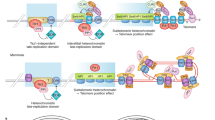
Key Points
-
Telomeres must overcome specific challenges to ensure their efficient replication.
-
In yeast cells, telomeres are replicated in late S phase in agreement with the late firing of subtelomeric origins. By contrast, in humans, subtelomeric origins might be activated earlier, although completion of replication is resumed very late because of delayed replication fork progression at the telomeric DNA repeats.
-
The unusual structures of telomeric chromatin hamper fork progression and may cause fork pause or arrest. We describe the events that allow the cell to alleviate these obstacles, pointing out the role of the telomeric DNA-binding proteins and of DNA-modifying enzymes.
-
Formation of the telomere overhang is a key event in telomere replication and for telomerase recruitment and activity. We describe the different events that lead to telomerase-independent overhang formation. Overhang formation requires fork passage and the leading and the lagging strand may be processed in different ways.
-
The erosion of telomeric DNA can be compensated for by elongation of telomeres by telomerase. We discuss the dynamic binding of telomerase and its associated proteins to telomeres during the cell cycle.
Abstract
The replication of the ends of linear chromosomes, or telomeres, poses unique problems, which must be solved to maintain genome integrity and to allow cell division to occur. Here, we describe and compare the timing and specific mechanisms that are required to initiate, control and coordinate synthesis of the leading and lagging strands at telomeres in yeasts, ciliates and mammals. Overall, it emerges that telomere replication relies on a strong synergy between the conventional replication machinery, telomere protection systems, DNA-damage-response pathways and chromosomal organization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McClintock, B. The production of homozygous deficient tissues with mutant characteristics by means of the aberrant mitotic behavior of ring-shaped chromosomes. Genetics 23, 315–376 (1938).
Sandell, L. L. & Zakian, V. A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell 75, 729–739 (1993).
van Steensel, B., Smogorzewska, A. & de Lange, T. TRF2 protects human telomeres from end-to-end fusions. Cell 92, 401–413 (1998).
de Lange, T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19, 2100–2110 (2005).
Brunori, M., Luciano, P., Gilson, E. & Geli, V. The telomerase cycle: normal and pathological aspects. J. Mol. Med. 83, 244–257 (2005).
Greider, C. W. & Blackburn, E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43, 405–413 (1985).
Cech, T. R. & Brehm, S. L. Replication of the extrachromosomal ribosomal RNA genes of Tetrahymena thermophilia. Nucleic Acids Res. 9, 3531–3543 (1981).
Wellinger, R. J., Wolf, A. J. & Zakian, V. A. Structural and temporal analysis of telomere replication in yeast. Cold Spring Harb. Symp. Quant. Biol. 58, 725–732 (1993).
Ivessa, A. S., Zhou, J. Q., Schulz, V. P., Monson, E. K. & Zakian, V. A. Saccharomyces Rrm3p, a 5′ to 3′ DNA helicase that promotes replication fork progression through telomeric and subtelomeric DNA. Genes Dev. 16, 1383–1396 (2002).
Makovets, S., Herskowitz, I. & Blackburn, E. H. Anatomy and dynamics of DNA replication fork movement in yeast telomeric regions. Mol. Cell. Biol. 24, 4019–4031 (2004).
Miller, K. M., Rog, O. & Cooper, J. P. Semi-conservative DNA replication through telomeres requires Taz1. Nature 440, 824–828 (2006). Before this study it was assumed that telomere-binding proteins impede replication fork progression. Conversely, this study shows that Taz1 is crucial for efficient replication fork progression through the telomere.
Zahler, A. M. & Prescott, D. M. DNA primase and the replication of the telomeres in Oxytricha nova. Nucleic Acids Res. 17, 6299–6317 (1989).
Ray, S., Karamysheva, Z., Wang, L., Shippen, D. E. & Price, C. M. Interactions between telomerase and primase physically link the telomere and chromosome replication machinery. Mol. Cell. Biol. 22, 5859–5868 (2002). A physical association of telomerase and primase is shown in Euplotes crassus , supporting the proposed coordinated regulation of telomeric G- and C-strand synthesis.
Dahlen, M., Sunnerhagen, P. & Wang, T. S. Replication proteins influence the maintenance of telomere length and telomerase protein stability. Mol. Cell. Biol. 23, 3031–3042 (2003).
Stevenson, J. B. & Gottschling, D. E. Telomeric chromatin modulates replication timing near chromosome ends. Genes Dev. 13, 146–151 (1999).
Poloumienko, A., Dershowitz, A., De, J. & Newlon, C. S. Completion of replication map of Saccharomyces cerevisiae chromosome III. Mol. Biol. Cell 12, 3317–3327 (2001).
