Key Points
-
Structural maintenance of chromosomes (SMC) proteins are highly conserved ATPases that have fundamental roles in higher-order chromosome organization and dynamics in organisms from bacteria to humans.
-
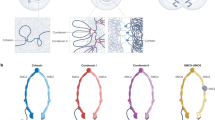
SMC dimers adopt a two-armed structure in which a central hinge domain connects long coiled-coil arms, each having an ATP-binding head domain at its distal end. The head domain of SMC proteins is structurally related to the ATP-binding cassette (ABC) domain of ABC transporters.
-
ATP binding and hydrolysis modulate the engagement and the disengagement of the head domains, respectively, and have an important role in regulating the dynamic interactions between SMC proteins and DNA.
-
The hinge domain is important for modulating the mechanochemical cycle of SMC proteins, implicating long-distance communication between the hinge and the head domains.
-
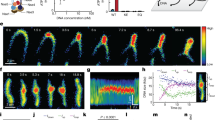
Condensin I has the capacity to introduce positive superhelical tension into DNA in an ATP-hydrolysis-dependent manner, possibly by organizing positive gyres or loops. A single-DNA-molecule assay reveals dynamic and reversible compaction of DNA supported by condensin I.
-
Cohesin might hold sister chromatids together by embracing two DNA duplexes within its ring-like structure, which is composed of SMC arms and a non-SMC 'kleisin' subunit.
-
SMC proteins might use a diverse array of intramolecular and intermolecular protein–protein interactions to tether, fold and manipulate the genome in an ATP-dependent manner.
Abstract
Structural maintenance of chromosomes (SMC) proteins are ubiquitous in organisms from bacteria to humans, and function as core components of the condensin and cohesin complexes in eukaryotes. SMC proteins adopt a V-shaped structure with two long arms, each of which has an ATP-binding head domain at the distal end. It is important to understand how these uniquely designed protein machines interact with DNA strands and how such interactions are modulated by the ATP-binding and -hydrolysis cycle. An emerging idea is that SMC proteins use a diverse array of intramolecular and intermolecular protein–protein interactions to actively fold, tether and manipulate DNA strands.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Losada, A. & Hirano, T. Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev. 19, 1269–1287 (2005).
Nasmyth, K. & Haering, C. H. The structure and function of SMC and kleisin complexes. Annu. Rev. Biochem. 74, 595–648 (2005).
Peterson, C. L. The SMC family: novel motor proteins for chromosome condensation? Cell 79, 389–392 (1994).
Hirano, T. Condensins: organizing and segregating the genome. Curr. Biol. 15, R265–R275 (2005).
Haering, C. H. & Nasmyth, K. Building and breaking bridges between sister chromatids. BioEssays 25, 1178–1191 (2003).
Lehmann, A. R. The role of SMC proteins in the responses to DNA damage. DNA Repair (Amst.) 4, 309–314 (2005).
Thanbichler, M., Wang, S. C. & Shapiro, L. The bacterial nucleoid: A highly organized and dynamic structure. J. Cell. Biochem. 96, 506–521 (2005).
Saitoh, N., Goldberg, I. G., Wood, E. R. & Earnshaw, W. C. ScII: an abundant chromosome scaffold protein is a member of a family of putative ATPases with an unusual predicted tertiary structure. J. Cell Biol. 127, 303–318 (1994). This study proposes, for the first time, that SMC proteins might be folded by antiparallel coiled-coil interactions, forming an ABC-like ATP-binding pocket at their ends.
Melby, T. E. G., Ciampaglio, C. N., Briscoe, G. & Erickson, H. P. The symmetrical structure of structural maintenance of chromosomes (SMC) and MukB proteins: long, antiparallel coiled coils, folded at a flexible hinge. J. Cell Biol. 142, 1595–1604 (1998). Electron microscopy shows that SMC proteins form a V-shaped dimer with two antiparallel coiled-coil arms.
Haering, C. H., Lowe, J., Hochwagen, A. & Nasmyth, K. Molecular architecture of SMC proteins and the yeast cohesin complex. Mol. Cell 9, 773–788 (2002). Biochemical and structural analyses reveal a tripartite ring-like structure of Smc1–Smc3–Scc1, providing a molecular basis for the ring model of sister-chromatid cohesion.
Hirano, M. & Hirano, T. Hinge-mediated dimerization of SMC protein is essential for its dynamic interaction with DNA. EMBO J. 21, 5733–5744 (2002).
Matoba, K., Yamazoe, M., Mayanagi, K., Morikawa, K. & Hiraga, S. Comparison of MukB homodimer versus MukBEF complex molecular architectures by electron microscopy reveals a higher-order multimerization. Biochem. Biophys. Res. Commun. 333, 694–702 (2005).
