activate macrophage antimicrobial activity that is capable of restricting the growth of Mycobacterium tuberculosis. Two pathways activated by IFNγ that are capable of killing M. tuberculosis are nitric oxide production and phagosome–lysosome fusion, which acidifies the bacterial phagosome. b | 'A revised view of T cell-mediated immunity' incorporates additional T cell subsets (CD4+ T cells, CD8+ T cells and unconventional T cells: γδ T cells, mucosal-associated invariant T (MAIT) cells and CD1-restricted T cells) and includes additional mechanisms by which T cells mediate killing of M. tuberculosis. These include additional cytokines (for example, granulocyte–macrophage colony-stimulating factor (GM-CSF)) and cytolysis of infected macrophages. The cytolytic mechanisms vary and can include cytotoxic granules, which can deliver antimicrobial peptides, such as granulysin, but can also deliver granzymes, which can trigger apoptotic cell death. Cytotoxic T lymphocyte (CTL) activity mediated by FAS ligand (FASL)–FAS or TNF can also lead to apoptosis. Apoptosis can have a beneficial effect on the outcome of infection, as infected apoptotic cells can be engulfed by bystander macrophages, which are capable of destroying the apoptotic cells, including any intracellular bacteria. Finally, several components of the innate response, including interleukin-1 (IL-1) and vitamins, can synergize with cytokines that are produced by T cells. c | 'Protective T cells and vaccination' focuses on the desired features of protective T cell responses. Rational vaccine design should aim to elicit protective T cells by optimizing their action on infected cells in several ways. Vaccine-elicited memory T cells must rapidly expand and generate secondary effector T cells that undergo sustained proliferation following activation. Whereas the functions of primary effector T cells are heterogeneously expressed, vaccination can lead to more homogenous expression of effector functions during the recall response. Such T cells, which are often identified as multifunctional T cells, may have a greater protective potential. Primed effector and memory T cells should efficiently traffic to sites of infection, but the kinetics of the response must be balanced with respect to T cell subsets and limit the potential for T cell exhaustion, excessive inflammatory pathology or an ineffective response that hinders T cell–target contact.
Shortcomings of the central dogma. Although IFNγ and CD4+ T cells are key components of the immune responses against mycobacteria, the intricacies of immunity to M. tuberculosis require that we reassess their roles. For example, the risk of active TB significantly increases during the first year after HIV infection, despite normal CD4+ T cell counts26, and progression to AIDS — which is characterized by a substantial loss of CD4+ T cells — does not correlate with the development of active TB26,27. HIV infection induces several immunological abnormalities, some of which are apparent even before CD4+ T cell numbers decline28. It is possible that alterations in CD4+ T cell function that are secondary to HIV infection increase TB susceptibility even before CD4+ T cell numbers decrease. However, this pattern of susceptibility is clearly different from other opportunistic infections, in which incidence correlates with the peripheral blood CD4+ T cell count in HIV patients29.
Similar complexity is observed in patients with MSMD: more than 300 cases of MSMD have been described, but M. tuberculosis infection was present in only four cases; the rest were infections with BCG or NTM species24. Although such bias might reflect the relative exposure to BCG or NTM compared with M. tuberculosis, IFNγ-activated pathways might be more important for immunity against NTM than against M. tuberculosis24,30. Although these rare cases of TB and immunodeficiency are instructive, most people who develop active TB have no obvious defects in their T cell compartment and generate M. tuberculosis-specific IFNγ responses. Thus, whereas HIV and MSMD patient data establish that T cells and IFNγ are required for immunity against M. tuberculosis, T cells that produce IFNγ do not seem to be sufficient to prevent active disease.
The shortcomings of the 'central dogma' also apply to disease progression and vaccine-induced protection in otherwise healthy people, as more T cells that secrete IFNγ, or greater IFNγ levels, do not correlate with protection31. In fact, patients whose T cells produce greater amounts of IFNγ are more likely to progress to active disease than patients with weaker responses32, which supports the idea that IFNγ levels correlate better with bacterial burden than with disease control. Such a correlation between increased M. tuberculosis bacterial burden and increased T cell IFNγ production has been observed in humans, non-human primates (NHPs) and mice32,33,34,35.
Similar conclusions can be drawn from vaccination studies36. BCG vaccination can elicit protective T cells in experimental animals, but IFNγ production by these T cells has not been predictive of vaccine-induced protection36,37. The only predictor of protection in mice that are vaccinated with BCG is an increased number of antigen-specific CD8+ T cells36. In some human studies, increased IFNγ production by T cells has been observed after BCG vaccination or adult re-vaccination, but protection was not evaluated38,39,40,41. One study in South African infants who were vaccinated with BCG addressed the relationship between vaccine-induced protection, T cell frequency and cytokine profile but found no correlation between the number of BCG-elicited T cells that produce IFNγ or multiple cytokines (such as IFNγ, IL-2 and tumour necrosis factor (TNF)) and the development of culture-positive TB42.
These data raise the question: if CD4+ T cells and IFNγ are important, why doesn't IFNγ production by CD4+ T cells correlate with protection? The idea that, because CD4+ T cells produce IFNγ, their IFNγ production must be important is an assumption that has little supporting data. Several studies have shown that CD4+ T cells protect mice against M. tuberculosis independently of IFNγ43,44,45,46,47. Transgenic CD4+ T cells, which are specific for the M. tuberculosis antigen ESAT6 (6 kDa early secretory antigenic target) retain their ability to protect mice against M. tuberculosis even when they are unable to produce IFNγ or TNF44. Similarly, the ability of IFNγ-null memory T cells to mediate protection is only slightly diminished compared with wild-type memory T cells45,48. These studies show that, although CD4+ T cells and IFNγ are important for M. tuberculosis control, T cell functions other than IFNγ production can mediate protection. Furthermore, it is not known how much IFNγ is needed, which cells are required to produce it and whether more is better36. Also, the inflammatory microenvironment in which IFNγ is produced might be important, as the balance between IFNγ and different cytokines, such as IL-10 and other T helper 2 (TH2) cell cytokines, is likely to influence disease outcome49. Therefore, it is crucial to identify factors that are required for resistance and that correlate with susceptibility in individuals with intact immune systems, as opposed to components of the immune response that are necessary for protection but that do not predict clinical outcome or disease state.
