Key Points
-
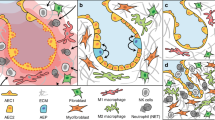
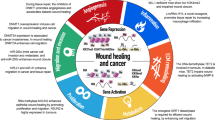
A series of clinical and preclinical findings suggest a relationship between wound repair and cancer: malignant tumours often develop at sites of chronic injury and permanent tissue damage through chronic inflammation is a major risk factor for the development of cancer.
-
Recent studies have highlighted important parallels between wound healing and cancer at the molecular and cellular level. For example, microarray analyses revealed strong similarities in the gene-expression profile of wounds and carcinomas. However, important differences were also observed, which might explain the altered metabolism, impaired differentiation capacity and invasive growth of tumour cells.
-
The wound-healing process occurs in three overlapping phases: inflammation, new tissue formation and tissue remodelling. This review summarizes the cellular and molecular events that occur during these phases and the similarities and differences to cancer.
-
The presence of a fibrin clot is a hallmark of early wounds and cancers and it initiates a healing response. This response is transient and self-limiting in wounds, but it becomes chronic in cancer.
-
Stromal cells in wounds and tumours, including fibroblasts and/or myofibroblasts, endothelial cells and inflammatory cells, are important regulators of migration and proliferation of normal epithelial cells in wounds and of malignant epithelial cells in tumours. The factors that are responsible for the stromal–epithelial cross-talk are similar in wounds and tumours and include cytokines or growth factors, matrix molecules and proteinases.
-
Most cancer therapies also inhibit the wound-healing process, but recent examples suggest that inhibition of tumour growth can be achieved without affecting the tissue-repair process.
Abstract
What is the relationship between the wound-healing process and the development of cancer? Malignant tumours often develop at sites of chronic injury, and tissue injury has an important role in the pathogenesis of malignant disease, with chronic inflammation being the most important risk factor. The development and functional characterization of genetically modified mice that lack or overexpress genes that are involved in repair, combined with gene-expression analysis in wounds and tumours, have highlighted remarkable similarities between wound repair and cancer. However, a few crucial differences were also observed, which could account for the altered metabolism, impaired differentiation capacity and invasive growth of malignant tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Reed, B. R. & Clark, R. A. Cutaneous tissue repair: practical implications of current knowledge. II. J. Am. Acad. Dermatol. 13, 919–941 (1985).
Virchow, R. Virchow, R. Aetiologie der neoplastischen Geschwulste/Pathogenie der neoplastischen Geschwulste. (Verlag von August Hirschwald, Berlin, Germany, 1863).
Dunham, L. J. Cancer in man at site of prior benign lesion of skin or mucous membrane: a review. Cancer Res. 32, 1359–1374 (1972).
Haddow, A. Molecular repair, wound healing, and carcinogenesis: tumor production a possible overhealing? Adv. Cancer Res. 16, 181–234 (1972).
Dvorak, H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659 (1986). In this paper, H. Dvorak formulated his famous hypothesis that tumours are wounds that do not heal, and he describes the underlying cellular basis.
Dolberg, D. S., Hollingsworth, R., Hertle, M. & Bissell, M. J. Wounding and its role in RSV-mediated tumor formation. Science 230, 676–678 (1985). The first demonstration that tumours preferentially grow at sites of wounding in retrovirus-infected chickens.
Martins-Green, M., Boudreau, N. & Bissell, M. J. Inflammation is responsible for the development of wound-induced tumors in chickens infected with Rous sarcoma virus. Cancer Res. 54, 4334–4341 (1994).
Schuh, A. C., Keating, S. J., Monteclaro, F. S., Vogt, P. K. & Breitman, M. L. Obligatory wounding requirement for tumorigenesis in v-jun transgenic mice. Nature 346, 756–760 (1990).
Drew, A. F., Liu, H., Davidson, J. M., Daugherty, C. C. & Degen, J. L. Wound-healing defects in mice lacking fibrinogen. Blood 97, 3691–3698 (2001).
Sakai, T. et al. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nature Med. 7, 324–330 (2001).
Weller, K., Foitzik, K., Paus, R., Syska, W. & Maurer, M. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 20, 2366–2368 (2006).
Leibovich, S. J. & Ross, R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am. J. Pathol. 78, 71–100 (1975).
Martin, P. & Leibovich, S. J. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 15, 599–607 (2005). Summarizes the positive and negative effects of inflammation on the wound-repair process.
