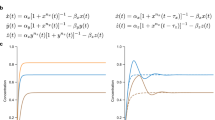
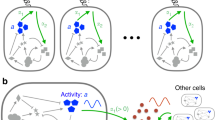
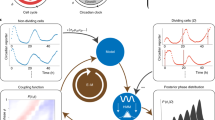
Key Points
-
Many physiological behaviours of cells are repeated periodically in time, such as hormone secretion, second messenger signalling, cell-division cycles and circadian rhythms.
-
Oscillatory behaviour is a systems-level property of the interactions of genes, proteins and metabolites in the cell.
-
All biochemical oscillators are characterized by negative feedback with time delay.
-
Time delay can be due to a chain of intermediates between the 'cause' and 'effect' of the negative-feedback loop or to a positive-feedback loop in the network.
-
For a biochemical reaction network to oscillate, the kinetics of the reactions must be sufficiently nonlinear and their timescales must be properly balanced, in quantitative terms that are predicted by theoretical analysis.
-
These conditions are satisfied by various specific network topologies that provide the basis for a classification of biochemical oscillators.
Abstract
Cellular rhythms are generated by complex interactions among genes, proteins and metabolites. They are used to control every aspect of cell physiology, from signalling, motility and development to growth, division and death. We consider specific examples of oscillatory processes and discuss four general requirements for biochemical oscillations: negative feedback, time delay, sufficient 'nonlinearity' of the reaction kinetics and proper balancing of the timescales of opposing chemical reactions. Positive feedback is one mechanism to delay the negative-feedback signal. Biological oscillators can be classified according to the topology of the positive- and negative-feedback loops in the underlying regulatory mechanism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pye, K. & Chance, B. Sustained sinusoidal oscillations of reduced pyridine nucleotide in a cell-free extract of Saccharomyces carlsbergensis. Proc. Natl Acad. Sci. USA 55, 888–894 (1966).
Hess, B. & Boiteux, A. Oscillatory phenomena in biochemistry. Annu. Rev. Biochem. 40, 237–258 (1971).
Gerisch, G., Fromm, H., Huesgen, A. & Wick, U. Control of cell-contact sites by cyclic AMP pulses in differentiating Dictyostelium cells. Nature 255, 547–549 (1975).
Olsen, L. F. & Degn, H. Oscillatory kinetics of the peroxidase-oxidase reaction in an open system. Experimental and theoretical studies. Biochim. Biophys. Acta 523, 321–334 (1978).
Olsen, L. F. & Degn, H. Chaos in an enzyme reaction. Nature 267, 177–178 (1977).
Higgins, J. Theory of oscillating reactions. Ind. Eng. Chem. 59, 19–62 (1967). An early but still useful review on biochemical oscillators with clear explanations of the underlying theory.
Prigogine, I., Lefever, R., Goldbeter, A. & Herschkowitz-Kaufman, M. Symmetry breaking instabilities in biological systems. Nature 223, 913–916 (1969).
Dunlap, J. C. Molecular bases for circadian clocks. Cell 96, 271–290 (1999).
Evans, T., Rosenthal, E. T., Youngblom, J., Distel, D. & Hunt, T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33, 389–396 (1983).
Gerhart, J., Wu, M. & Kirschner, M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J. Cell Biol. 98, 1247–1255 (1984).
Elowitz, M. B. & Leibler, S. A synthetic oscillatory network of transcriptional regulators. Nature 403, 335–338 (2000). The first example of a synthetic GRN with oscillatory dynamics. The oscillation arises from a three-component negative-feedback loop.
Chance, B., Pye, E. K., Ghosh, A. K. & Hess, B. Biological and Biochemical Oscillators (Academic Press, New York, 1973).
Gray, P. & Scott, S. K. Chemical Oscillations and Instabilities (Clarendon Press, Oxford, 1994).
Goldbeter, A. Biochemical Oscillations and Cellular Rhythms (Cambridge University Press, Cambridge, 1996).