The idea that IFNγ is necessary but not sufficient for bacterial control following mycobacterial infection is supported by multiple studies in mice. For example, several knockout mice (such as TNF-, granulocyte–macrophage colony-stimulating factor (GM-CSF)-, IL-1- and IL-6-null mice) die rapidly following M. tuberculosis infection, similarly to IFNγ-null animals50,51,52,53. As these mice produce IFNγ, their failure to control M. tuberculosis indicates that additional pathways besides IFNγ production are essential for immunity. Although the data from knockout mice and MSMD families is irrefutable, more mechanistic insights into the protective pathways that lead to M. tuberculosis control are needed. It is also important to remember that, although IFNγ inhibits M. tuberculosis replication in mouse macrophages54, it is not sufficient to control M. tuberculosis growth in human macrophages55,56. Similarly, nitric oxide (NO) production by murine macrophages can kill M. tuberculosis, but its production by human alveolar macrophages and its role in controlling M. tuberuclosis in these cells is controversial57,58. These observations reinforce the idea that we must look beyond the CD4+ T cell–IFNγ central dogma (Fig. 2a) to identify other immunological functions that protect against M. tuberculosis.
Reassessing protective immunity
In recent years, many studies have looked past the central dogma and have shown that different pathways are involved in protective immunity during TB. These studies reveal characteristics of protective T cells that should be incorporated in the design of new vaccines against M. tuberculosis (Fig. 2b).
Other mediators that activate macrophages. The cytokines TNF, GM-CSF and IL-1β and vitamins C and D are all implicated as mediators that activate macrophages to control the growth of M. tuberculosis. Mice that lack TNF are highly susceptible to M. tuberculosis infection, and TNF production by T cells has been shown to be important for resistance against M. tuberculosis50,59. TNF synergizes with IFNγ in stimulating NO production by macrophages, maintaining granuloma structure and limiting immunopathology, possibly by modulating IL-10 levels, by inhibition of TH2 cell responses and by limiting neutrophil infiltration60,61,62. The widespread use of TNF blockers to treat patients with autoimmune diseases for which TNF is a pathogenic factor, such as rheumatoid arthritis, has resulted in many cases of reactivated latent TB. This establishes TNF as an important mediator of resistance to M. tuberculosis in people63.
Mice lacking GM-CSF are highly susceptible to M. tuberculosis, and GM-CSF treatment of mouse and human macrophages restricts the intracellular growth of M. tuberculosis and Mycobacterium avium52,64,65,66. GM-CSF is produced by a range of cells, including leukocytes, epithelial cells and fibroblasts, and loss of this cytokine leads to abnormalities in surfactant recycling and the development of a lung disease that resembles human pulmonary alveolar proteinosis67. Overexpressing GM-CSF in epithelial cells reverses these lung abnormalities, but susceptibility to M. tuberculosis remains, which suggests that GM-CSF production by other cells — perhaps T cells — contributes to protection in mice. This idea is supported by the observation that iNKT cell production of GM-CSF contributes to host resistance against tuberculosis66. In addition, the presence of GM-CSF-specific autoantibodies that block GM-CSF function has been linked to both cryptococcal meningitis and pulmonary TB in otherwise healthy people, which indicates that GM-CSF has an important role in host defences against infection in people68.
Mice that lack IL-1β — a pro-inflammatory cytokine that is produced by macrophages — or its receptor are highly susceptible to M. tuberculosis infection, and IL-1β directly inhibits intracellular growth of M. tuberculosis47,51,69,70,71. Although mice that lack IL-1β die prematurely from infection, IL-1β can also be detrimental by recruiting pathogenic TH17 cells and neutrophils to the lungs, which results in tissue inflammation46,72,73. IL-1β also activates human macrophages to control bacterial replication69,71,74.
Stimulation with either IFNγ or a ligand that triggers Toll-like receptor (TLR2) and TLR1 induces the nuclear vitamin D receptor (VDR) and enzymes that catalyse the conversion of vitamin D to its bioactive form22,75. Signalling through VDR elicits the production of the human cathelicidin LL-37, which is an antimicrobial peptide that directly kills M. tuberculosis76. Beyond its role in cathelicidin production, vitamin D is involved in autophagy, phagosome–lysosome fusion and IL-1β production22,77,78. Dissecting the role of vitamin D has been challenging. Multiple studies show decreased levels of bioactive vitamin D in patients with TB, but whether this is a cause or an effect of TB is unknown, and whether Vitamin D supplementation benefits treatment is still uncertain79.
Vitamin C might be important for immunity against M. tuberculosis, as vitamin C affects M. tuberculosis survival and growth80. Given the established association between malnutrition and susceptibility to TB, it is important to determine whether specific nutritional deficiencies contribute to susceptibility to M. tuberculosis.
Killing of infected macrophages. In addition to cytokine production, T cells — particularly CD8+ T cells — kill cells that they recognize as 'foreign'. CD8+ T cells that have the capacity to kill target cells are called cytotoxic T lymphocytes (CTLs). M. tuberculosis elicits CD8+ T cell responses in people and in animal models, and these CD8+ T cells function as CTLs in vivo81,82,83,84. Three different molecular pathways mediate CTL activity: exocytosis of cytotoxic granules containing proteins that cause the lysis and apoptosis of target cells, such as perforin, granulysin and granzymes; FAS (also known as CD95) or FAS ligand (FASL; also known as CD95L), which are cell surface proteins that mediate death signalling; and TNF84. The increased susceptibility of Fas−/−, FasL−/− and perforin−/− mice to M. tuberculosis corroborate the importance of these pathways for immunity85,86. Importantly, perforin is required for CTL-mediated protection84. Human CD8+ T cells also require perforin to restrict M. tuberculosis growth, and granulysin is an important granule constituent87. Other than perforin, the crucial effector molecules for mouse CD8+ T cells are unknown87.
How killing of infected macrophages by CD8+ T cells impairs M. tuberculosis survival is an active area of investigation. All three killing pathways induce target cell apoptosis, which is associated with reduced bacterial viability88. The engulfment of apoptotic, infected cells by uninfected macrophages — which is a process known as efferocytosis — leads to the rapid association of the bacteria trapped in the phagocytosed apoptotic cell (known as the 'efferosome') with lysosomes and the killing of M. tuberculosis89.
