Key Points
-
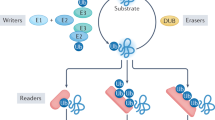
Small ubiquitin-like modifier (SUMO) proteins are structurally closely related to ubiquitin and can be covalently conjugated to other proteins to regulate their activity, intracellular localization or interaction partners.
-
Many viral proteins can be sumoylated, can influence the sumoylation of cellular proteins or can engage with regulatory pathways that have SUMO-dependent control points.
-
Promyelocytic leukaemia nuclear bodies in uninfected cell nuclei are major hubs of sumoylation and have significant antiviral roles. Several viruses disrupt the antiviral functions of these nuclear bodies through SUMO-related mechanisms.
-
There are emerging roles for SUMO pathways in intrinsic and innate immune defences against viral infection.
Abstract
Post-translational modification by members of the small ubiquitin-like modifier (SUMO) family of proteins is important for the regulation of many cellular proteins and pathways. As obligate parasites, viruses must engage with the host cell throughout their replication cycles, and it is therefore unsurprising that there are many examples of interplay between viral proteins and the host sumoylation system. This article reviews recent advances in this field, summarizing information on sumoylated viral proteins, the varied ways in which viruses engage with SUMO-related pathways, and the consequences of these interactions for viral replication and engagement with innate and intrinsic immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Boddy, M. N., Howe, K., Etkin, L. D., Solomon, E. & Freemont, P. S. PIC 1, a novel ubiquitin-like protein which interacts with the PML component of a multiprotein complex that is disrupted in acute promyelocytic leukaemia. Oncogene 13, 971–982 (1996).
Gareau, J. R. & Lima, C. D. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nature Rev. Mol. Cell Biol. 11, 861–871 (2010). A comprehensive recent review of the SUMO pathway.
Hay, R. T. SUMO: a history of modification. Mol. Cell 18, 1–12 (2005).
Kamitani, T., Nguyen, H. P. & Yeh, E. T. Preferential modification of nuclear proteins by a novel ubiquitin-like molecule. J. Biol. Chem. 272, 14001–14004 (1997).
Mahajan, R., Delphin, C., Guan, T., Gerace, L. & Melchior, F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88, 97–107 (1997).
Matunis, M. J., Coutavas, E. & Blobel, G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 135, 1457–1470 (1996).
Shen, Z., Pardington-Purtymun, P. E., Comeaux, J. C., Moyzis, R. K. & Chen, D. J. UBL1, a human ubiquitin-like protein associating with human RAD51/RAD52 proteins. Genomics 36, 271–279 (1996).
Wang, Y. & Dasso, M. SUMOylation and deSUMOylation at a glance. J. Cell Sci. 122, 4249–4252 (2009).
Wimmer, P., Schreiner, S. & Dobner, T. Human pathogens and the host cell SUMOylation system. J. Virol. 86, 642–654 (2012).
Hickey, C. M., Wilson, N. R. & Hochstrasser, M. Function and regulation of SUMO proteases. Nature Rev. Mol. Cell Biol. 13, 755–766 (2012).
Bergink, S. & Jentsch, S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature 458, 461–467 (2009).
Bernardi, R. & Pandolfi, P. P. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nature Rev. Mol. Cell Biol. 8, 1006–1016 (2007).
Cuchet-Lourenco, D. et al. SUMO pathway dependent recruitment of cellular repressors to herpes simplex virus type 1 genomes. PLoS Pathog. 7, e1002123 (2011).
Negorev, D. & Maul, G. G. Cellular proteins localized at and interacting within ND10/PML nuclear bodies/PODs suggest functions of a nuclear depot. Oncogene 20, 7234–7242 (2001).
Everett, R. D. & Chelbi-Alix, M. K. PML and PML nuclear bodies: implications in antiviral defence. Biochimie 89, 819–830 (2007).
Everett, R. D. Interactions between DNA viruses, ND10 and the DNA damage response. Cell. Microbiol. 8, 365–374 (2006).
Geoffroy, M. C. & Chelbi-Alix, M. K. Role of promyelocytic leukemia protein in host antiviral defense. J. Interferon Cytokine Res. 31, 145–158 (2011).
Hofmann, H., Floss, S. & Stamminger, T. Covalent modification of the transactivator protein IE2-p86 of human cytomegalovirus by conjugation to the ubiquitin-homologous proteins SUMO-1 and hSMT3b. J. Virol. 74, 2510–2524 (2000).