Deng, Z. et al. Telomeric proteins regulate episomal maintenance of Epstein-Barr virus origin of plasmid replication. Mol. Cell 9, 493–503 (2002).
Amiard, S. et al. A topological mechanism for TRF2-enhanced strand invasion. Nature Struct. Mol. Biol. 14, 147–154 (2007).
Verdun, R. E. & Karlseder, J. The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell 127, 709–720 (2006). Shows that telomere ends need to be recognized as damaged DNA in order for end replication to be completed and for a telomere-specific structure to be formed at chromosome ends after replication.
Raghuraman, M. K. et al. Replication dynamics of the yeast genome. Science 294, 115–121 (2001).
Gottschling, D. E., Aparicio, O. M., Billington, B. L. & Zakian, V. A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63, 751–762 (1990).
Ferguson, B. M. & Fangman, W. L. A position effect on the time of replication origin activation in yeast. Cell 68, 333–339 (1992).
Zappulla, D. C., Sternglanz, R. & Leatherwood, J. Control of replication timing by a transcriptional silencer. Curr. Biol. 12, 869–875 (2002).
Pryde, F. E. & Louis, E. J. Limitations of silencing at native yeast telomeres. EMBO J. 18, 2538–2550 (1999).
Wyrick, J. J. et al. Chromosomal landscape of nucleosome-dependent gene expression and silencing in yeast. Nature 402, 418–421 (1999).
Cosgrove, A. J., Nieduszynski, C. A. & Donaldson, A. D. Ku complex controls the replication time of DNA in telomere regions. Genes Dev. 16, 2485–2490 (2002).
Hiraga, S., Robertson, E. D. & Donaldson, A. D. The Ctf18 RFC-like complex positions yeast telomeres but does not specify their replication time. EMBO J. 25, 1505–1514 (2006).
Raghuraman, M. K., Brewer, B. J. & Fangman, W. L. Cell cycle-dependent establishment of a late replication program. Science 276, 806–809 (1997).
Wang, Y., Vujcic, M. & Kowalski, D. DNA replication forks pause at silent origins near the HML locus in budding yeast. Mol. Cell. Biol. 21, 4938–4948 (2001).
Zou, Y., Gryaznov, S. M., Shay, J. W., Wright, W. E. & Cornforth, M. N. Asynchronous replication timing of telomeres at opposite arms of mammalian chromosomes. Proc. Natl Acad. Sci. USA 101, 12928–12933 (2004).
Tan, M., Jahn, C. L. & Price, C. M. Origin usage during Euplotes ribosomal DNA amplification. Eukaryot. Cell 2, 115–122 (2003).
Wright, W. E., Tesmer, V. M., Liao, M. L. & Shay, J. W. Normal human telomeres are not late replicating. Exp. Cell Res. 251, 492–499 (1999).
Hultdin, M. et al. Replication timing of human telomeric DNA and other repetitive sequences analyzed by fluorescence in situ hybridization and flow cytometry. Exp. Cell Res. 271, 223–229 (2001).
Ofir, R., Wong, A. C., McDermid, H. E., Skorecki, K. L. & Selig, S. Position effect of human telomeric repeats on replication timing. Proc. Natl Acad. Sci. USA 96, 11434–11439 (1999).
Marcand, S., Brevet, V. & Gilson, E. Progressive cis-inhibition of telomerase upon telomere elongation. EMBO J. 18, 3509–3519 (1999).
Teixeira, M. T., Arneric, M., Sperisen, P. & Lingner, J. Telomere length homeostasis is achieved via a switch between telomerase-extendible and -nonextendible states. Cell 117, 323–335 (2004). Shows that telomerase does not act on every telomere in each cell cycle and that it exhibits a preference for short telomeres.
Bianchi, A. & Shore, D. Early replication of short telomeres in budding yeast. Cell 128, 1051–1062 (2007).
Shirahige, K. et al. Regulation of DNA-replication origins during cell-cycle progression. Nature 395, 618–621 (1998).
Santocanale, C. & Diffley, J. F. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 395, 615–618 (1998).
Feng, W. et al. Genomic mapping of single-stranded DNA in hydroxyurea-challenged yeasts identifies origins of replication. Nature Cell Biol. 8, 148–155 (2006).
Longhese, M. P., Paciotti, V., Neecke, H. & Lucchini, G. Checkpoint proteins influence telomeric silencing and length maintenance in budding yeast. Genetics 155, 1577–1591 (2000).
Crabbe, L., Verdun, R. E., Haggblom, C. I. & Karlseder, J. Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science 306, 1951–1953 (2004). Reports that cells that lack WRN show deletion of telomeres that were replicated by lagging-strand synthesis, suggesting that WRN is necessary for the efficient replication of G-rich telomeric DNA.