T cells orchestrate granuloma formation. In addition to detecting infected macrophages, T cells have a key role in the formation of granulomas. T cell-derived cytokines (such as TNF) and chemokines (such as CC-chemokine ligand 3 (CCL3)) recruit inflammatory macrophages, neutrophils and B cells to the granuloma90. IFNγ and TNF maintain granuloma architecture in mice and people17,63,91,92,93. The importance of CD4+ T cells in shaping the granuloma microenvironment is inferred from HIV-positive subjects who form dysfunctional granulomas that fail to contain M. tuberculosis94 and from studies in guinea pigs and rabbits95,96. Recent imaging studies in people and NHPs indicate that granulomas function autonomously and are more dynamic than previously appreciated8,97,98. Granulomas change over time independently of each other with respect to size and metabolic activity: some shrink, whereas others expand. Although CD4+ T cells promote granuloma formation early after M. tuberculosis infection, they also contribute to transmission by promoting granuloma necrosis accompanied by erosion into airways during later disease stages99.
Balancing pro- and anti-inflammatory signals. In many chronic infections, including TB, immune-mediated tissue injury is more detrimental than the pathogen itself. Therefore, there are mechanisms to counter-regulate pro-inflammatory immune cells and prevent the harmful effects of excessive inflammation; however, these effects might also dampen protective immunity.
Foxp3+ (CD4+CD25+) regulatory T cells (TRegs) suppress inflammation and limit immune responses by producing immunosuppressive cytokines, such as IL-10 and transforming growth factor β (TGFβ), and by directly interacting with other cells via inhibitory cell surface molecules100. TRegs are generated following M. tuberculosis infection in humans, NHPs and mice101,102,103,104. In mice, TReg elimination can improve protective immunity, as shown by the survival of fewer bacteria; however, whether this occurs at the risk of greater tissue injury has not been addressed104,105,106.
Chronic antigen stimulation and exposure to inflammatory cytokines leads to a state of T cell exhaustion that is manifested by a progressive loss of T cell function over time, which has been best documented during chronic viral infection. There is great interest in the mediators of exhaustion, as blocking them might 're-invigorate' T cell immunity and promote pathogen clearance during chronic infection. One such mediator, programmed cell death 1 (PD-1), is a cell surface receptor that is expressed by antigen-activated T cells. Interaction of PD-1 with its ligands transduces a signal that inhibits T cell proliferation and cytokine production107. Disruption of the PD-1–ligand interaction, by the use of neutralizing antibodies or knockout of PD-1 in mice, increases the number and function of M. tuberculosis-specific T cells in the lungs of infected mice108,109,110. However, in the absence of PD-1 signalling, dysregulation of CD4+ T cells leads to increased bacterial burden, the destruction of lung tissue and the death of infected mice108,110. These data suggest that T cell exhaustion might be a beneficial regulatory mechanism that prevents overt immunopathology.
Neutrophils have an early protective role against M. tuberculosis in the lungs by producing IL-1β, TNF, defensins, cathelicidins, lipocalin, NADPH oxidase and superoxides76,111,112,113. Neutrophils also participate in T cell priming, including cross-presentation of class I-restricted antigens, which is a process that is important for the stimulation of CD8+ T cells by intracellular pathogens114. However, when the short-lived neutrophils die, the pro-inflammatory contents of their granules can be released; thus, an excess of neutrophils can promote tissue damage.
Although IFNγ is a pro-inflammatory cytokine, it also limits inflammation, at least in part by the direct and indirect inhibition of neutrophils. IFNγ can have anti-proliferative effects on T cells and modulate their function, including inhibiting CD4+ T cell production of IL-17, which is a cytokine that drives neutrophilic inflammation115. In addition, IFNγ acts directly on neutrophils to inhibit their accumulation in the lung46,72. In fact, we view neutrophil infiltrates in the lung as a sign of failed TH1 cell immunity, which leads to accelerated tissue destruction during chronic M. tuberculosis infection46. Similarly, NO restrains inflammation by inhibiting IL-1β production by macrophages. Whereas NO production by mouse macrophages mediates the antimicrobial activity of IFNγ, NO also inhibits NLRP3 inflammasome assembly, which curtails the production of IL-1β116.
Collectively, these data support the idea that T cells are uniquely positioned to influence the balance of pro- and anti-inflammatory signals. These results strengthen the idea that the function of CD4+ T cells and IFNγ is broader than activating macrophages and is necessary for optimal immunity during M. tuberculosis infection. Thus, IFNγ functions as a key negative regulator of innate immunity, including neutrophils and IL-1β, both of which might be beneficial early in M. tuberculosis infection but have detrimental effects if they persist into the chronic phase of infection. The anti-inflammatory role of T cells might prevent over-exuberant protective responses that cause harmful immunopathology and tissue damage during chronic infection46,116. Measuring surrogates of pro- or anti-inflammatory signals — for example, by expression profiling117 or by measuring the monocyte/lymphocyte ratio118 in peripheral blood — could help to identify individuals who are at risk of developing active TB.
Other cells participate in the immune response to M. tuberculosis. Although it is generally accepted that conventional CD4+ and CD8+ T cells mediate protection against M. tuberculosis, many other cell types participate in the immune response (see Box 2 for the contribution of non-conventional T cells). The TB mouse model is CD4+ T cell-centric and it is difficult to prove a role even for conventional CD8+ T cells. Other T cell subsets are not present or are qualitatively different in the mouse compared to humans. Similarly, the contribution of B cells and antibody-mediated immunity needs further clarification119. Thus, these different cell types need to be investigated in other models. Both CD8+ T cells and non-conventional T cells seem to have a quantitatively greater role in immunity to M. tuberculosis in NHPs than in other animal models81,120. Understanding the roles of these different cell types during M. tuberculosis infection might provide opportunities to discover new protective effector functions and to develop methods to augment their function as part of new vaccination or treatment strategies.
Lessons for developing T cell vaccines
Is natural immunity against TB sufficient? Many pathogens do not elicit protective immunity, including common pathogens that cause urinary tract infections (such as enteric Gram-positive and Gram-negative bacteria), pathogens that cause sexually transmitted infections such as Chlamydia trachomatis, Neisseria gonorrhea and Treponema pallidum and group A streptococci that cause pharyngitis. Other pathogens, such as poxviruses, induce long-term protection — an observation that is the basis of vaccination. It is still unclear why some pathogens, but not others, induce protective immunity against reinfection. Which is the case for TB? If only around 10% of infected people develop active disease during their lifetime, one must concede that natural immunity works well, even if it doesn't lead to sterilization. What about the 10% who develop symptomatic disease? Although progression to symptomatic disease can sometimes be attributed to acquired immunodeficiency (caused by AIDS, TNF blockade, corticosteroids, autoantibodies etc.), in many cases, immunocompetent individuals also develop active TB, which indicates failure of their immune systems to control the infection. Why does the immune system fail to enforce latency and enable active disease to emerge in immunocompetent individuals?