Sadanari, H., Yamada, R., Ohnishi, K., Matsubara, K. & Tanaka, J. SUMO-1 modification of the major immediate-early (IE) 1 and 2 proteins of human cytomegalovirus is regulated by different mechanisms and modulates the intracellular localization of the IE1, but not IE2, protein. Arch. Virol. 150, 1763–1782 (2005).
Spengler, M. L., Kurapatwinski, K., Black, A. R. & Azizkhan-Clifford, J. SUMO-1 modification of human cytomegalovirus IE1/IE72. J. Virol. 76, 2990–2996 (2002).
Xu, Y. et al. Proteasome-independent disruption of PML oncogenic domains (PODs), but not covalent modification by SUMO-1, is required for human cytomegalovirus immediate-early protein IE1 to inhibit PML-mediated transcriptional repression. J. Virol. 75, 10683–10695 (2001).
Gawn, J. M. & Greaves, R. F. Absence of IE1 p72 protein function during low-multiplicity infection by human cytomegalovirus results in a broad block to viral delayed-early gene expression. J. Virol. 76, 4441–4455 (2002).
Kang, H. et al. Inhibition of SUMO-independent PML oligomerization by the human cytomegalovirus IE1 protein. J. Gen. Virol. 87, 2181–2190 (2006).
Kim, Y. E. et al. Human cytomegalovirus infection causes degradation of Sp100 proteins that suppress viral gene expression. J. Virol. 85, 11928–11937 (2011).
Lee, H. R. et al. Ability of the human cytomegalovirus IE1 protein to modulate sumoylation of PML correlates with its functional activities in transcriptional regulation and infectivity in cultured fibroblast cells. J. Virol. 78, 6527–6542 (2004). An excellent and thorough functional analysis of the interplay between viral and host sumoylated proteins.
Tavalai, N., Adler, M., Scherer, M., Riedl, Y. & Stamminger, T. Evidence for a dual antiviral role of the major nuclear domain 10 component Sp100 during the immediate-early and late phases of the human cytomegalovirus replication cycle. J. Virol. 85, 9447–9458 (2011).
Nevels, M., Brune, W. & Shenk, T. SUMOylation of the human cytomegalovirus 72-kilodalton IE1 protein facilitates expression of the 86-kilodalton IE2 protein and promotes viral replication. J. Virol. 78, 7803–7812 (2004).
Huh, Y. H. et al. Binding STAT2 by the acidic domain of human cytomegalovirus IE1 promotes viral growth and is negatively regulated by SUMO. J. Virol. 82, 10444–10454 (2008).
Marchini, A., Liu, H. & Zhu, H. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75, 1870–1878 (2001).
Lee, J. M. et al. PIAS1 enhances SUMO-1 modification and the transactivation activity of the major immediate-early IE2 protein of human cytomegalovirus. FEBS Lett. 555, 322–328 (2003).
Lee, H. R. & Ahn, J. H. Sumoylation of the major immediate-early IE2 protein of human cytomegalovirus Towne strain is not required for virus growth in cultured human fibroblasts. J. Gen. Virol. 85, 2149–2154 (2004).
Berndt, A., Hofmann-Winkler, H., Tavalai, N., Hahn, G. & Stamminger, T. Importance of covalent and noncovalent SUMO interactions with the major human cytomegalovirus transactivator IE2p86 for viral infection. J. Virol. 83, 12881–12894 (2009).
Sinigalia, E. et al. The human cytomegalovirus DNA polymerase processivity factor UL44 is modified by SUMO in a DNA-dependent manner. PLoS ONE 7, e49630 (2012).
Scherer, M. et al. SUMO pathway-mediated enhancement of human cytomegalovirus replication correlates with a recruitment of SUMO-1/3 proteins to viral replication compartments. J. Gen. Virol. 13 Feb 2013 (doi:10.1099/vir.0.051078-0).
Hagemeier, S. R. et al. Sumoylation of the Epstein-Barr virus BZLF1 protein inhibits its transcriptional activity and is regulated by the virus-encoded protein kinase. J. Virol. 84, 4383–4394 (2010).