Bai, Y. & Murnane, J. P. Telomere instability in a human tumor cell line expressing a dominant-negative WRN protein. Hum. Genet. 113, 337–347 (2003).
Shen, J. & Loeb, L. A. Unwinding the molecular basis of the Werner syndrome. Mech. Ageing Dev. 122, 921–944 (2001).
Du, X. et al. Telomere shortening exposes functions for the mouse Werner and Bloom syndrome genes. Mol. Cell. Biol. 24, 8437–8446 (2004).
Chang, S. et al. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nature Genet. 36, 877–882 (2004).
Bankhead, T., Kobryn, K. & Chaconas, G. Unexpected twist: harnessing the energy in positive supercoils to control telomere resolution. Mol. Microbiol. 62, 895–905 (2006).
Ivessa, A. S. & Zakian, V. A. To fire or not to fire: origin activation in Saccharomyces cerevisiae ribosomal DNA. Genes Dev. 16, 2459–2464 (2002).
Azvolinsky, A., Dunaway, S., Torres, J. Z., Bessler, J. B. & Zakian, V. A. The S. cerevisiae Rrm3p DNA helicase moves with the replication fork and affects replication of all yeast chromosomes. Genes Dev. 20, 3104–3116 (2006).
Schmidt, K. H. & Kolodner, R. D. Suppression of spontaneous genome rearrangements in yeast DNA helicase mutants. Proc. Natl Acad. Sci. USA 103, 18196–18201 (2006).
Opresko, P. L. et al. The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol. Cell 14, 763–74 (2004).
Makarov, V. L., Hirose, Y. & Langmore, J. P. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell 88, 657–666 (1997).
Dionne, I. & Wellinger, R. J. Cell cycle-regulated generation of single-stranded G-rich DNA in the absence of telomerase. Proc. Natl Acad. Sci. USA 93, 13902–13907 (1996).
Klobutcher, L. A., Swanton, M. T., Donini, P. & Prescott, D. M. All gene-sized DNA molecules in four species of hypotrichs have the same terminal sequence and an unusual 3′ terminus. Proc. Natl Acad. Sci. USA 78, 3015–3019 (1981).
Larrivee, M., LeBel, C. & Wellinger, R. J. The generation of proper constitutive G-tails on yeast telomeres is dependent on the MRX complex. Genes Dev. 18, 1391–1396 (2004). Demonstrates that G-tails are present outside S phase on normal yeast telomeres, and that Mre11 is essential to form this constitutive end structure.
Chai, W., Du, Q., Shay, J. W. & Wright, W. E. Human telomeres have different overhang sizes at leading versus lagging strands. Mol. Cell 21, 427–435 (2006). Shows that human diploid cells have longer G overhangs at telomeres generated by lagging-strand synthesis than by leading-strand synthesis, which suggests that leading and lagging daughter telomeres are generated differently.
Dionne, I. & Wellinger, R. J. Processing of telomeric DNA ends requires the passage of a replication fork. Nucleic Acids Res. 26, 5365–5371 (1998).
Hemann, M. T. & Greider, C. W. G-strand overhangs on telomeres in telomerase-deficient mouse cells. Nucleic Acids Res. 27, 3964–3969 (1999).
Ohki, R., Tsurimoto, T. & Ishikawa, F. In vitro reconstitution of the end replication problem. Mol. Cell. Biol. 21, 5753–5766 (2001).
Ira, G. et al. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431, 1011–1017 (2004).
Frank, C. J., Hyde, M. & Greider, C. W. Regulation of telomere elongation by the cyclin-dependent kinase CDK1. Mol. Cell 24, 423–432 (2006).
Vodenicharov, M. D. & Wellinger, R. J. DNA degradation at unprotected telomeres in yeast is regulated by the CDK1 (Cdc28/Clb) cell-cycle kinase. Mol. Cell 24, 127–137 (2006). References 61 and 62 report evidence that cyclin-dependent kinase Cdk1/Cdc28 activity is required for the generation of 3′ single-strand overhangs at telomeres in S. cerevisiae.
Negrini, S., Ribaud, V., Bianchi, A. & Shore, D. DNA breaks are masked by multiple Rap1 binding in yeast: implications for telomere capping and telomerase regulation. Genes Dev. 21, 292–302 (2007).
Takata, H., Tanaka, Y. & Matsuura, A. Late S phase-specific recruitment of Mre11 complex triggers hierarchical assembly of telomere replication proteins in Saccharomyces cerevisiae. Mol. Cell 17, 573–583 (2005).
van Overbeek, M. & de Lange, T. Apollo, an Artemis-related nuclease, interacts with TRF2 and protects human telomeres in S phase. Curr. Biol. 16, 1295–1302 (2006).