People who have previously been treated for TB are at higher risk of developing additional episodes of disease121,122,123,124,125. Can this be attributed to relapse after inadequate treatment? Or do these individuals have a defect in immunity that might explain why they developed disease in the first place? If these are the patients who we are trying to protect with vaccination, we need to understand why they are susceptible to TB. This is important, as vaccines that aim to augment typical immune responses might fail to protect people with immune defects. Such people might not respond normally to vaccines or they might be resistant to their effects, suggesting that natural immunity in these individuals is defective.
As an example, after aerosol M. tuberculosis infection, C3HeB/FeJ mice develop necrotic granulomatous lesions and die rapidly. However, these mice have robust T cell responses126,127. The genetic basis for their susceptibility has been mapped to several loci, and the dominant locus, Ipr1, is preferentially expressed by macrophages and alters their death modality128. Macrophages that express the resistant allele of Ipr1 are more prone to apoptosis following intracellular infection, whereas macrophages that express the susceptible allele undergo necrosis, which is associated with higher bacterial loads and more tissue destruction. This is an important insight as some people might develop active TB because their macrophages are unable to control intracellular M. tuberculosis growth, rather than because they have dysfunctional T cells. Similarly, an increase in type I and type II interferon-inducible genes is found in the peripheral blood of individuals with active pulmonary TB117. Surprisingly, this gene signature is mostly accounted for by changes in neutrophil gene expression. These data support the theory that, just as for macrophages, alterations in neutrophil functions can have an effect on disease susceptibility and progression. Thus, even vaccines that elicit strong T cell responses might not be effective at protecting such people from TB because their macrophages, neutrophils or other cell types cannot respond appropriately to the T cell signals. Without understanding why people are susceptible to disease, we cannot predict how to protect them.
Finally, instead of mimicking natural immunity, vaccine-induced protection against TB might require 'uncommon' or 'unnatural' immunity, as recently discussed by the Gates Foundation (grand challenges TB vaccine accelerator; see further information). For example, such unnatural protective immunity is induced after tetanus toxoid vaccination, but is not observed after natural infection with Clostridium tetani129. Examples of unnatural immunity to M. tuberculosis are iNKT cells (Box 2). These cells are dispensable for protection against primary infection in immunocompetent mice, but their activation can prolong the survival of inbred strains of susceptible mice130. It might be possible to induce such unnatural or uncommon immunity — for example, by engineering BCG to express the bacterial toxins listeriolysin or perfringolysin, which alter the route of antigen presentation and lead to more efficient stimulation of CD8+ T cells131,132,133. Incorporating into vaccine design such strategies that stimulate a broader immune response than current vaccines might have a greater effect on the induction of protective immunity than incorporating strategies that only stimulate a stronger response to a single antigen.
Quantity versus quality. The goal of vaccination is to elicit a population of long-lived memory T cells that, after M. tuberculosis challenge, will rapidly proliferate, acquire optimal effector functions, traffic to the lungs, recognize M. tuberculosis-infected cells, control bacterial replication and lead to sterilization (Fig. 2c). We assume that successful vaccination will elicit CD4+ and CD8+ T cells, which are specific for one or more mycobacterial antigen or antigens and whose functions will include IFNγ production (that is, a TH1 cell response). However, TH1 cell responses are unable to sterilize the host during active disease, and as we cannot define protective immunity, there is no way to measure successful immunization other than empirically quantifying changes in pathogen burden after challenge or, in people, after natural exposure — an approach that is slow and cumbersome.
For infections that can be prevented by humoral immunity, antibody titres correlate with protective immunity. For T cell-based vaccines, we are not sure whether the number of elicited T cells will correlate with protection or whether a change in one of the many functions that T cells perform will be a more useful measure. For example, re-exposure to antigen in vivo induces CD8+ T cells to more frequently and persistently co-express effector molecules (such as perforin, granzyme A, granzyme B, FASL and IFNγ) and to more efficiently kill than CD8+ T cells that are stimulated by the antigen the first time134. This suggests that an important function of T cell vaccination is to induce and coordinate the expression of effector molecules. Similarly, several different types of intermediate to long-term antigen-specific T cells (central, effector and tissue-resident memory cells) persist after infection or vaccination, with the potential to rapidly respond to infectious challenge135; however, it is unclear which population (or populations) are most effective in preventing TB.
Collectively, the data summarized in this Opinion article suggest that vaccines that elicit large numbers of T cells with the capacity to only produce IFNγ might not be optimal for protecting against TB. We should be looking for changes in T cell function, rather than numbers, as key factors that will lead to a substantial breakthrough in vaccine design. Furthermore, as several studies have shown different pathways to be involved in protective immunity against TB (such as those mediated by IL-1β, GM-CSF, vitamin C, vitamin D and cytolysis), vaccine design should aim at arming T cells with the capacity to modulate such pathways in cells that are infected with M. tuberculosis (Fig. 2c). Finally, we must avoid the trap of thinking that there is one type of T cell that will mediate protection alone. The host response to M. tuberculosis elicits many different types of T cells and, even if all of them do not kill M. tuberculosis, it is likely that they all have a role in orchestrating a successful immune response.
Conclusion
An important obstacle to vaccine development is our incomplete understanding of what constitutes protective immunity against M. tuberculosis. It is difficult to define the goals of vaccination without first knowing what the immune system is capable of. Although a vaccine that prevents infection is everyone's first choice, the consensus seems to be that a vaccine that enforces latency and prevents transmission is a more realistic goal. However, would we feel differently if we understood why some people do not become infected despite repeated exposure? Or why some granulomas function autonomously, with some apparently able to control and eradicate M. tuberculosis and others not98?
This lack of knowledge supports our main suggestion for vaccine design: that characterization of additional immune mediators and cell types, even ones that seem to have minor roles during natural infection, is an essential first step. An effective vaccine might need to engage multiple immune mechanisms that are activated during a typical infection and might need to skew the host response in ways that are not seen during natural infection.