Murata, T. et al. Transcriptional repression by sumoylation of Epstein-Barr virus BZLF1 protein correlates with association of histone deacetylase. J. Biol. Chem. 285, 23925–23935 (2010).
Izumiya, Y. et al. Kaposi's sarcoma-associated herpesvirus K-bZIP represses gene transcription via SUMO modification. J. Virol. 79, 9912–9925 (2005).
Adamson, A. L. & Kenney, S. Epstein-Barr virus immediate-early protein BZLF1 is SUMO-1 modified and disrupts promyelocytic leukemia bodies. J. Virol. 75, 2388–2399 (2001).
Verger, A., Perdomo, J. & Crossley, M. Modification with SUMO. A role in transcriptional regulation. EMBO Rep. 4, 137–142 (2003).
Marcos-Villar, L. et al. Covalent modification by SUMO is required for efficient disruption of PML oncogenic domains by Kaposi's sarcoma-associated herpesvirus latent protein LANA2. J. Gen. Virol. 92, 188–194 (2011).
Endter, C., Kzhyshkowska, J., Stauber, R. & Dobner, T. SUMO-1 modification required for transformation by adenovirus type 5 early region 1B 55-kDa oncoprotein. Proc. Natl Acad. Sci. USA 98, 11312–11317 (2001).
Kindsmuller, K. et al. Intranuclear targeting and nuclear export of the adenovirus E1B-55K protein are regulated by SUMO1 conjugation. Proc. Natl Acad. Sci. USA 104, 6684–6689 (2007).
Wimmer, P. et al. Cross-talk between phosphorylation and SUMOylation regulates transforming activities of an adenoviral oncoprotein. Oncogene 21 May 2012 (doi:10.1038/onc.2012.187).
Wimmer, P. et al. SUMO modification of E1B-55K oncoprotein regulates isoform-specific binding to the tumour suppressor protein PML. Oncogene 29, 5511–5522 (2010).
Palacios, S. et al. Quantitative SUMO-1 modification of a vaccinia virus protein is required for its specific localization and prevents its self-association. Mol. Biol. Cell 16, 2822–2835 (2005).
Lamsoul, I. et al. Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-κB activation by the human T-cell leukemia virus tax oncoprotein. Mol. Cell. Biol. 25, 10391–10406 (2005).
Xu, K. et al. Modification of nonstructural protein 1 of influenza A virus by SUMO1. J. Virol. 85, 1086–1098 (2011).
Chen, S. C. et al. Sumoylation-promoted enterovirus 71 3C degradation correlates with a reduction in viral replication and cell apoptosis. J. Biol. Chem. 286, 31373–31384 (2011).
Kfoury, Y. et al. Tax ubiquitylation and SUMOylation control the dynamic shuttling of Tax and NEMO between Ubc9 nuclear bodies and the centrosome. Blood 117, 190–199 (2011).
Li, R. et al. SUMO binding by the Epstein-Barr virus protein kinase BGLF4 is crucial for BGLF4 function. J. Virol. 86, 5412–5421 (2012).
Gonzalez-Santamaria, J. et al. Regulation of vaccinia virus E3 protein by small ubiquitin-like modifier proteins. J. Virol. 85, 12890–12900 (2011).
Kim, E. T., Kim, Y. E., Huh, Y. H. & Ahn, J. H. Role of noncovalent SUMO binding by the human cytomegalovirus IE2 transactivator in lytic growth. J. Virol. 84, 8111–8123 (2010).
Li, S. J. & Hochstrasser, M. A new protease required for cell-cycle progression in yeast. Nature 398, 246–251 (1999).
Shin, E. J. et al. DeSUMOylating isopeptidase: a second class of SUMO protease. EMBO Rep. 13, 339–346 (2012).
Muller, S. & Dobner, T. The adenovirus E1B-55K oncoprotein induces SUMO modification of p53. Cell Cycle 7, 754–758 (2008).
Pennella, M. A., Liu, Y., Woo, J. L., Kim, C. A. & Berk, A. J. Adenovirus E1B 55-kilodalton protein is a p53-SUMO1 E3 ligase that represses p53 and stimulates its nuclear export through interactions with promyelocytic leukemia nuclear bodies. J. Virol. 84, 12210–12225 (2010).
Chang, P. C. et al. Kaposi's sarcoma-associated herpesvirus (KSHV) encodes a SUMO E3 ligase that is SIM-dependent and SUMO-2/3-specific. J. Biol. Chem. 285, 5266–5273 (2010).