Lenain, C. et al. The Apollo 5′ exonuclease functions together with TRF2 to protect telomeres from DNA repair. Curr. Biol. 16, 1303–1310 (2006).
Parenteau, J. & Wellinger, R. J. Accumulation of single-stranded DNA and destabilization of telomeric repeats in yeast mutant strains carrying a deletion of RAD27. Mol. Cell. Biol. 19, 4143–4152 (1999).
Adams Martin, A., Dionne, I., Wellinger, R. J. & Holm, C. The function of DNA polymerase α at telomeric G tails is important for telomere homeostasis. Mol. Cell. Biol. 20, 786–796 (2000).
Tomita, K. et al. Fission yeast Dna2 is required for generation of the telomeric single-strand overhang. Mol. Cell. Biol. 24, 9557–9567 (2004).
Hubscher, U., Maga, G. & Spadari, S. Eukaryotic DNA polymerases. Annu. Rev. Biochem. 71, 133–163 (2002).
Qi, H. & Zakian, V. A. The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase α and the telomerase-associated est1 protein. Genes Dev. 14, 1777–88 (2000).
Grossi, S., Puglisi, A., Dmitriev, P. V., Lopes, M. & Shore, D. Pol12, the B subunit of DNA polymerase α, functions in both telomere capping and length regulation. Genes Dev. 18, 992–1006 (2004).
Carson, M. J. & Hartwell, L. CDC17: an essential gene that prevents telomere elongation in yeast. Cell 42, 249–257 (1985).
Wellinger, R. J., Wolf, A. J. & Zakian, V. A. Saccharomyces telomeres acquire single-strand TG1–3 tails late in S phase. Cell 72, 51–60 (1993).
Bertuch, A. A. & Lundblad, V. The Ku heterodimer performs separable activities at double-strand breaks and chromosome termini. Mol. Cell. Biol. 23, 8202–8215 (2003).
Churikov, D., Wei, C. & Price, C. M. Vertebrate POT1 restricts G-overhang length and prevents activation of a telomeric DNA damage checkpoint but is dispensable for overhang protection. Mol. Cell. Biol. 26, 6971–6982 (2006).
Baumann, P. Are mouse telomeres going to pot? Cell 126, 33–36 (2006).
Zhu, X. D. et al. ERCC1/XPF removes the 3′ overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Mol. Cell 12, 1489–1498 (2003).
Celli, G. B. & de Lange, T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nature Cell Biol. 7, 712–718 (2005).
Rossi, M. L., Purohit, V., Brandt, P. D. & Bambara, R. A. Lagging strand replication proteins in genome stability and DNA repair. Chem. Rev. 106, 453–473 (2006).
Budd, M. E., Reis, C. C., Smith, S., Myung, K. & Campbell, J. L. Evidence suggesting that Pif1 helicase functions in DNA replication with the Dna2 helicase/nuclease and DNA polymerase δ. Mol. Cell. Biol. 26, 2490–2500 (2006).
Qiu, J., Qian, Y., Frank, P., Wintersberger, U. & Shen, B. Saccharomyces cerevisiae RNase H(35) functions in RNA primer removal during lagging-strand DNA synthesis, most efficiently in cooperation with Rad27 nuclease. Mol. Cell. Biol. 19, 8361–8371 (1999).
Jeong, H. S., Backlund, P. S., Chen, H. C., Karavanov, A. A. & Crouch, R. J. RNase H2 of Saccharomyces cerevisiae is a complex of three proteins. Nucleic Acids Res. 32, 407–414 (2004).
Reveal, P. M., Henkels, K. M. & Turchi, J. J. Synthesis of the mammalian telomere lagging strand in vitro. J. Biol. Chem. 272, 11678–11681 (1997).
Fan, X. & Price, C. M. Coordinate regulation of G- and C strand length during new telomere synthesis. Mol. Biol. Cell 8, 2145–2155 (1997).
Jacob, N. K., Kirk, K. E. & Price, C. M. Generation of telomeric G strand overhangs involves both G and C strand cleavage. Mol. Cell 11, 1021–1032 (2003).
Sfeir, A. J., Chai, W., Shay, J. W. & Wright, W. E. Telomere-end processing the terminal nucleotides of human chromosomes. Mol. Cell 18, 131–138 (2005).
Hockemeyer, D., Sfeir, A. J., Shay, J. W., Wright, W. E. & de Lange, T. POT1 protects telomeres from a transient DNA damage response and determines how human chromosomes end. EMBO J. 24, 2667–2678 (2005).
Stansel, R. M., de Lange, T. & Griffith, J. D. T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J. 20, 5532–5540 (2001).
Fouche, N. et al. The basic domain of TRF2 directs binding to DNA junctions irrespective of the presence of TTAGGG repeats. J. Biol. Chem. 281, 37486–37495 (2006).