TB is a chronic disease, and M. tuberculosis evades detection by antibodies by occupying an intracellular niche. Thus, a vaccine that generates CD4+ and CD8+ central memory T cells with high proliferative potential, as well as a cohort of potent CD8+ effector memory and resident memory T cells that are poised to rapidly kill infected cells in the lungs can be expected to be an ideal T cell vaccine candidate for disease prevention. Alternative approaches that — for example — stimulate unconventional T cell subsets and B cell and antibody responses alongside conventional T cells should be further investigated. However, we think that continuing to develop T cell vaccines aimed at boosting childhood BCG vaccination by solely varying the antigen will probably continue to fail. It is not enough to target specific antigens without a better understanding of how vaccines modulate T cell subsets and their functions and trafficking. IFNγ production by CD4+ T cells is essential in certain situations but it will probably not be sufficient as a protective response after vaccination. We think that the premise that IFNγ production by CD4+ T cells is required for protection during infection has never been conclusively shown, and the assumed role of IFNγ in vaccine-induced immunity is made on the basis of an over-interpretation of published data. In addition, we think that it is important to make vaccines that elicit multiple T cell subsets that express diverse protective functions. For example, we predict that a vaccine that elicits CD4+ T cells that produce GM-CSF and IFNγ, and CD8+ T cells that function as cytolytic effectors, in addition to producing IFNγ, would be more protective than vaccines that elicit only IFNγ. Finally, we must broaden the ways in which vaccine candidates are evaluated and the biomarkers that are used to measure their effects. Without defined correlates of protection, this will be challenging. Ongoing efforts to expand the ways in which vaccine candidates are evaluated and to embrace the diversity and heterogeneity of T cells need to be supported.
References
Russell, D. G., Barry, C. E. 3rd & Flynn, J. L. Tuberculosis: what we don't know can, and does, hurt us. Science 328, 852–856 (2010).
Cooper, A. M., Mayer-Barber, K. D. & Sher, A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 4, 252–260 (2011).
Chackerian, A. A., Alt, J. M., Perera, T. V., Dascher, C. C. & Behar, S. M. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect. Immun. 70, 4501–4509 (2002).
Wolf, A. J. et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 205, 105–115 (2008).
Reiley, W. W. et al. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc. Natl Acad. Sci. USA 105, 10961–10966 (2008).
Flynn, J. L., Chan, J. & Lin, P. L. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 4, 271–278 (2011).
Horsburgh, C. R. Jr. & Rubin, E. J. Clinical practice. Latent tuberculosis infection in the United States. New Engl. J. Med. 364, 1441–1448 (2011).
Barry, C. E. et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nature Rev. Microbiol. 7, 845–855 (2009).
Fine, P. E. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346, 1339–1345 (1995).
North, R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell. Immunol. 7, 166–176 (1973).
Orme, I. M., Roberts, A. D., Griffin, J. P. & Abrams, J. S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J. Immunol. 151, 518–525 (1993).
Shimokata, K., Kishimoto, H., Takagi, E. & Tsunekawa, H. Determination of the T-cell subset producing gamma-interferon in tuberculous pleural effusion. Microbiol. Immunol. 30, 353–361 (1986).
Mogues, T., Goodrich, M. E., Ryan, L., LaCourse, R. & North, R. J. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193, 271–280 (2001).
Behar, S. M., Dascher, C. C., Grusby, M. J., Wang, C. R. & Brenner, M. B. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J. Exp. Med. 189, 1973–1980 (1999).
Geldmacher, C., Zumla, A. & Hoelscher, M. Interaction between HIV and Mycobacterium tuberculosis: HIV-1-induced CD4 T-cell depletion and the development of active tuberculosis. Curr. Opin. HIV AIDS 7, 268–275 (2012).
Flynn, J. L. et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178, 2249–2254 (1993).
Cooper, A. M. et al. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178, 2243–2247 (1993).
Chan, J., Xing, Y., Magliozzo, R. S. & Bloom, B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175, 1111–1122 (1992).
MacMicking, J. D., Taylor, G. A. & McKinney, J. D. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science 302, 654–659 (2003).
Gutierrez, M. G. et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119, 753–766 (2004).
MacMicking, J. D. et al. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl Acad. Sci. USA 94, 5243–5248 (1997).
Fabri, M. et al. Vitamin D is required for IFN-γ-mediated antimicrobial activity of human macrophages. Sci. Transl. Med. 3, 104ra102 (2011).
Liu, P. T., Stenger, S., Tang, D. H. & Modlin, R. L. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 179, 2060–2063 (2007).
Casanova, J. L. & Abel, L. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20, 581–620 (2002).
Cooper, A. M., Magram, J., Ferrante, J. & Orme, I. M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 186, 39–45 (1997).
Sonnenberg, P. et al. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J. Infect. Dis. 191, 150–158 (2005).
Murray, J. et al. Cause of death and presence of respiratory disease at autopsy in an HIV-1 seroconversion cohort of southern African gold miners. AIDS 21, S97–S104 (2007).
Miedema, F. et al. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J. Clin. Invest. 82, 1908–1914 (1988).
Geldmacher, C. & Koup, R. A. Pathogen-specific T cell depletion and reactivation of opportunistic pathogens in HIV infection. Trends Immunol. 33, 207–214 (2012).
Alcais, A., Fieschi, C., Abel, L. & Casanova, J. L. Tuberculosis in children and adults: two distinct genetic diseases. J. Exp. Med. 202, 1617–1621 (2005).
Wallis, R. S. et al. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect. Dis. 13, 362–372 (2013).
Diel, R., Loddenkemper, R., Niemann, S., Meywald-Walter, K. & Nienhaus, A. Negative and positive predictive value of a whole-blood interferon-γ release assay for developing active tuberculosis: an update. Am. J. Respir. Crit. Care Med. 183, 88–95 (2011).
Mattila, J. T., Diedrich, C. R., Lin, P. L., Phuah, J. & Flynn, J. L. Simian immunodeficiency virus-induced changes in T cell cytokine responses in cynomolgus macaques with latent Mycobacterium tuberculosis infection are associated with timing of reactivation. J. Immunol. 186, 3527–3537 (2011).
Lazarevic, V., Nolt, D. & Flynn, J. L. Long-term control of Mycobacterium tuberculosis infection is mediated by dynamic immune responses. J. Immunol. 175, 1107–1117 (2005).
Abebe, F., Mustafa, T., Nerland, A. H. & Bjune, G. A. Cytokine profile during latent and slowly progressive primary tuberculosis: a possible role for interleukin-15 in mediating clinical disease. Clin. Exp. Immunol. 143, 180–192 (2006).
Mittrücker, H.-W. et al. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc. Natl Acad. Sci. USA 104, 12434–12439 (2007).
Mollenkopf, H.-J., Hahnke, K. & Kaufmann, S. H. E. Transcriptional responses in mouse lungs induced by vaccination with Mycobacterium bovis BCG and infection with Mycobacterium tuberculosis. Microbes Infect. 8, 136–144 (2006).