Mascarenhas, J. et al. Dynamic assembly, localization and proteolysis of the Bacillus subtilis SMC complex. BMC Cell Biol. 6, 28 (2005).
Anderson, D. E., Losada, A., Erickson, H. P. & Hirano, T. Condensin and cohesin display different arm conformations with characteristic hinge angles. J. Cell Biol. 156, 419–424 (2002).
Yoshimura, S. H. et al. Condensin architecture and interaction with DNA: regulatory non-SMC subunits bind to the head of SMC heterodimer. Curr. Biol. 12, 508–513 (2002).
Hirano, M. & Hirano, T. Positive and negative regulation of SMC–DNA interactions by ATP and accessory proteins. EMBO J. 23, 2664–2673 (2004).
Dervyn, E. et al. The bacterial condensin/cohesin-like protein complex acts in DNA repair and regulation of gene expression. Mol. Microbiol. 51, 1629–1640 (2004).
Yamazoe, M. et al. Complex formation of MukB, MukE and MukF proteins involved in chromosome partitioning in Escherichia coli. EMBO J. 18, 5873–5884 (1999).
Haering, C. H. et al. Structure and stability of cohesin's Smc1–kleisin interaction. Mol. Cell 15, 951–964 (2004).
Lammens, A., Schele, A. & Hopfner, K.-P. Structural biochemistry of ATP-driven dimerization and DNA-stimulated activation of SMC ATPases. Curr. Biol. 14, 1778–1782 (2004).
Hopfner, K.-P. et al. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101, 789–800 (2000). Crystallization of the Rad50 head domain provides the first compelling evidence for the dimerization of two ABC domains in a nucleotide-sandwich manner.
Smith, P. C. et al. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol. Cell 10, 139–149 (2002).
Locher, K. P., Lee, A. T. & Rees, D. C. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science 296, 1091–1098 (2002).
Hirano, M., Anderson, D. E., Erickson, H. P. & Hirano, T. Bimodal activation of SMC ATPase by intra- and inter-molecular interactions. EMBO J. 20, 3238–3250 (2001).
Hopfner, K.-P. et al. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature 418, 562–566 (2002).
Chiu, A., Revenkova, E. & Jessberger, R. DNA interaction and dimerization of eukaryotic SMC hinge domains. J. Biol. Chem. 279, 26233–26242 (2004).
Sergeant, J. et al. Composition and architecture of the Schizosaccharomyces pombe Rad18 (Smc5–6) complex. Mol. Cell. Biol. 25, 172–184 (2005).
Hirano, M. & Hirano, T. Opening closed arms: long-distance activation of SMC ATPase by hinge–DNA interaction. Mol. Cell 21, 175–186 (2006). Comprehensive mutational analyses of BsSMC demonstrate that the hinge domain has a crucial role in modulating the mechanochemical cycle of SMC proteins.
Hirano, M. & Hirano, T. ATP-dependent aggregation of single-stranded DNA by a bacterial SMC homodimer. EMBO J. 17, 7139–7148 (1998).
Chen, J., Sharma, S., Quiocho, F. A. & Davidson, A. L. Trapping the transition state of an ATP-binding cassette transporter: evidence for a concerted mechanism of maltose transport. Proc. Natl Acad. Sci. USA. 98, 1525–1530 (2001).
Moreno-Herrero, F. et al. Mesoscale conformational changes in the DNA-repair complex Rad50–Mre11–Nbs1 upon binding DNA. Nature 437, 440–443 (2005). Atomic force microscopy provides evidence that DNA binding by the Rad50 head domains might induce a conformational change in its coiled-coil domains, converting the apex–apex interaction from the intramolecular mode to the intermolecular mode.
Mascarenhas, J., Soppa, J., Strunnikov, A. V. & Graumann, P. L. Cell cycle-dependent localization of two novel prokaryotic chromosome segregation and condensation proteins in Bacillus subtilis that interact with SMC protein. EMBO J. 21, 3108–3118 (2002).
Soppa, J. et al. Discovery of two novel families of proteins that are proposed to interact with prokaryotic SMC proteins, and characterization of the Bacillus subtilis family members ScpA and ScpB. Mol. Microbiol. 45, 59–71 (2002).
Volkov, A., Mascarenhas, J., Andrei-Selmer, C., Ulrich, H. D. & Graumann, P. L. A prokaryotic condensin/cohesin-like complex can actively compact chromosomes from a single position on the nucleoid and binds to DNA as a ring-like structure. Mol. Cell. Biol. 23, 5638–5650 (2003).