Bailey, S. M., Cornforth, M. N., Kurimasa, A., Chen, D. J. & Goodwin, E. H. Strand-specific postreplicative processing of mammalian telomeres. Science 293, 2462–2465 (2001).
Wang, R. C., Smogorzewska, A. & de Lange, T. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 119, 355–368 (2004).
Gotta, M. et al. The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. J. Cell Biol. 134, 1349–1363 (1996).
Heun, P., Laroche, T., Shimada, K., Furrer, P. & Gasser, S. M. Chromosome dynamics in the yeast interphase nucleus. Science 294, 2181–2186 (2001).
Marcand, S., Brevet, V., Mann, C. & Gilson, E. Cell cycle restriction of telomere elongation. Curr. Biol. 10, 487–490 (2000). Shows that in budding yeast cells that progress synchronously through the cell cycle, telomere elongation coincides with the time of telomere replication.
Diede, S. J. & Gottschling, D. E. Telomerase-mediated telomere addition in vivo requires DNA primase and DNA polymerases α and δ. Cell 99, 723–733 (1999). Shows that the essential DNA polymerase-α and -δ and DNA primase are required for telomerase function, indicating that telomeric DNA synthesis by telomerase is tightly coregulated with the production of the opposite strand.
Taggart, A. K., Teng, S. C. & Zakian, V. A. Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science 297, 1023–1026 (2002). This study correlates the timing of telomere elongation in budding yeast with the binding at the telomeres of several proteins that are involved in telomere elongation, including the telomerase holoenzyme.
Schramke, V. et al. RPA regulates telomerase action by providing Est1p access to chromosome ends. Nature Genet. 36, 46–54 (2004). Shows that in budding yeast, RPA binds to telomeres at the end of S phase and is required for telomerase action.
Bianchi, A., Negrini, S. & Shore, D. Delivery of yeast telomerase to a DNA break depends on the recruitment functions of Cdc13 and Est1. Mol. Cell 16, 139–146 (2004).
Osterhage, J. L., Talley, J. M. & Friedman, K. L. Proteasome-dependent degradation of Est1p regulates the cell cycle-restricted assembly of telomerase in Saccharomyces cerevisiae. Nature Struct. Mol. Biol. 13, 720–728 (2006).
Goudsouzian, L. K., Tuzon, C. T. & Zakian, V. A. S. cerevisiae Tel1p and Mre11p are required for normal levels of Est1p and Est2p telomere association. Mol. Cell 24, 603–610 (2006).
Stellwagen, A. E., Haimberger, Z. W., Veatch, J. R. & Gottschling, D. E. Ku interacts with telomerase RNA to promote telomere addition at native and broken chromosome ends. Genes Dev. 17, 2384–2395 (2003).
Fisher, T. S., Taggart, A. K. & Zakian, V. A. Cell cycle-dependent regulation of yeast telomerase by Ku. Nature Struct. Mol. Biol. 11, 1198–1205 (2004).
Evans, S. K. & Lundblad, V. Est1 and Cdc13 as comediators of telomerase access. Science 286, 117–120 (1999).
Nugent, C. I., Hughes, T. R., Lue, N. F. & Lundblad, V. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science 274, 249–252 (1996).
Pennock, E., Buckley, K. & Lundblad, V. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell 104, 387–396 (2001).
Grandin, N., Damon, C. & Charbonneau, M. Cdc13 cooperates with the yeast Ku proteins and Stn1 to regulate telomerase recruitment. Mol. Cell. Biol. 20, 8397–8408 (2000).
Seto, A. G., Zaug, A. J., Sobel, S. G., Wolin, S. L. & Cech, T. R. Saccharomyces cerevisiae telomerase is an Sm small nuclear ribonucleoprotein particle. Nature 401, 177–180 (1999).
Seto, A. G., Livengood, A. J., Tzfati, Y., Blackburn, E. H. & Cech, T. R. A bulged stem tethers Est1p to telomerase RNA in budding yeast. Genes Dev. 16, 2800–2812 (2002).
Peterson, S. E. et al. The function of a stem-loop in telomerase RNA is linked to the DNA repair protein Ku. Nature Genet. 27, 64–67 (2001).
Dandjinou, A. T. et al. A phylogenetically based secondary structure for the yeast telomerase RNA. Curr. Biol. 14, 1148–1158 (2004).
Zappulla, D. C. & Cech, T. R. Yeast telomerase RNA: a flexible scaffold for protein subunits. Proc. Natl Acad. Sci. USA 101, 10024–10029 (2004). Based on the interactions of yeast telomerase RNA TLC1 with Est1, Ku and Sm proteins, this study proposes that TLC1 provides a flexible tether for these proteins.