Eming, S. A., Krieg, T. & Davidson, J. M. Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 127, 514–525 (2007).
Martin, P. et al. Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Curr. Biol. 13, 1122–1128 (2003).
Dovi, J. V., He, L. K. & DiPietro, L. A. Accelerated wound closure in neutrophil-depleted mice. J. Leukoc. Biol. 73, 448–455 (2003).
Kumin, A. et al. Peroxiredoxin 6 is required for blood vessel integrity in wounded skin. J. Cell Biol. 179, 747–760 (2007).
de Visser, K. E., Eichten, A. & Coussens, L. M. Paradoxical roles of the immune system during cancer development. Nature Rev. Cancer 6, 24–37 (2006).
Balkwill, F., Charles, K. A. & Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7, 211–217 (2005). References18 and 19 summarize the current knowledge on the role of inflammation in cancer development and progression.
Allavena, P., Sica, A., Garlanda, C. & Mantovani, A. The yin-yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol. Rev. 222, 155–161 (2008).
Condeelis, J. & Pollard, J. W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124, 263–266 (2006). Summarizes the different functions of macrophages in cancer that are also relevant for the wound-healing process.
Eming, S. A. et al. Accelerated wound closure in mice deficient for interleukin-10. Am. J. Pathol. 170, 188–202 (2007).
Hussain, S. P., Hofseth, L. J. & Harris, C. C. Radical causes of cancer. Nature Rev. Cancer 3, 276–285 (2003).
Marigo, I., Dolcetti, L., Serafini, P., Zanovello, P. & Bronte, V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 222, 162–179 (2008).
Nagaraj, S. et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nature Med. 13, 828–835 (2007).
Albini, A. & Sporn, M. B. The tumour microenvironment as a target for chemoprevention. Nature Rev. Cancer 7, 139–147 (2007).
Muller-Decker, K., Hirschner, W., Marks, F. & Furstenberger, G. The effects of cyclooxygenase isozyme inhibition on incisional wound healing in mouse skin. J. Invest. Dermatol. 119, 1189–1195 (2002).
Subbaramaiah, K. & Dannenberg, A. J. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol. Sci. 24, 96–102 (2003).
Braun, S. et al. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol. Cell. Biol. 22, 5492–5505 (2002).
Yu, X. & Kensler, T. Nrf2 as a target for cancer chemoprevention. Mutat. Res. 591, 93–102 (2005).
Martin, P. Wound healing—aiming for perfect skin regeneration. Science 276, 75–81 (1997).
Grose, R. et al. A crucial role of β 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129, 2303–2315 (2002).
White, D. E. et al. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 6, 159–170 (2004).
Romer, J. et al. Impaired wound healing in mice with a disrupted plasminogen gene. Nature Med. 2, 287–292 (1996).
Lund, L. R. et al. Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J. 18, 4645–4656 (1999).
Egeblad, M. & Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nature Rev. Cancer 2, 161–174 (2002).
Florin, L., Maas-Szabowski, N., Werner, S., Szabowski, A. & Angel, P. Increased keratinocyte proliferation by JUN-dependent expression of PTN and SDF-1 in fibroblasts. J. Cell Sci. 118, 1981–1989 (2005).
Avniel, S. et al. Involvement of the CXCL12/CXCR4 pathway in the recovery of skin following burns. J. Invest. Dermatol. 126, 468–476 (2006).
Ceradini, D. J. et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Med. 10, 858–864 (2004).
Orimo, A. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348 (2005). Describes the important role of carcinoma-associated fibroblasts for tumour growth and the underlying mechanisms of action.
Chmielowiec, J. et al. c-Met is essential for wound healing in the skin. J. Cell Biol. 177, 151–162 (2007).
Werner, S. & Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83, 835–870 (2003).
Benvenuti, S. & Comoglio, P. M. The MET receptor tyrosine kinase in invasion and metastasis. J. Cell Physiol. 213, 316–325 (2007).
Sibilia, M. et al. The epidermal growth factor receptor: from development to tumorigenesis. Differentiation 75, 770–787 (2007).
Sano, S., Chan, K. S. & Digiovanni, J. Impact of Stat3 activation upon skin biology: a dichotomy of its role between homeostasis and diseases. J. Dermatol. Sci. 50, 1–14 (2008).