Querido, E. et al. Degradation of p53 by adenovirus E4orf6 and E1B55K proteins occurs via a novel mechanism involving a cullin-containing complex. Genes Dev. 15, 3104–3117 (2001).
Rodriguez, M. S. et al. SUMO-1 modification activates the transcriptional response of p53. EMBO J. 18, 6455–6461 (1999).
Sohn, S. Y. & Hearing, P. Adenovirus regulates sumoylation of Mre11-Rad50-Nbs1 components through a paralog-specific mechanism. J. Virol. 86, 9656–9665 (2012). Clear examples of virus-induced increases in sumoylation of specific cellular proteins.
Stracker, T. H., Carson, C. T. & Weitzman, M. D. Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature 418, 348–352 (2002).
Boutell, C. & Everett, R. D. The regulation of alphaherpesvirus infections by the ICP0 family of proteins. J. Gen. Virol. 94, 465–481 (2013).
Everett, R. D. in Alphaherpesviruses: Molecular Virology. (ed. Weller, S. K.) 39–50 (Caister Academic Press, 2011).
Hagglund, R. & Roizman, B. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J. Virol. 78, 2169–2178 (2004).
Everett, R. D. et al. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 80, 7995–8005 (2006).
Cuchet-Lourenco, D., Vanni, E., Glass, M., Orr, A. & Everett, R. D. Herpes simplex virus 1 ubiquitin ligase ICP0 interacts with PML isoform I and induces its SUMO-independent degradation. J. Virol. 86, 11209–11222 (2012).
Boutell, C. et al. A viral ubiquitin ligase has substrate preferential SUMO targeted ubiquitin ligase activity that counteracts intrinsic antiviral defence. PLoS Pathog. 7, e1002245 (2011). The first example of a viral protein with STUbL-like properties.
Everett, R. D. et al. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72, 6581–6591 (1998). The first demonstration of viral engagement with the SUMO pathway.
Muller, S. & Dejean, A. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 73, 5137–5143 (1999).
Everett, R. D., McNair, C., Grant, L., Boutell, C. & Orr, A. Comparison of the biological and biochemical activities of several members of the herpes simplex virus ICP0 family of proteins. J. Virol. 84, 3476–3487 (2009).
Parkinson, J. & Everett, R. D. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J. Virol. 74, 10006–10017 (2000).
Wang, L. et al. Disruption of PML nuclear bodies is mediated by ORF61 SUMO-interacting motifs and required for varicella-zoster virus pathogenesis in skin. PLoS Pathog. 7, e1002157 (2011). An analysis of the role of SIMs within a viral protein in an in vivo model system.
Kyratsous, C. A., Walters, M. S., Panagiotidis, C. A. & Silverstein, S. J. Complementation of a herpes simplex virus ICP0 null mutant by varicella-zoster virus ORF61p. J. Virol. 83, 10637–10643 (2009).
Everett, R. D., Bell, A. J., Lu, Y. & Orr, A. The replication defect of ICP0-null mutant HSV-1 can be largely complemented by the combined activities of HCMV proteins IE1 and pp71. J. Virol. 87, 978–990 (2013).
Doucas, V. et al. Adenovirus replication is coupled with the dynamic properties of the PML nuclear structure. Genes Dev. 10, 196–207 (1996).
Leppard, K. N. & Everett, R. D. The adenovirus type 5 E1b 55K and E4 Orf3 proteins associate in infected cells and affect ND10 components. J. Gen. Virol. 80, 997–1008 (1999).
Boggio, R., Colombo, R., Hay, R. T., Draetta, G. F. & Chiocca, S. A mechanism for inhibiting the SUMO pathway. Mol. Cell 16, 549–561 (2004). The finding that a viral protein can comprehensively dysregulate the host sumoylation system.
Boggio, R., Passafaro, A. & Chiocca, S. Targeting SUMO E1 to ubiquitin ligases: a viral strategy to counteract sumoylation. J. Biol. Chem. 282, 15376–15382 (2007).
Heaton, P. R., Deyrieux, A. F., Bian, X. L. & Wilson, V. G. HPV E6 proteins target Ubc9, the SUMO conjugating enzyme. Virus Res. 158, 199–208 (2011).