Zappulla, D. C., Goodrich, K. & Cech, T. R. A miniature yeast telomerase RNA functions in vivo and reconstitutes activity in vitro. Nature Struct. Mol. Biol. 12, 1072–1077 (2005).
Gao, H., Cervantes, R. B., Mandell, E. K., Otero, J. H. & Lundblad, V. RPA-like proteins mediate yeast telomere function. Nature Struct. Mol. Biol. 14, 208–214 (2007).
Grossi, S., Bianchi, A., Damay, P. & Shore, D. Telomere formation by rap1p binding site arrays reveals end-specific length regulation requirements and active telomeric recombination. Mol. Cell. Biol. 21, 8117–8128 (2001).
Boule, J. B., Vega, L. R. & Zakian, V. A. The yeast Pif1p helicase removes telomerase from telomeric DNA. Nature 438, 57–61 (2005). Suggests that Pif1 RNA/DNA helicase activity limits telomerase action by displacing active telomerase from DNA ends.
Eugster, A. et al. The finger subdomain of yeast telomerase cooperates with Pif1p to limit telomere elongation. Nature Struct. Mol. Biol. 13, 734–739 (2006).
Marcand, S., Gilson, E. & Shore, D. A protein-counting mechanism for telomere length regulation in yeast. Science 275, 986–990 (1997).
Naito, T., Matsuura, A. & Ishikawa, F. Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nature Genet. 20, 203–206 (1998).
Chan, S. W., Chang, J., Prescott, J. & Blackburn, E. H. Altering telomere structure allows telomerase to act in yeast lacking ATM kinases. Curr. Biol. 11, 1240–1250 (2001).
Greenwell, P. W. et al. TEL1, a gene involved in controlling telomere length in S. cerevisiae, is homologous to the human ataxia telangiectasia gene. Cell 82, 823–829 (1995).
Viscardi, V., Baroni, E., Romano, M., Lucchini, G. & Longhese, M. P. Sudden telomere lengthening triggers a Rad53-dependent checkpoint in Saccharomyces cerevisiae. Mol. Biol. Cell 14, 3126–3143 (2003).
Nakamura, T. M., Moser, B. A. & Russell, P. Telomere binding of checkpoint sensor and DNA repair proteins contributes to maintenance of functional fission yeast telomeres. Genetics 161, 1437–1452 (2002).
Sabourin, M., Tuzon, C.T. & Zakian, V.A. Telomerase and Tel1p preferentially associate with short telomeres in S. cerevisiae. Mol. Cell 27, 550–561 (2007).
Bianchi, A. & Shore, D. Increased association of telomerase with short telomeres in yeast. Genes Dev. 21, 1726–1730 (2007).
Hector, R.E. et al. Tel1p preferentially associates with short telomeres to stimulate their elongation. Mol. Cell (in the press).
Arneriç, M. & Lingner, J. Tel1p kinase and subtelomere bound Tbf1p mediate preferential elongation of short telomeres by telomerase in yeast. EMBO Rep. (in the press).
Chang, M., Arneric, M., & Lingner, J. Telomerase repeat addition processivity is increased at critically short telomeres in a Tel1-dependent manner in Saccharomyces cerevisiae. Genes Dev. (in the press).
Berthiau, A. S. et al. Subtelomeric proteins negatively regulate telomere elongation in budding yeast. EMBO J. 25, 846–856 (2006).
Hediger, F., Berthiau, A. S., van Houwe, G., Gilson, E. & Gasser, S. M. Subtelomeric factors antagonize telomere anchoring and Tel1-independent telomere length regulation. EMBO J. 25, 857–867 (2006).
Paeschke, K., Simonsson, T., Postberg, J., Rhodes, D. & Lipps, H. J. Telomere end-binding proteins control the formation of G-quadruplex DNA structures in vivo. Nature Struct. Mol. Biol. 12, 847–854 (2005).
Loayza, D., Parsons, H., Donigian, J., Hoke, K. & de Lange, T. DNA binding features of human POT1: a nonamer 5′-TAGGGTTAG-3′ minimal binding site, sequence specificity, and internal binding to multimeric sites. J. Biol. Chem. 279, 13241–13248 (2004).
Ye, J. Z. et al. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 18, 1649–1654 (2004).
Colgin, L. M., Baran, K., Baumann, P., Cech, T. R. & Reddel, R. R. Human POT1 facilitates telomere elongation by telomerase. Curr. Biol. 13, 942–946 (2003).
Armbruster, B. N. et al. Rescue of an hTERT mutant defective in telomere elongation by fusion with hPot1. Mol. Cell. Biol. 24, 3552–3561 (2004).
Kelleher, C., Kurth, I. & Lingner, J. Human protection of telomeres 1 (POT1) is a negative regulator of telomerase activity in vitro. Mol. Cell. Biol. 25, 808–818 (2005).