Amendt, C., Schirmacher, P., Weber, H. & Blessing, M. Expression of a dominant negative type II TGF-β receptor in mouse skin results in an increase in carcinoma incidence and an acceleration of carcinoma development. Oncogene 17, 25–34 (1998).
Ashcroft, G. S. et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nature Cell Biol. 1, 260–266 (1999).
Wakefield, L. M. & Roberts, A. B. TGF-β signaling: positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 12, 22–29 (2002).
Werner, S. et al. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science 266, 819–822 (1994). The first study to use transgenic mice to study wound repair.
Grose, R. et al. The role of fibroblast growth factor receptor 2b in skin homeostasis and cancer development. EMBO J. 26, 1268–1278 (2007).
Tse, J. C. & Kalluri, R. Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J. Cell Biochem. 101, 816–829 (2007).
Mani, S. A. et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Janda, E. et al. Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 156, 299–314 (2002).
Radisky, D. C. et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436, 123–127 (2005).
Ting, S. B. et al. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science 308, 411–413 (2005).
Ferrara, N. & Kerbel, R. S. Angiogenesis as a therapeutic target. Nature 438, 967–974 (2005).
Tepper, O. M. et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 105, 1068–1077 (2005).
Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971). In this paper, J. Folkman formulates the important hypothesis that angiogenesis is a prerequisite for tumour growth.
Paavonen, K., Puolakkainen, P., Jussila, L., Jahkola, T. & Alitalo, K. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am. J. Pathol. 156, 1499–1504 (2000).
Saharinen, P., Tammela, T., Karkkainen, M. J. & Alitalo, K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 25, 387–395 (2004).
Liersch, R. & Detmar, M. Lymphangiogenesis in development and disease. J. Thromb. Haemost. 98, 304–310 (2007).
Ferrara, N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin. Oncol. 29, 10–14 (2002).
Rossiter, H. et al. Loss of vascular endothelial growth factor A activity in murine epidermal keratinocytes delays wound healing and inhibits tumor formation. Cancer Res. 64, 3508–3516 (2004).
Hong, Y. K. et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the α1β1 and α2β1 integrins. FASEB J. 18, 1111–1113 (2004).
Larcher, F., Murillas, R., Bolontrade, M., Conti, C. J. & Jorcano, J. L. VEGF/VPF overexpression in skin of transgenic mice induces angiogenesis, vascular hyperpermeability and accelerated tumor development. Oncogene 17, 303–311 (1998).
Hirakawa, S. et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 201, 1089–1099 (2005).
Carmeliet, P. et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nature Med. 7, 575–583 (2001).
Cianfarani, F. et al. Placenta growth factor in diabetic wound healing: altered expression and therapeutic potential. Am. J. Pathol. 169, 1167–1182 (2006).
Marcellini, M. et al. Increased melanoma growth and metastasis spreading in mice overexpressing placenta growth factor. Am. J. Pathol. 169, 643–654 (2006).
Saaristo, A. et al. Vascular endothelial growth factor-C accelerates diabetic wound healing. Am. J. Pathol. 169, 1080–1087 (2006).
Hirakawa, S. et al. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 109, 1010–1017 (2007).
Streit, M. et al. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 19, 3272–3282 (2000).
Streit, M. et al. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am. J. Pathol. 155, 441–452 (1999).
Luster, A. D., Cardiff, R. D., MacLean, J. A., Crowe, K. & Granstein, R. D. Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc. Assoc. Am. Physicians 110, 183–196 (1998).
Arenberg, D. A. et al. Interferon-γ-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J. Exp. Med. 184, 981–992 (1996).
O'Reilly, M. S. et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285 (1997).
Bloch, W. et al. The angiogenesis inhibitor endostatin impairs blood vessel maturation during wound healing. FASEB J. 14, 2373–2376 (2000).
Lange-Asschenfeldt, B. et al. The angiogenesis inhibitor vasostatin does not impair wound healing at tumor-inhibiting doses. J. Invest. Dermatol. 117, 1036–1041 (2001).
Kamba, T. & McDonald, D. M. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br. J. Cancer 96, 1788–1795 (2007).
Opalenik, S. R. & Davidson, J. M. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J. 19, 1561–1563 (2005).
Werner, S. & Smola, H. Paracrine regulation of keratinocyte proliferation and differentiation. Trends Cell Biol. 11, 143–146 (2001).