Epstein, I. R. & Pojman, J. A. An Introduction to Nonlinear Chemical Dynamics (Oxford University Press, Oxford, 1998).
Berridge, M. J. & Rapp, P. E. A comparative survey of the function, mechanism and control of cellular oscillators. J. Exp. Biol. 81, 217–279 (1979).
Goldbeter, A. Computational approaches to cellular rhythms. Nature 420, 238–245 (2002).
Kholodenko, B. N. Cell-signalling dynamics in time and space. Nature Rev. Mol. Cell Biol. 7, 165–176 (2006).
Hardin, P. E., Hall, J. C. & Rosbash, M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343, 536–540 (1990). One of the first demonstrations that a transcriptional negative-feedback loop is at the heart of the circadian clock.
Gallego, M. & Virshup, D. M. Post-translational modifications regulate the ticking of the circadian clock. Nature Rev. Mol. Cell Biol. 8, 139–148 (2007).
Mackey, M. C. & Glass, L. Oscillation and chaos in physiological control systems. Science 197, 287–289 (1977). A classic study of oscillations in a time-delayed negative-feedback loop with application to physiological control systems.
Griffith, J. S. Mathematics of cellular control processes. I. Negative feedback to one gene. J. Theor. Biol. 20, 202–208 (1968).
Goodwin, B. C. Oscillatory behavior in enzymatic control processes. Adv. Enzyme Regul. 3, 425–438 (1965). The first theoretical investigation of oscillations in a transcriptional negative-feedback loop.
Goodwin, B. C. An entrainment model for timed enzyme syntheses in bacteria. Nature 209, 479–481 (1966).
Masters, M. & Donachie, W. D. Repression and the control of cyclic enzyme synthesis in Bacillus subtilis. Nature 209, 476–479 (1966).
Goldbeter, A. A model for circadian oscillations in the Drosophila period protein (PER). Proc. Biol. Sci. 261, 319–324 (1995).
Leloup, J. C. & Goldbeter, A. Modeling the circadian clock: from molecular mechanism to physiological disorders. Bioessays 30, 590–600 (2008).
Thomas, R. & D'Ari, R. Biological Feedback (CRC Press, Boca Raton, 1990).
Laurent, M. & Kellershohn, N. Multistability: a major means of differentiation and evolution in biological systems. Trends Biochem. Sci. 24, 418–422 (1999).
Tyson, J. J., Hong, C. I., Thron, C. D. & Novak, B. A simple model of circadian rhythms based on dimerization and proteolysis of PER and TIM. Biophys. J. 77, 2411–2417 (1999).
Segel, L. A. Biological Kinetics (Cambridge University Press, Cambridge, 1991).
Monod, J., Wyman, J. & Changeux, J. P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12, 88–118 (1965).
Goldbeter, A. & Koshland, D. E., Jr. An amplified sensitivity arising from covalent modification in biological systems. Proc. Natl Acad. Sci. USA 78, 6840–6844 (1981).
Gunawardena, J. Multisite protein phosphorylation makes a good threshold but can be a poor switch. Proc. Natl Acad. Sci. USA 102, 14617–14622 (2005).
Thron, C. D. Mathematical analysis of binary activation of a cell cycle kinase which down-regulates its own inhibitor. Biophys. Chem. 79, 95–106 (1999).
Kim, S. Y. & Ferrell, J. E., Jr. Substrate competition as a source of ultrasensitivity in the inactivation of Wee1. Cell 128, 1133–1145 (2007).
Lahav, G. et al. Dynamics of the p53–Mdm2 feedback loop in individual cells. Nature Genet. 36, 147–150 (2004).
Lev Bar-Or, R. et al. Generation of oscillations by the p53–Mdm2 feedback loop: a theoretical and experimental study. Proc. Natl Acad. Sci. USA 97, 11250–11255 (2000).