Lei, M., Zaug, A. J., Podell, E. R. & Cech, T. R. Switching human telomerase on and off with hPOT1 protein in vitro. J. Biol. Chem. 280, 20449–20456 (2005).
Kim, S. H. et al. TIN2 mediates functions of TRF2 at human telomeres. J. Biol. Chem. 279, 43799–43804 (2004).
Liu, D. et al. PTOP interacts with POT1 and regulates its localization to telomeres. Nature Cell Biol. 6, 673–680 (2004).
Ye, J. Z. et al. TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. J. Biol. Chem. 279, 47264–47271 (2004).
O'Connor, M. S., Safari, A., Xin, H., Liu, D. & Songyang, Z. A critical role for TPP1 and TIN2 interaction in high-order telomeric complex assembly. Proc. Natl Acad. Sci. USA 103, 11874–11879 (2006).
Houghtaling, B. R., Cuttonaro, L., Chang, W. & Smith, S. A dynamic molecular link between the telomere length regulator TRF1 and the chromosome end protector TRF2. Curr. Biol. 14, 1621–1631 (2004).
Murzin, A. G. OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J. 12, 861–867 (1993).
Wang, F. et al. The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature 445, 506–510 (2007).
Xin, H. et al. TPP1 is a homologue of ciliate TEBP-β and interacts with POT1 to recruit telomerase. Nature 445, 559–562 (2007). References 144 and 145 show that the human telomeric proteins TPP1 and POT1 form a complex that regulates telomerase access to the telomere and increases the processivity of the telomerase core enzyme.
Ancelin, K. et al. Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol. Cell. Biol. 22, 3474–3487 (2002).
Loayza, D. & De Lange, T. POT1 as a terminal transducer of TRF1 telomere length control. Nature 423, 1013–1018 (2003).
Gottschling, D. E. & Cech, T. R. Chromatin structure of the molecular ends of Oxytricha macronuclear DNA: phased nucleosomes and a telomeric complex. Cell 38, 501–510 (1984).
Wright, J. H., Gottschling, D. E. & Zakian, V. A. Saccharomyces telomeres assume a non-nucleosomal chromatin structure. Genes Dev. 6, 197–210 (1992).
Teixeira, M. T. & Gilson, E. Telomere maintenance, function and evolution: the yeast paradigm. Chromosome Res. 13, 535–548 (2005).
Blasco, M. A. The epigenetic regulation of mammalian telomeres. Nature Rev. Genet. 8, 299–309 (2007).
Baur, J. A., Zou, Y., Shay, J. W. & Wright, W. E. Telomere position effect in human cells. Science 292, 2075–2077 (2001).
Koering, C. E. et al. Human telomeric position effect is determined by chromosomal context and telomeric chromatin integrity. EMBO Rep. 3, 1055–1061 (2002).
Chong, L. et al. A human telomeric protein. Science 270, 1663–1667 (1995).
Broccoli, D., Smogorzewska, A., Chong, L. & de Lange, T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nature Genet. 17, 231–235 (1997).
Bilaud, T. et al. Telomeric localization of TRF2, a novel human telobox protein. Nature Genet. 17, 236–239 (1997).
Griffith, J. D. et al. Mammalian telomeres end in a large duplex loop. Cell 97, 503–514 (1999).
Opresko, P. L. et al. POT1 stimulates RecQ helicases WRN and BLM to unwind telomeric DNA substrates. J. Biol. Chem. 280, 32069–32080 (2005).
Zaug, A. J., Podell, E. R. & Cech, T. R. Human POT1 disrupts telomeric G-quadruplexes allowing telomerase extension in vitro. Proc. Natl Acad. Sci. USA 102, 10864–10869 (2005).
Griffith, J., Bianchi, A. & de Lange, T. TRF1 promotes parallel pairing of telomeric tracts in vitro. J. Mol. Biol. 278, 79–88 (1998).
Bae, N. S. & Baumann, P. A RAP1/TRF2 complex inhibits nonhomologous end-joining at human telomeric DNA ends. Mol. Cell 26, 323–334 (2007).
Bryan, T. M., Englezou, A., Dalla-Pozza, L., Dunham, M. A. & Reddel, R. R. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nature Med. 3, 1271–1274 (1997).
Marciniak, R. A. et al. A novel telomere structure in a human alternative lengthening of telomeres cell line. Cancer Res. 65, 2730–2737 (2005).
McEachern, M. J. & Haber, J. E. Break-induced replication and recombinational telomere elongation in yeast. Annu. Rev. Biochem. 75, 111–135 (2006).
Biessmann, H. & Mason, J. M. Telomere maintenance without telomerase. Chromosoma 106, 63–69 (1997).