Schleiffer, A. et al. Kleisins: a superfamily of bacterial and eukaryotic SMC protein partners. Mol. Cell 11, 571–575 (2003).
Fennell-Fezzie, R., Gradia, S. D., Akey, D. & Berger, J. M. The MukF subunit of Escherichia coli condensin: architecture and functional relationship to kleisins. EMBO J. 24, 1921–1930 (2005).
Kim, J.-S. et al. Crystal structure of ScpB from Chlorobium tepidum, a protein involved in chromosome partitioning. Protein 62, 322–328 (2006).
Hopfner, K.-P. & Tainer, J. A. Rad50/SMC proteins and ABC transporters: unifying concepts from high-resolution structures. Curr. Opin. Struct. Biol. 13, 249–255 (2003).
Chen, J., Lu, G., Lin, J., Davidson, A. L. & Quiocho, F. A. A tweezer-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol. Cell 12, 651–661 (2003).
Ono, T. et al. Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell 115, 109–121 (2003).
Stray, J. E. & Lindsley, J. E. Biochemical analysis of the yeast condensin Smc2/4 complex: an ATPase that promotes knotting of circular DNA. J. Biol. Chem. 278, 26238–26248 (2003).
Stray, J. E., Crisona, N. J., Belotserkovskii, B. P., Lindsley, J. E. & Cozzarelli, N. R. The Saccharomyces cerevisiase Smc2/4 condensin compacts DNA into (+) chiral structures without net supercoiling. J. Biol. Chem. 280, 34723–34734 (2005).
Sutani, T. & Yanagida, M. DNA renaturation activity of the SMC complex implicated in chromosome condensation. Nature 388, 798–801 (1997).
Sakai, A., Hizume, K., Sutani, T., Takeyasu, K. & Yanagida, M. Condensin but not cohesin SMC heterodimer induces DNA reannealing through protein–protein assembly. EMBO J. 22, 2764–2775 (2003).
Kimura, K. & Hirano, T. Dual roles of the 11S regulatory subcomplex in condensin functions. Proc. Natl Acad. Sci. USA. 97, 11972–11977 (2000).
Kimura, K. & Hirano, T. ATP-dependent positive supercoiling of DNA by 13S condensin: a biochemical implication for chromosome condensation. Cell 90, 625–634 (1997). This study shows that condensin I has the ability to introduce positive supercoils into closed circular DNA in the presence of ATP and topoisomerase I.
Kimura, K., Hirano, M., Kobayashi, R. & Hirano, T. Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science 282, 487–490 (1998).
Hagstrom, K. A., Holmes, V. F., Cozzarelli, N. R. & Meyer, B. J. C. elegans condensin promotes mitotic chromosome architecture, centromere organization, and sister chromatid segregation during mitosis and meiosis. Genes Dev. 16, 729–742 (2002).
Kimura, K., Rybenkov, V. V., Crisona, N. J., Hirano, T. & Cozzarelli, N. R. 13S condensin actively reconfigures DNA by introducing global positive writhe: implications for chromosome condensation. Cell 98, 239–248 (1999).
Bazett-Jones, D. P., Kimura, K. & Hirano, T. Efficient supercoiling of DNA by a single condensin complex as revealed by electron spectroscopic imaging. Mol. Cell 9, 1183–1190 (2002).
Strick, T. R., Kawaguchi, T. & Hirano, T. Real-time detection of single-molecule DNA compaction by condensin I. Curr. Biol. 14, 874–880 (2004). A DNA nanomanipulation assay demonstrates that condensin I supports dynamic and reversible compaction of a single DNA molecule in an ATP-hydrolysis-dependent manner.
Kireeva, N., Lakonishok, M., Kireev, I., Hirano, T. & Belmont, A. S. Visualization of early chromosome condensation: a hierarchical folding, axial glue model of chromosome structure. J. Cell Biol. 166, 775–785 (2004).
Maeshima, K. & Laemmli, U. K. A two-step scaffolding model for mitotic chromosome assembly. Dev. Cell 4, 467–480 (2003).
Hudson, D. F., Vagnarelli, P., Gassmann, R. & Earnshaw, W. C. Condensin is required for nonhistone protein assembly and structural integrity of vertebrate chromosomes. Dev. Cell 5, 323–336 (2003).
Csankovszki, G., McDonel, P. & Meyer, B. J. Recruitment and spreading of the C. elegans dosage compensation complex along X chromosomes. Science 303, 1182–1185 (2004).
Neuwald, A. F. & Hirano, T. HEAT repeats associated with condensins, cohesins and other chromosome-related complexes. Genome Res. 10, 1445–1452 (2000).