Ma, L. et al. A plausible model for the digital response of p53 to DNA damage. Proc. Natl Acad. Sci. USA 102, 14266–14271 (2005).
Monk, N. A. Oscillatory expression of Hes1, p53, and NF-κB driven by transcriptional time delays. Curr. Biol. 13, 1409–1413 (2003).
Nelson, D. E. et al. Oscillations in NF-κB signaling control the dynamics of gene expression. Science 306, 704–708 (2004).
Cheong, R., Hoffmann, A. & Levchenko, A. Understanding NF-κB signaling via mathematical modeling. Mol. Syst. Biol. 4, 192 (2008).
Hoffmann, A., Levchenko, A., Scott, M. L. & Baltimore, D. The IκB–NF-κB signaling module: temporal control and selective gene activation. Science 298, 1241–1245 (2002).
Franck, U. F. Kinetic feedback processes in physico-chemical oscillatory systems. Faraday Symp. Chem. Soc. 9, 137–149 (1974).
Rössler, O. E. A principle for chemical multivibration. J. Theor. Biol. 36, 413–417 (1972).
Gierer, A. & Meinhardt, H. A theory of biological pattern formation. Kybernetik 12, 30–39 (1972).
Novak, B. & Tyson, J. J. Numerical analysis of a comprehensive model of M-phase control in Xenopus oocyte extracts and intact embryos. J. Cell Sci. 106, 1153–1168 (1993).
Pomerening, J. R., Kim, S. Y. & Ferrell, J. E., Jr. Systems-level dissection of the cell-cycle oscillator: bypassing positive feedback produces damped oscillations. Cell 122, 565–578 (2005). Convincing experimental evidence that amplification (positive feedback) makes the cell-cycle oscillator (a negative-feedback loop) more robust.
Novak, B. & Tyson, J. J. Modeling the control of DNA replication in fission yeast. Proc. Natl Acad. Sci. USA 94, 9147–9152 (1997).
Stricker, J. et al. A fast, robust, and tunable synthetic gene oscillator. Nature (in the press).
Tsai, T. Y. et al. Robust, tunable biological oscillations from interlinked positive and negative feedback loops. Science 321, 126–129 (2008).
Goldbeter, A. & Lefever, R. Dissipative structures for an allosteric model. Application to glycolytic oscillations. Biophys. J. 12, 1302–1315 (1972).
Higgins, J. A chemical mechanism for oscillation of glycolytic intermediates in yeast cells. Proc. Natl Acad. Sci. USA 51, 989–994 (1964).
Sel'kov, E. E. Self-oscillation in glycolysis. 1. A simple kinetic model. Eur. J. Biochem. 4, 79–86 (1968).
Martiel, J. L. & Goldbeter, A. A model based on receptor desensitization for cyclic AMP signaling in Dictyostelium cells. Biophys. J. 52, 807–828 (1987).
Tyson, J. J. & Novak, B. Regulation of the eukaryotic cell cycle: molecular antagonism, hysteresis, and irreversible transitions. J. Theor. Biol. 210, 249–263 (2001).
Tyson, J. J. & Novak, B. Temporal organization of the cell cycle. Curr. Biol. 18, R759–R768 (2008).
Ciliberto, A., Novak, B. & Tyson, J. J. Steady states and oscillations in the p53/Mdm2 network. Cell Cycle 4, 488–493 (2005).
Zhang, T., Brazhnik, P. & Tyson, J. J. Exploring mechanisms of the DNA-damage response: p53 pulses and their possible relevance to apoptosis. Cell Cycle 6, 85–94 (2007).
Rust, M. J., Markson, J. S., Lane, W. S., Fisher, D. S. & O'Shea, E. K. Ordered phosphorylation governs oscillation of a three-protein circadian clock. Science 318, 809–812 (2007).
Borisuk, M. T. & Tyson, J. J. Bifurcation analysis of a model of mitotic control in frog eggs. J. Theor. Biol. 195, 69–85 (1998).