Pardue, M. L. et al. Two retrotransposons maintain telomeres in Drosophila. Chromosome Res. 13, 443–453 (2005).
Morrish, T. A. et al. Endonuclease-independent LINE-1 retrotransposition at mammalian telomeres. Nature 446, 208–212 (2007).
Salas, T. R. et al. Human replication protein A unfolds telomeric G-quadruplexes. Nucleic Acids Res. 34, 4857–4865 (2006).
Muftuoglu, M. et al. Telomere repeat binding factor 2 interacts with base excision repair proteins and stimulates DNA synthesis by DNA polymerase β. Cancer Res. 66, 113–124 (2006).
Acknowledgements
We would like to thank M.-J. Giraud-Panis, T. Teixeira, A. Londono-Vallejo and P. Luciano for critical reading and helpful discussions. The E.G. and V.G. laboratories are supported by 'La Ligue Nationale contre le Cancer' ('Equipes labellisées'). We apologize for all the important papers that could not be cited due to space limitations.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Cellular senescence
-
A permanent form of cell-cycle arrest that can be induced by different types of exogenous or endogenous stress. Replicative senescence is triggered by an excessive telomere shortening that is the consequence of multiple rounds of cell division and is considered to be an intrinsic mechanism for limiting the proliferative lifespan of normal somatic cells.
- Reverse transcriptase
-
An enzyme that copies single-stranded RNA into single-stranded DNA.
- Replisome
-
A multiprotein complex at the junction of the DNA replication fork that contains all the enzymes that are required for DNA replication.
- Primase
-
The enzyme that synthesizes an RNA primer for initiation of DNA replication. Primase is associated with DNA polymerase-α to form a four-subunit complex. The polymerase-α–primase complex functions in the initiation of DNA replication at chromosomal origins and in the discontinuous synthesis of Okazaki fragments on the lagging strand of the replication fork.
- OB fold
-
An N-terminal oligonucleotide/oligosaccharide binding (OB) motif. The five-stranded β-sheet forms a closed β-barrel, which is capped by an α-helix located between the third and fourth strands. The OB fold is frequently used for the specific recognition of single-stranded nucleic acids.
- Origin recognition complex
-
A heteromeric six-subunit protein complex that binds to DNA at replication origin sites and functions as a scaffold for the assembly of pre-replicative complexes in the G1 phase of the cell cycle.
- D-loop
-
The displacement loop structure that results from the displacement of a duplex DNA by a homologous single-stranded DNA.
- Position effect
-
The influence of the chromosomal context on various DNA transactions, including transcription, replication and recombination. It often refers to the repression that is conferred by heterochromatin proximity.
- Sir proteins
-
The silent information regulators (Sir)-2, -3 and -4 are the structural constituents of a particular type of silent chromatin in budding yeast. At telomeres, Sir3 and Sir4 interact with the telomere-binding protein Rap1, can self-associate, and bind to deacetylated and demethylated N-terminal tails of histones H3 and H4 of subtelomeric nucleosomes. The deacetylase activity of Sir2 is required to spread the Sir complex along the chromatin toward the centromere.
- t-loop
-
A structure adopted by telomeres that may result from invasion of the 3′ overhang into duplex DNA.
- G quadruplex
-
A four-stranded structure that is held together by square planes of four guanines ('G-quartets'), associated through Hoogsteen base pairing. Once such structures form they are extremely stable and are likely to need enzymatic activity to be unwound in vivo.
- RecQ helicase
-
One of a family of evolutionarily conserved helicases, mutations of which can lead to hereditary cancer-predisposition syndromes in humans. Helicases use the energy of ATP hydrolysis to unwind duplex DNA.
- DNA topoisomerase
-
An enzyme that changes DNA supercoiling by inserting or removing superhelical twists.
Rights and permissions
About this article
Cite this article
Gilson, E., Géli, V. How telomeres are replicated. Nat Rev Mol Cell Biol 8, 825–838 (2007). https://doi.org/10.1038/nrm2259
Issue Date:
DOI: https://doi.org/10.1038/nrm2259
This article is cited by
-
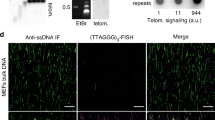
PARP1 allows proper telomere replication through TRF1 poly (ADP-ribosyl)ation and helicase recruitment
Communications Biology (2023)
-
Non-canonical telomere protection role of FOXO3a of human skeletal muscle cells regulated by the TRF2-redox axis
Communications Biology (2023)
-
Break-induced replication orchestrates resection-dependent template switching
Nature (2023)
-
Genetics of human telomere biology disorders
Nature Reviews Genetics (2023)
-
Telomere DNA length regulation is influenced by seasonal temperature differences in short-lived but not in long-lived reef-building corals
Nature Communications (2023)