Ledl, A., Schmidt, D. & Muller, S. Viral oncoproteins E1A and E7 and cellular LxCxE proteins repress SUMO modification of the retinoblastoma tumor suppressor. Oncogene 24, 3810–3818 (2005).
Bieniasz, P. D. Intrinsic immunity: a front-line defense against viral attack. Nature Immunol. 5, 1109–1115 (2004).
Chu, Y. & Yang, X. SUMO E3 ligase activity of TRIM proteins. Oncogene 30, 1108–1116 (2011). A pointer to the potential future importance of TRIM proteins and SUMO in intrinsic and innate immunity.
Napolitano, L. M., Jaffray, E. G., Hay, R. T. & Meroni, G. Functional interactions between ubiquitin E2 enzymes and TRIM proteins. Biochem. J. 434, 309–319 (2011).
Everett, R. D. & Murray, J. ND10 components relocate to sites associated with herpes simplex virus type 1 nucleoprotein complexes during virus infection. J. Virol. 79, 5078–5089 (2005).
Everett, R. D., Parsy, M. L. & Orr, A. Analysis of the functions of herpes simplex virus type 1 regulatory protein ICP0 that are critical for lytic infection and derepression of quiescent viral genomes. J. Virol. 83, 4963–4977 (2009).
Lukashchuk, V., Orr, A. & Everett, R. D. Regulation of ICP0 null mutant HSV-1 infection by ND10 components ATRX and hDaxx. J. Virol. 84, 4026–4040 (2010).
Maul, G. G. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays 20, 660–667 (1998).
Arriagada, G., Muntean, L. N. & Goff, S. P. SUMO-interacting motifs of human TRIM5α are important for antiviral activity. PLoS Pathog. 7, e1002019 (2011). A study showing the involvement of SIMs in intrinsic antiviral defences.
Lukic, Z. & Campbell, E. M. The cell biology of TRIM5α. Curr. HIV/AIDS Rep. 9, 73–80 (2012).
Lukic, Z., Goff, S. P., Campbell, E. M. & Arriagada, G. Role of SUMO-1 and SUMO interacting motifs in rhesus TRIM5α-mediated restriction. Retrovirology 10, 10 (2013).
Brandariz-Nunez, A. et al. Contribution of SUMO-interacting motifs and SUMOylation to the antiretroviral properties of TRIM5α. Virology 435, 463–471 (2012).
Liang, Q. et al. Tripartite motif-containing protein 28 is a small ubiquitin-related modifier E3 ligase and negative regulator of IFN regulatory factor 7. J. Immunol. 187, 4754–4763 (2011).
Uchil, P. D. et al. TRIM protein mediated regulation of inflammatory and innate immune signaling and its association with antiretroviral activity. J. Virol. 87, 257–272 (2012).
Randall, R. E. & Goodbourn, S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89, 1–47 (2008).
Fu, J., Xiong, Y., Xu, Y., Cheng, G. & Tang, H. MDA5 is SUMOylated by PIAS2β in the upregulation of type I interferon signaling. Mol. Immunol. 48, 415–422 (2011).
Mi, Z., Fu, J., Xiong, Y. & Tang, H. SUMOylation of RIG-I positively regulates the type I interferon signaling. Protein Cell 1, 275–283 (2010).
Ran, Y. et al. SENP2 negatively regulates cellular antiviral response by deSUMOylating IRF3 and conditioning it for ubiquitination and degradation. J. Mol. Cell. Biol. 3, 283–292 (2011).
Begitt, A., Droescher, M., Knobeloch, K. P. & Vinkemeier, U. SUMO conjugation of STAT1 protects cells from hyperresponsiveness to IFNγ. Blood 118, 1002–1007 (2011).
Van Nguyen, T. et al. SUMO-specific protease 1 is critical for early lymphoid development through regulation of STAT5 activation. Mol. Cell 45, 210–221 (2012).
Han, K. J., Jiang, L. & Shu, H. B. Regulation of IRF2 transcriptional activity by its sumoylation. Biochem. Biophys. Res. Commun. 372, 772–778 (2008).
Kubota, T. et al. Virus infection triggers SUMOylation of IRF3 and IRF7, leading to the negative regulation of type I interferon gene expression. J. Biol. Chem. 283, 25660–25670 (2008).