Radisky, D. C., Kenny, P. A. & Bissell, M. J. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J. Cell Biochem. 101, 830–839 (2007).
Gaggioli, C. et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nature Cell Biol. 9, 1392–1400 (2007).
Direkze, N. C. et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 64, 8492–8495 (2004).
Iyer, V. R. et al. The transcriptional program in the response of human fibroblasts to serum. Science 283, 83–87 (1999).
Cooper, L., Johnson, C., Burslem, F. & Martin, P. Wound healing and inflammation genes revealed by array analysis of 'macrophageless' PU.1 null mice. Genome Biol. 6, R5 (2005).
Li, W. et al. Extracellular heat shock protein-90α: linking hypoxia to skin cell motility and wound healing. EMBO J. 26, 1221–1233 (2007).
Eustace, B. K. et al. Functional proteomic screens reveal an essential extracellular role for hsp90 α in cancer cell invasiveness. Nature Cell Biol. 6, 507–514 (2004).
Ostman, A. PDGF receptors-mediators of autocrine tumor growth and regulators of tumor vasculature and stroma. Cytokine Growth Factor Rev. 15, 275–286 (2004).
Gao, Z. et al. Deletion of the PDGFR-β gene affects key fibroblast functions important for wound healing. J. Biol. Chem. 280, 9375–9389 (2005).
Pietras, K. et al. STI571 enhances the therapeutic index of epothilone B by a tumor-selective increase of drug uptake. Clin. Cancer Res. 9, 3779–3787 (2003).
Rajkumar, V. S. et al. Platelet-derived growth factor-β receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am. J. Pathol. 169, 2254–2265 (2006).
Distler, J. H. et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 56, 311–322 (2007).
Steed, D. L. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plast. Reconstr Surg. 117, S143–S149 (2006).
Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C. & Brown, R. A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature Rev. Mol. Cell Biol. 3, 349–363 (2002).
Shah, M., Foreman, D. M. & Ferguson, M. W. Neutralisation of TGF-β 1 and TGF-β 2 or exogenous addition of TGF-β 3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 108, 985–1002 (1995).
Cheon, S. S. et al. β-catenin regulates wound size and mediates the effect of TGF-β in cutaneous healing. FASEB J. 20, 692–701 (2006).
Cheon, S. S. et al. β-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc. Natl Acad. Sci. USA 99, 6973–6978 (2002).
Alman, B. A., Li, C., Pajerski, M. E., Diaz-Cano, S. & Wolfe, H. J. Increased β-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors). Am. J. Pathol. 151, 329–334 (1997).
Bhowmick, N. A. et al. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303, 848–851 (2004).
Stover, D. G., Bierie, B. & Moses, H. L. A delicate balance: TGF-β and the tumor microenvironment. J. Cell Biochem. 101, 851–861 (2007).
Mori, R., Shaw, T. J. & Martin, P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J. Exp. Med. 205, 43–51 (2008).
El-Tanani, M. K. et al. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 17, 463–474 (2006).
Esteller, M. Epigenetics in cancer. N. Engl. J. Med. 358, 1148–1159 (2008).
Chang, H. Y. et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2, E7 (2004). Reports on the parallels in the gene-expression pattern between malignant tumours and serum-treated fibroblasts.
Riss, J. et al. Cancers as wounds that do not heal: differences and similarities between renal regeneration/repair and renal cell carcinoma. Cancer Res. 66, 7216–7224 (2006).
Pedersen, T. X. et al. Laser capture microdissection-based in vivo genomic profiling of wound keratinocytes identifies similarities and differences to squamous cell carcinoma. Oncogene 22, 3964–3976 (2003).
Thorey, I. S. et al. Transgenic mice reveal novel activities of growth hormone in wound repair, angiogenesis, and myofibroblast differentiation. J. Biol. Chem. 279, 26674–26684 (2004).
Acknowledgements
We are grateful to M. Detmar, ETH Zürich, Switzerland, P. Martin, University of Bristol, UK, and A. Knuth, University of Zürich, Switzerland, for helpful suggestions and critical comments on the manuscript; to U. Scheier for help with the figures and the references; and to I. Thorey for providing the micrographs shown in Figure 4 and 5. Work in the laboratory of S.W. is supported by the ETH Zürich, the Swiss National Science Foundation (grant 3100A0-109340/1), Oncosuisse (grant OCS-02017-02-2007), and the European Union (grant Ulcertherapy). M.S. is supported by an EMBO postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
41580_2008_BFnrm2455_MOESM2_ESM.pdf
Supplementary information S1 (table) | Approved cancer drugs that target growth factors or their receptors: Effects on cancer and wound healing (PDF 161 kb)
Related links
Glossary
- Scar
-
A connective tissue replacement following the wounding of the dermis.