Oliveira, E. S., Marinho, J. M. & Barbosa, T. Interferon-gamma production by mononuclear cells in Bacille Calmette-Guérin-revaccinated healthy volunteers predicted long-term antimycobacterial responses in a randomized controlled trial. Vaccine 31, 3778–3782 (2013).
Soares, A. P. et al. Bacillus Calmette-Guérin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J. Immunol. 180, 3569–3577 (2008).
Murray, R. A. et al. Bacillus Calmette Guerin vaccination of human newborns induces a specific, functional CD8+ T cell response. J. Immunol. 177, 5647–5651 (2006).
Andersen, A. et al. The immunological effect of revaccination with Bacille Calmette-Guérin vaccine at 19 months of age. Vaccine 31, 2137–2144 (2013).
Kagina, B. M. et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am. J. Respir. Crit. Care Med. 182, 1073–1079 (2010).
Cowley, S. C. & Elkins, K. L. CD4+ T cells mediate IFN-γ-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo. J. Immunol. 171, 4689–4699 (2003).
Gallegos, A. M. et al. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 7, e1002052 (2011).
Miller, H. E. & Robinson, R. T. Early control of Mycobacterium tuberculosis infection requires il12rb1 expression by rag1-dependent lineages. Infect. Immun. 80, 3828–3841 (2012).
Nandi, B. & Behar, S. M. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J. Exp. Med. 208, 2251–2262 (2011).
Jayaraman, P. et al. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J. Exp. Med. 207, 2343–2354 (2010).
Green, A. M., Difazio, R. & Flynn, J. L. IFN-γ from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J. Immunol. 190, 270–277 (2013).
Pitt, J. M. et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains TH1, TH17, and innate lymphoid IFN-γ and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J. Immunol. 189, 4079–4087 (2012).
Flynn, J. L. et al. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2, 561–572 (1995).
Mayer-Barber, K. D. et al. Caspase-1 independent IL-1β production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184, 3326–3330 (2010).
Gonzalez-Juarrero, M. et al. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J. Leukoc. Biol. 77, 914–922 (2005).
Ladel, C. H. et al. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect. Immun. 65, 4843–4849 (1997).
Chan, J., Tanaka, K., Carroll, D., Flynn, J. & Bloom, B. R. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 63, 736–740 (1995).
Jung, J. Y. et al. The intracellular environment of human macrophages that produce nitric oxide promotes growth of mycobacteria. Infect. Immun. 81, 3198–3209 (2013).
Weinberg, J. B. et al. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood 86, 1184–1195 (1995).
Nathan, C. & Shiloh, M. U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl Acad. Sci. USA 97, 8841–8848 (2000).
Aston, C., Rom, W. N., Talbot, A. T. & Reibman, J. Early inhibition of mycobacterial growth by human alveolar macrophages is not due to nitric oxide. Am. J. Respiratory Crit. Care Med. 157, 1943–1950 (1998).
Saunders, B. M., Briscoe, H. & Britton, W. J. T cell-derived tumour necrosis factor is essential, but not sufficient, for protection against Mycobacterium tuberculosis infection. Clin. Exp. Immunol. 137, 279–287 (2004).
Mohan, V. P. et al. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect. Immun. 69, 1847–1855 (2001).
Tobin, D. M. et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 140, 717–730 (2010).
Bean, A. G. et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162, 3504–3511 (1999).
Keane, J. et al. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N. Engl. J. Med. 345, 1098–1104 (2001).
Denis, M. & Ghadirian, E. Granulocyte–macrophage colony-stimulating factor restricts growth of tubercle bacilli in human macrophages. Immunol. Lett. 24, 203–206 (1990).
Bermudez, L. E. & Young, L. S. Recombinant granulocyte-macrophage colony-stimulating factor activates human macrophages to inhibit growth or kill Mycobacterium avium complex. J. Leukoc. Biol. 48, 67–73 (1990).
Rothchild, A. C., Jayaraman, P., Nunes-Alves, C. & Behar, S. M. iNKT cell production of GM-CSF controls Mycobacterium tuberculosis. PLoS Pathog. 10, e1003805 (2014).
Trapnell, B. C. & Whitsett, J. A. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu. Rev. Physiol. 64, 775–802 (2002).
Rosen, L. B. et al. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J. Immunol. 190, 3959–3966 (2013).
Jayaraman, P. et al. IL-1β promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation. J. Immunol. 190, 4196–4204 (2013).
Qiu, Y. et al. Tim-3-expressing CD4+ and CD8+ T cells in human tuberculosis (TB) exhibit polarized effector memory phenotypes and stronger anti-TB effector functions. PLoS Pathog. 8, e1002984 (2012).
Sada-Ovalle, I. et al. The Tim3-galectin 9 pathway induces antibacterial activity in human macrophages infected with Mycobacterium tuberculosis. J. Immunol. 189, 5896–5902 (2012).
Desvignes, L. & Ernst, J. D. Interferon-γ-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity 31, 974–985 (2009).
Cruz, A. et al. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J. Exp. Med. 207, 1609–1616 (2010).
Novikov, A. et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1β production in human macrophages. J. Immunol. 187, 2540–2547 (2011).
Liu, P. T. et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311, 1770–1773 (2006).
Martineau, A. R. et al. IFN-γ- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J. Immunol. 178, 7190–7198 (2007).
Verway, M. et al. Vitamin D induces interleukin-1β expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog. 9, e1003407 (2013).
Yuk, J. M. et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 6, 231–243 (2009).
Ralph, A. P., Lucas, R. M. & Norval, M. Vitamin D and solar ultraviolet radiation in the risk and treatment of tuberculosis. Lancet Infect. Dis. 13, 77–88 (2013).
Vilcheze, C., Hartman, T., Weinrick, B. & Jacobs, W. R. Jr. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nature Commun. 4, 1881 (2013).
Chen, C. Y. et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 5, e1000392 (2009).
Skinner, M. A., Parlane, N., McCarthy, A. & Buddle, B. M. Cytotoxic T-cell responses to Mycobacterium bovis during experimental infection of cattle with bovine tuberculosis. Immunology 110, 234–241 (2003).
Woodworth, J. S. & Behar, S. M. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit. Rev. Immunol. 26, 317–352 (2006).
Woodworth, J. S., Wu, Y. & Behar, S. M. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J. Immunol. 181, 8595–8603 (2008).
Turner, J. et al. CD8- and CD95/95L-dependent mechanisms of resistance in mice with chronic pulmonary tuberculosis. Am. J. Respir. Cell. Mol. Biol. 24, 203–209 (2001).