Nakagawa, K. & Yokosawa, H. PIAS3 induces SUMO-1 modification and transcriptional repression of IRF-1. FEBS Lett. 530, 204–208 (2002).
Liu, Y., Bridges, R., Wortham, A. & Kulesz-Martin, M. NF-κB repression by PIAS3 mediated RelA SUMOylation. PLoS ONE 7, e37636 (2012).
Desterro, J. M., Rodriguez, M. S. & Hay, R. T. SUMO-1 modification of IκBα inhibits NF-κB activation. Mol. Cell 2, 233–239 (1998).
Liu, B. et al. PIAS1 selectively inhibits interferon-inducible genes and is important in innate immunity. Nature Immunol. 5, 891–898 (2004).
Tahk, S. et al. Control of specificity and magnitude of NF-κB and STAT1-mediated gene activation through PIASy and PIAS1 cooperation. Proc. Natl Acad. Sci. USA 104, 11643–11648 (2007).
Chang, T. H., Xu, S., Tailor, P., Kanno, T. & Ozato, K. The small ubiquitin-like modifier-deconjugating enzyme sentrin-specific peptidase 1 switches IFN regulatory factor 8 from a repressor to an activator during macrophage activation. J. Immunol. 189, 3548–3556 (2012).
Chang, T. H. et al. Ebola Zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLoS Pathog. 5, e1000493 (2009). An example of a virus using the sumoylation system to block antiviral innate immunity.
Bentz, G. L., Shackelford, J. & Pagano, J. S. Epstein-Barr virus latent membrane protein 1 regulates the function of interferon regulatory factor 7 by inducing its sumoylation. J. Virol. 86, 12251–12261 (2012).
Bruderer, R. et al. Purification and identification of endogenous polySUMO conjugates. EMBO Rep. 12, 142–148 (2011).
Tirard, M. et al. In vivo localization and identification of SUMOylated proteins in the brain of His6-HA-SUMO1 knock-in mice. Proc. Natl Acad. Sci. USA 109, 21122–21127 (2012).
Pal, S., Santos, A., Rosas, J. M., Ortiz-Guzman, J. & Rosas-Acosta, G. Influenza A virus interacts extensively with the cellular SUMOylation system during infection. Virus Res. 158, 12–27 (2011). A paper demonstrating massive upregulation of host cell sumoylation in response to viral infection.
Wu, C. Y., Jeng, K. S. & Lai, M. M. The SUMOylation of matrix protein M1 modulates the assembly and morphogenesis of influenza A virus. J. Virol. 85, 6618–6628 (2011).
Cohen, P. & Tcherpakov, M. Will the ubiquitin system furnish as many drug targets as protein kinases? Cell 143, 686–693 (2010).
Shen, T. H., Lin, H. K., Scaglioni, P. P., Yung, T. M. & Pandolfi, P. P. The mechanisms of PML-nuclear body formation. Mol. Cell 24, 331–339 (2006).
Ishov, A. M. et al. PML is critical for ND10 formation and recruits the PML-interacting protein Daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147, 221–234 (1999).
Lewis, P. W., Elsaesser, S. J., Noh, K. M., Stadler, S. C. & Allis, C. D. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc. Natl Acad. Sci. USA 107, 14075–14080 (2010).
Li, H. et al. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol. Cell. Biol. 20, 1784–1796 (2000).
Ishov, A. M., Vladimirova, O. V. & Maul, G. G. Heterochromatin and ND10 are cell-cycle regulated and phosphorylation-dependent alternate nuclear sites of the transcription repressor Daxx and SWI/SNF protein ATRX. J. Cell Sci. 117, 3807–3820 (2004).
Cuchet, D. et al. PML isoforms I and II participate in PML-dependent restriction of HSV-1 replication. J. Cell Sci. 124, 280–291 (2011).
Ullmann, R., Chien, C. D., Avantaggiati, M. L. & Muller, S. An acetylation switch regulates SUMO-dependent protein interaction networks. Mol. Cell 46, 759–770 (2012).
Weidtkamp-Peters, S. et al. Dynamics of component exchange at PML nuclear bodies. J. Cell Sci. 121, 2731–2743 (2008).
Chelbi-Alix, M. K. & de Thé, H. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18, 935–941 (1999).