- Keloid
-
An overgrowth of scar tissue beyond the original wound edge.
- Stroma
-
A connective tissue component of an organ (or tumour), which includes fibroblasts, blood and lymphatic vessels, inflammatory cells and extracellular matrix.
- Granulation tissue
-
A highly vascularized and cell-rich tissue that replaces the fibrin clot in a skin wound.
- Sarcoma
-
A cancer that arises from mesenchymal cells.
- Warburg effect
-
The observation that most cancer cells predominantly produce energy by anaerobic glycolysis, which results in lactate formation.
- Keratinocyte
-
The epithelial cell of the skin.
- Epidermis
-
The outer, protective, non-vascular layer of the skin that covers the dermis.
- Re-epithelialization
-
Regeneration of the injured epidermis in a skin wound.
- Dermis
-
The connective tissue layer of the skin that is located below the epidermis.
- Complement
-
A group of more than 20 serum proteins, some of which can be serially activated and participate in a cascade that results in cell lysis.
- Reactive oxygen species
-
(ROS). Molecules or ions that are formed by the incomplete one-electron reduction of oxygen. ROS include singlet oxygen, superoxides, peroxides, hydroxyl radicals and hypochlorous acid.
- PU.1
-
A member of the ETS family of transcription factors that is required for the development of multiple haematopoietic lineages.
- Peroxiredoxins
-
A family of six thiol proteins that detoxify hydrogen peroxide, lipid hydroperoxides and — in the case of peroxiredoxin-6 — also peroxinitrite.
- Angiogenesis
-
The sprouting of new vessels from pre-existing vessels.
- Matrix metalloproteinases
-
(MMPs). Zinc-dependent endopeptidases that cleave different extracellular matrix proteins and also growth factors, chemokines, cell-surface receptors and other proteins.
- Myeloid-derived suppressor cells
-
(MDSCs). Heterogeneous mixture of immature myeloid cells that are potent inhibitors of anti-tumour immunity. In mice they are generally defined by the markers CD11b and GR1.
- Cyclooxygenases
-
Enzymes responsible for the formation of prostaglandins, prostacyclins and thromboxanes.
- NRF2
-
A Leu-zipper transcription factor that activates the expression of a battery of cytoprotective genes.
- Lamellipodium
-
A flattened projection from the cell surface, generally associated with cell migration.
- Epithelial–mesenchymal transition
-
(EMT). A developmental programme in which epithelial cells lose cell–cell adhesion, acquire a fibroblast-like morphology and increase their motility.
- Carcinoma
-
A cancer that arises from epithelial cells.
- STAT3
-
A protein that transduces the signal from activated cytokine or growth-factor receptors to the nucleus.
- Psoriasis
-
An inflammatory skin disease that is associated with keratinocyte hyperproliferation and abnormal differentiation.
- SMAD3
-
A signalling protein that is activated by the type I transforming growth factor-β receptor, and which transduces the signal from the plasma membrane to the nucleus.
- Pericyte
-
A mesenchymal cell that is associated with the wall of small blood vessels.
Rights and permissions
About this article
Cite this article
Schäfer, M., Werner, S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol 9, 628–638 (2008). https://doi.org/10.1038/nrm2455
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2455
This article is cited by
-
Bridging tissue repair and epithelial carcinogenesis: epigenetic memory and field cancerization
Cell Death & Differentiation (2024)
-
All-in-one smart dressing for simultaneous angiogenesis and neural regeneration
Journal of Nanobiotechnology (2023)
-
PTP4A1 promotes oral squamous cell carcinoma (OSCC) metastasis through altered mitochondrial metabolic reprogramming
Cell Death Discovery (2023)
-
A microfluidic-based PDAC organoid system reveals the impact of hypoxia in response to treatment
Cell Death Discovery (2023)
-
Effects of dexmedetomidine on A549 non-small cell lung cancer growth in a clinically relevant surgical xenograft model
Scientific Reports (2023)