Sousa, A. O. et al. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc. Natl Acad. Sci. USA 97, 4204–4208 (2000).
Stenger, S. et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282, 121–125 (1998).
Behar, S. M., Divangahi, M. & Remold, H. G. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nature Rev. Microbiol. 8, 668–674 (2010).
Martin, C. J. et al. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe 12, 289–300 (2012).
Saunders, B. M. et al. Transmembrane TNF is sufficient to initiate cell migration and granuloma formation and provide acute, but not long-term, control of Mycobacterium tuberculosis infection. J. Immunol. 174, 4852–4859 (2005).
Botha, T. & Ryffel, B. Reactivation of latent tuberculosis infection in TNF-deficient mice. J. Immunol. 171, 3110–3118 (2003).
Saunders, B. M., Frank, A. A., Orme, I. M. & Cooper, A. M. CD4 is required for the development of a protective granulomatous response to pulmonary tuberculosis. Cell. Immunol. 216, 65–72 (2002).
Ehlers, S., Kutsch, S., Ehlers, E. M., Benini, J. & Pfeffer, K. Lethal granuloma disintegration in mycobacteria-infected TNFRp55−/− mice is dependent on T cells and IL-12. J. Immunol. 165, 483–492 (2000).
Lawn, S. D., Butera, S. T. & Shinnick, T. M. Tuberculosis unleashed: the impact of human immunodeficiency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect. 4, 635–646 (2002).
Ly, L. H., Russell, M. I. & McMurray, D. N. Cytokine profiles in primary and secondary pulmonary granulomas of Guinea pigs with tuberculosis. Am. J. Respir. Cell. Mol. Biol. 38, 455–462 (2008).
Manabe, Y. C. et al. The aerosol rabbit model of TB latency, reactivation and immune reconstitution inflammatory syndrome. Tuberculosis (Edinb.) 88, 187–196 (2008).
Via, L. E. et al. Differential virulence and disease progression following Mycobacterium tuberculosis complex infection of the common marmoset (Callithrix jacchus). Infection Immun. 81, 2909–2919 (2013).
Lin, P. L. Sterilization of granulomas is common in both active and latent tuberculosis despite extensive within-host variability in bacterial killing. Nature Med. 20, 75–79 (2013).
Kwan, C. K. & Ernst, J. D. HIV and tuberculosis: a deadly human syndemic. Clin. Microbiol. Rev. 24, 351–376 (2011).
Josefowicz, S. Z., Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Guyot-Revol, V., Innes, J. A., Hackforth, S., Hinks, T. & Lalvani, A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am. J. Respir. Crit. Care Med. 173, 803–810 (2006).
Ribeiro-Rodrigues, R. et al. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin. Exp. Immunol. 144, 25–34 (2006).
Green, A. M. et al. CD4 regulatory T cells in a cynomolgus macaque model of Mycobacterium tuberculosis infection. J. Infect. Dis. 202, 533–541 (2010).
Scott-Browne, J. P. et al. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J. Exp. Med. 204, 2159–2169 (2007).
Kursar, M. et al. Cutting edge: regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J. Immunol. 178, 2661–2665 (2007).
Shafiani, S., Tucker-Heard, G., Kariyone, A., Takatsu, K. & Urdahl, K. B. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J. Exp. Med. 207, 1409–1420 (2010).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Lazar-Molnar, E. et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc. Natl Acad. Sci. USA 107, 13402–13407 (2010).
Jurado, J. O. et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J. Immunol. 181, 116–125 (2008).
Barber, D. L., Mayer-Barber, K. D., Feng, C. G., Sharpe, A. H. & Sher, A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J. Immunol. 186, 1598–1607 (2011).
Pedrosa, J. et al. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68, 577–583 (2000).
Rivas-Santiago, B. et al. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect. Immun. 76, 935–941 (2008).
Yang, C. T. et al. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe 12, 301–312 (2012).
Blomgran, R. & Ernst, J. D. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J. Immunol. 186, 7110–7119 (2011).
Cruz, A. et al. Cutting edge: IFN-γ regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J. Immunol. 177, 1416–1420 (2006).
Mishra, B. B. et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nature Immunol. 14, 52–60 (2013).
Berry, M. P. R. et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977 (2010).
Naranbhai, V. et al. Ratio of monocytes to lymphocytes in peripheral blood identifies adults at risk of incident tuberculosis among HIV-infected adults initiating antiretroviral therapy. J. Infect. Dis. 209, 500–509 (2013).
Maglione, P. J. & Chan, J. How B cells shape the immune response against Mycobacterium tuberculosis. Eur. J. Immunol. 39, 676–686 (2009).
Huang, D. et al. Immune distribution and localization of phosphoantigen-specific Vγ2Vδ2 T cells in lymphoid and nonlymphoid tissues in Mycobacterium tuberculosis infection. Infect. Immun. 76, 426–436 (2008).
Millet, J. P. et al. Tuberculosis recurrence after completion treatment in a European city: reinfection or relapse? PLoS ONE 8, e64898 (2013).
Millet, J. P. et al. Tuberculosis recurrence and its associated risk factors among successfully treated patients. J. Epidemiol. Commun. Health 63, 799–804 (2009).
Crofts, J. P., Andrews, N. J., Barker, R. D., Delpech, V. & Abubakar, I. Risk factors for recurrent tuberculosis in England and Wales, 1998–2005. Thorax 65, 310–314 (2010).
Dobler, C. C., Crawford, A. B., Jelfs, P. J., Gilbert, G. L. & Marks, G. B. Recurrence of tuberculosis in a low-incidence setting. Eur. Respir. J. 33, 160–167 (2009).
van Rie, A. et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N. Engl. J. Med. 341, 1174–1179 (1999).
Kamath, A. B., Alt, J., Debbabi, H. & Behar, S. M. Toll-like receptor 4-defective C3H/HeJ mice are not more susceptible than other C3H substrains to infection with Mycobacterium tuberculosis. Infection Immun. 71, 4112–4118 (2003).
Kamath, A. B., Alt, J., Debbabi, H., Taylor, C. & Behar, S. M. The major histocompatibility complex haplotype affects T-cell recognition of mycobacterial antigens but not resistance to Mycobacterium tuberculosis in C3H mice. Infect. Immun. 72, 6790–6798 (2004).
Pan, H. et al. Ipr1 gene mediates innate immunity to tuberculosis. Nature 434, 767–772 (2005).