Rössler, O. E. Chaos in abstract kinetics: two prototypes. Bull. Math. Biol. 39, 275–289 (1977).
Snoussi, E. H. Necessary conditions for multistationarity and stable periodicity. J. Biol. Sys. 6, 3–9 (1998).
Goldbeter, A. Mechanism for oscillatory synthesis of cyclic AMP in Dictyostelium discoideum. Nature 253, 540–542 (1975).
Meyer, T. & Stryer, L. Molecular model for receptor-stimulated calcium spiking. Proc. Natl Acad. Sci. USA 85, 5051–5055 (1988).
Garmendia-Torres, C., Goldbeter, A. & Jacquet, M. Nucleocytoplasmic oscillations of the yeast transcription factor Msn2: evidence for periodic PKA activation. Curr. Biol. 17, 1044–1049 (2007).
Jacquet, M., Renault, G., Lallet, S., De Mey, J. & Goldbeter, A. Oscillatory nucleocytoplasmic shuttling of the general stress response transcriptional activators Msn2 and Msn4 in Saccharomyces cerevisiae. J. Cell Biol. 161, 497–505 (2003).
Lewis, J. Autoinhibition with transcriptional delay: a simple mechanism for the zebrafish somitogenesis oscillator. Curr. Biol. 13, 1398–1408 (2003).
Glass, L. & Mackey, M.C. Pathological conditions resulting from instabilities in physiological control systems. Ann. N.Y. Acad. Sci. 316, 214–235 (1979).
Acknowledgements
J.J.T. acknowledges financial support from the National Institutes of Health and the hospitality of Merton College, Oxford, UK, during the writing of this review. B.N. acknowledges support from the Biotechnology and Biological Sciences Research Council and from the European Commission Seventh Framework Programme (EC FP7). Our understanding of biochemical oscillations has developed over many years of delightful conversations with A. Goldbeter, L. Segel, A. Winfree, M. Mackey and L. Glass.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Related links
Glossary
- Somites
-
Early segmentations of the body of a vertebrate embryo that are laid down in a temporally and spatially periodic pattern.
- Robustness
-
The notion that a control system should function reliably in the face of expected perturbations from outside the control system and from inevitable internal fluctuations.
- Entrainment
-
The process whereby two interacting oscillating systems, which have different periods when running independently, assume the same period. The two oscillators may fall into synchrony, but other phase relationships are also possible.
- Bistability
-
A reaction network with two coexisting stable steady states (separated by an unstable steady state). Which stable state the network adopts depends on the initial concentrations of the reacting species.
- Hysteresis
-
A property of systems with bistability. The control system can be switched from one stable state to the other by a transient signal, and switched back again by a different transient signal. Hence, the state of the system depends not only on its present conditions, but also on its recent history.
- Chemical chaos
-
Refers to oscillatory chemical systems with aperiodic unpredictable behaviour. Chaos requires at least three interacting components.
Rights and permissions
About this article
Cite this article
Novák, B., Tyson, J. Design principles of biochemical oscillators. Nat Rev Mol Cell Biol 9, 981–991 (2008). https://doi.org/10.1038/nrm2530
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2530
This article is cited by
-
Identification of genes with oscillatory expression in glioblastoma: the paradigm of SOX2
Scientific Reports (2024)
-
Dynamics of Changes in the cAMP/cGMP Concentration Ratio in the Thymus and Spleen of Laboratory Mice during Vaccination against Plague and Tularemia against the Background of Immunomodulation
Bulletin of Experimental Biology and Medicine (2024)
-
An oscillating reaction network with an exact closed form solution in the time domain
BMC Bioinformatics (2023)
-
Systematic analysis of negative and positive feedback loops for robustness and temperature compensation in circadian rhythms
npj Systems Biology and Applications (2023)
-
A catalytically active oscillator made from small organic molecules
Nature (2023)