Sivachandran, N., Sarkari, F. & Frappier, L. Epstein-Barr nuclear antigen 1 contributes to nasopharyngeal carcinoma through disruption of PML nuclear bodies. PLoS Pathog. 4, e1000170 (2008).
Ling, P. D., Tan, J., Sewatanon, J. & Peng, R. Murine gammaherpesvirus 68 open reading frame 75c tegument protein induces the degradation of PML and is essential for production of infectious virus. J. Virol. 82, 8000–8012 (2008).
Full, F., Reuter, N., Zielke, K., Stamminger, T. & Ensser, A. Herpesvirus saimiri antagonizes nuclear domain 10-instituted intrinsic immunity via an ORF3-mediated selective degradation of cellular protein Sp100. J. Virol. 86, 3541–3553 (2012).
Ling, P. D. et al. Mediation of Epstein-Barr virus EBNA-LP transcriptional coactivation by Sp100. EMBO J. 24, 3565–3575 (2005).
Tavalai, N. & Stamminger, T. New insights into the role of the subnuclear structure ND10 for viral infection. Biochim. Biophys. Acta 1783, 2207–2221 (2008).
Glass, M. & Everett, R. D. Components of promyelocytic leukemia nuclear bodies (ND10) act cooperatively to repress herpesvirus infection. J. Virol. 87, 2174–2185 (2013).
Acknowledgements
Work in the R.D.E. and C.B. laboratories is funded by the UK Medical Research Council. B.G.H. acknowledges funding support from the UK Medical Research Council, the University of Glasgow, UK, and the European Commission (Framework Programme 7 Marie Curie career integration grant 321703 as part of project UBIFLU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Supplementary information
Supplementary Table 1
Summary of identified SUMO modified viral proteins (PDF 317 kb)
Supplementary Table 2
Interactions of viral proteins with components of the SUMO modification pathway (PDF 314 kb)
Glossary
- Small ubiquitin-like modifier
-
(SUMO). A family of small proteins that are related to ubiquitin and that can be conjugated to substrate proteins through isopeptide linkages.
- Protein inhibitor of activated STAT
-
(PIAS). A family of small ubiquitin-like modifier (SUMO) E3 ligases with RING-related catalytic domains.
- SUMO-targeted ubiquitin ligases
-
(STUbLs). A class of ubiquitin E3 ligases that interact with small ubiquitin-like modifier (SUMO) and ubiquitylate sumoylated proteins.
- STAT
-
(Signal transducer and activator of transcription). A family of proteins that engage in both interferon-regulated signal transduction in the cytoplasm and, after phosphorylation and nuclear translocation, the assembly of transcriptional activation complexes on the promoters of interferon-stimulated genes.
- Interferon
-
(IFN). A family of small proteins that are synthesized in response to pathogens and which initiate signal transduction pathways in infected and surrounding cells to induce innate immune responses.
- RING finger
-
A protein motif in which two zinc atoms are coordinated by Cys and His residues in a cross-braced fold that frequently confers ubiquitin or small ubiquitin-like modifier (SUMO) E3 ligase activity.
- Pattern recognition receptors
-
(PRRs). Proteins that recognize pathogen-associated molecular signals in the initial stages of innate immune responses.
- Interferon-regulatory factor
-
(IRF). A family of proteins that are components of transcription complexes which regulate the transcription of interferon-responsive genes.
Rights and permissions
About this article
Cite this article
Everett, R., Boutell, C. & Hale, B. Interplay between viruses and host sumoylation pathways. Nat Rev Microbiol 11, 400–411 (2013). https://doi.org/10.1038/nrmicro3015
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3015
This article is cited by
-
SUMOylation of SAMHD1 at Lysine 595 is required for HIV-1 restriction in non-cycling cells
Nature Communications (2021)
-
Self-guarding of MORC3 enables virulence factor-triggered immunity
Nature (2021)
-
Sumoylation of Human Parainfluenza Virus Type 3 Phosphoprotein Correlates with A Reduction in Viral Replication
Virologica Sinica (2021)
-
Interaction of PIAS1 with PRRS virus nucleocapsid protein mediates NF-κB activation and triggers proinflammatory mediators during viral infection
Scientific Reports (2019)
-
RanBP2 regulates the anti-retroviral activity of TRIM5α by SUMOylation at a predicted phosphorylated SUMOylation motif
Communications Biology (2018)