Johnson, S. Antibody responses to clostridial infection in humans. Clin. Infect. Dis. 25, S173–177 (1997).
Chackerian, A., Alt, J., Perera, V. & Behar, S. M. Activation of NKT cells protects mice from tuberculosis. Infection Immun. 70, 6302–6309 (2002).
Sun, R. et al. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine 27, 4412–4423 (2009).
Rao, M. et al. The tuberculosis vaccine candidate Bacillus Calmette-Guérin ΔureC::hly coexpressing human interleukin-7 or -18 enhances antigen-specific T cell responses in mice. PLoS ONE 8, e78966 (2013).
Desel, C. et al. Recombinant BCG ΔureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J. Infecti. Diseases 204, 1573–1584 (2011).
Peixoto, A. et al. CD8 single-cell gene coexpression reveals three different effector types present at distinct phases of the immune response. J. Exp. Med. 204, 1193–1205 (2007).
Jameson, S. C. & Masopust, D. Diversity in T cell memory: an embarrassment of riches. Immunity 31, 859–871 (2009).
Ottenhoff, T. H. M. & Kaufmann, S. H. E. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog. 8, e1002607 (2012).
Brennan, M. J., Stone, M. R. & Evans, T. A rational vaccine pipeline for tuberculosis. Int. J. Tuberculosis Lung Dis. 16, 1566–1573 (2012).
Tameris, M. D. et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381, 1021–1028 (2013).
McShane, H. et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nature Med. 10, 1240–1244 (2004).
Scriba, T. J. et al. Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. Eur. J. Immunol. 40, 279–290 (2010).
Vavassori, S. et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nature Immunol. 14, 908–916 (2013).
D'Souza, C. D. et al. A novel nonclassic β2-microglobulin-restricted mechanism influencing early lymphocyte accumulation and subsequent resistance to tuberculosis in the lung. Am. J. Respir. Cell. Mol. Biol. 23, 188–193 (2000).
Sugawara, I. et al. Mycobacterial infection in natural killer T cell knockout mice. Tuberculosis (Edinb.) 82, 97–104 (2002).
Khader, S. A. et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-γ responses if IL-12p70 is available. J. Immunol. 175, 788–795 (2005).
Montoya, C. J. et al. Invariant NKT cells from HIV-1 or Mycobacterium tuberculosis-infected patients express an activated phenotype. Clin. Immunol. 127, 1–6 (2008).
Sutherland, J. S. et al. High granulocyte/lymphocyte ratio and paucity of NKT cells defines TB disease in a TB-endemic setting. Tuberculosis (Edinb.) 89, 398–404 (2009).
Im, J. S. et al. Alteration of the relative levels of iNKT cell subsets is associated with chronic mycobacterial infections. Clin. Immunol. 127, 214–224 (2008).
Van Rhijn, I. et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nature Immunol. 14, 706–713 (2013).
Kasmar, A. G. et al. CD1b tetramers bind αβ T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. J. Exp. Med. 208, 1741–1747 (2011).
Ly, D. et al. CD1c tetramers detect ex vivo T cell responses to processed phosphomycoketide antigens. J. Exp. Med. 210, 729–741 (2013).
Gold, M. C. et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 8, e1000407 (2010).
Le Bourhis, L. et al. Antimicrobial activity of mucosal-associated invariant T cells. Nature Immunol. 11, 701–708 (2010).
Havlir, D. V., Ellner, J. J., Chervenak, K. A. & Boom, W. H. Selective expansion of human γδ T cells by monocytes infected with live Mycobacterium tuberculosis. J. Clin. Invest. 87, 729–733 (1991).
Scriba, T. J. et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 180, 1962–1970 (2008).
Sada-Ovalle, I., Chiba, A., Gonzales, A., Brenner, M. B. & Behar, S. M. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-γ, and kill intracellular bacteria. PLoS Pathog. 4, e1000239 (2008).
Gold, M. C. et al. Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunol. 6, 35–44 (2013).
Lockhart, E., Green, A. M. & Flynn, J. L. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 177, 4662–4669 (2006).
Sada-Ovalle, I., Sköld, M., Tian, T., Besra, G. S. & Behar, S. M. α-galactosylceramide as a therapeutic agent for pulmonary Mycobacterium tuberculosis infection. Am. J. Respiratory Crit. Care Med. 182, 841–847 (2010).
Chen, C. Y. et al. Phosphoantigen/IL2 expansion and differentiation of Vγ2Vδ2 T cells increase resistance to tuberculosis in nonhuman primates. PLoS Pathog. 9, e1003501 (2013).
Gansert, J. L. et al. Human NKT cells express granulysin and exhibit antimycobacterial activity. J. Immunol. 170, 3154–3161 (2003).
Venkataswamy, M. M. et al. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guerin. J. Immunol. 183, 1644–1656 (2009).
Shen, Y. et al. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science 295, 2255–2258 (2002).
Umemura, M. et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 178, 3786–3796 (2007).
Okamoto Yoshida, Y. et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J. Immunol. 184, 4414–4422 (2010).
Khader, S. A. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nature Immunol. 8, 369–377 (2007).
Gopal, R. et al. IL-23-dependent IL-17 drives TH1-cell responses following Mycobacterium bovis BCG vaccination. Eur. J. Immunol. 42, 364–373 (2012).
Acknowledgements
The authors are supported by the following grants from the US National Institutes of Heath (NIH) and US National Institute of Allergy and Infectious Diseases (NIAID): R21AI100766, R01AI085669, R01AI098637, and R01AI106725.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Nunes-Alves, C., Booty, M., Carpenter, S. et al. In search of a new paradigm for protective immunity to TB. Nat Rev Microbiol 12, 289–299 (2014). https://doi.org/10.1038/nrmicro3230
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3230
This article is cited by
-
Key advances in vaccine development for tuberculosis—success and challenges
npj Vaccines (2023)
-
Elevation in the counts of IL-35-producing B cells infiltrating into lung tissue in mycobacterial infection is associated with the downregulation of Th1/Th17 and upregulation of Foxp3+Treg
Scientific Reports (2020)
-
Type I interferon-driven susceptibility to Mycobacterium tuberculosis is mediated by IL-1Ra
Nature Microbiology (2019)
-
Moving tuberculosis vaccines from theory to practice
Nature Reviews Immunology (2019)
-
CCR4-dependent reduction in the number and suppressor function of CD4+Foxp3+ cells augments IFN-γ-mediated pulmonary inflammation and aggravates tuberculosis pathogenesis
Cell Death & Disease (2018)