Groves, M. R. & Barford, D. Topological characteristics of helical repeat proteins. Curr. Opin. Struct. Biol. 9, 383–389 (1999).
Uhlmann, F., Lottspeich, F. & Nasmyth, K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400, 37–42 (1999).
Gruber, S., Haering, C. H. & Nasmyth, K. Chromosomal cohesin forms a ring. Cell 112, 765–777 (2003). Genetic engineering of cohesin subunits in vivo provides evidence for the ring model. Artificial cleavage of either Smc1 or Scc1 releases cohesin from chromosomes and promotes the separation of sister chromatids.
Ivanov, D. & Nasmyth, K. A topological interaction between cohesin rings and a circular minichromosome. Cell 122, 849–860 (2005).
Losada, A. & Hirano, T. Intermolecular DNA interactions stimulated by the cohesin complex in vitro: implications for sister chromatid cohesion. Curr. Biol. 11, 268–272 (2001).
Kagansky, A., Freeman, L., Lukyanov, D. & Strunnikov, A. Histone tail-independent chromatin binding activity of recombinant cohesin holocomplex. J. Biol. Chem. 279, 3382–3388 (2004).
Hirano, T. SMC-mediated chromosome mechanics: a conserved scheme from bacteria to vertebrates? Genes Dev. 13, 11–19 (1999).
Ciosk, R. et al. Cohesin's binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol. Cell 5, 243–254 (2000).
Weitzer, S., Lehane, C. & Uhlmann, F. A model for ATP hydrolysis-dependent binding of cohesin to DNA. Curr. Biol. 13, 1930–1940 (2003).
Arumugam, P. et al. ATP hydrolysis is required for cohesin's association with chromosomes. Curr. Biol. 13, 1941–1953 (2003).
Chang, C.-R., Wu, C.-S., Hom, Y. & Gartenberg, M. R. Targeting of cohesin by transcriptionally silent chromatin. Genes Dev. 19, 3031–3042 (2005).
Blat, Y. & Kleckner, N. Cohesins bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell 98, 249–259 (1999).
Laloraya, S., Guacci, V. & Koshland, D. Chromosomal addresses of the cohesin component Mcd1p. J. Cell Biol. 151, 1047–1056 (2000).
Tanaka, T., Cosma, M. P., Wirth, K. & Nasmyth, K. Identification of cohesin association sites at centromeres and along chromosome arms. Cell 98, 847–858 (1999).
Nonaka, N. et al. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol. 4, 89–93 (2002).
Huang, C. E., Milutinovich, M. & Koshland, D. Rings, bracelet or snaps: fashionable alternatives for Smc complexes. Phil. Trans. R. Soc. Lond. B. Biol. Sci. 360, 537–542 (2005).
Gillespie, P. J. & Hirano, T. Scc2 couples replication licensing to sister chromatid cohesion in Xenopus egg extracts. Curr. Biol. 14, 1598–1603 (2004).
Takahashi, T. S., Yiu, P., Chou, M. F., Gygi, S. & Walter, J. C. Recruitment of Xenopus Scc2 and cohesin to chromatin requires the pre-replication complex. Nature Cell Biol. 6, 991–996 (2004).
Uhlmann, F. & Nasmyth, K. Cohesion between sister chromatids must be established during DNA replication. Curr. Biol. 8, 1095–1101 (1998).
Lengronne, A. et al. Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature 430, 573–578 (2004).
Glynn, E. F. et al. Genome-wide mapping of the cohesin complex in the yeast Saccharomyces cerevisiae. PLoS Biol. 2, e259 (2004).
Kobayashi, T. & Ganley, A. R. Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats. Science 309, 1581–1584 (2005).
Rollins, R. A., Korom, M., Aulner, N., Martens, A. & Dorsett, D. Drosophila Nipped-B protein supports sister chromatid cohesion and opposes the stromalin/Scc3 cohesion factor to facilitate long-range activation of the cut gene. Mol. Cell. Biol. 24, 3100–3111 (2004).
Dorsett, D. et al. Effects of sister chromatid cohesion proteins on cut gene expression during wing development in Drosophila. Development 132, 4743–4753 (2005).
Unal, E. et al. DNA damage response pathway uses histone modification to assemble a double-strand break-specific cohesion domain. Mol. Cell 16, 991–1002 (2004).
Strom, L., Lindroos, H. B., Shirahige, K. & Sjogren, C. Postreplicative recruitment of cohesin to double-strand breaks is required for DNA repair. Mol. Cell 16, 1003–1015 (2004).
Acknowledgements
I thank the members of the Hirano laboratory for critically reading the manuscript. I am also grateful to many colleagues in the field for stimulating discussions. The work from the author's laboratory was supported by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Related links
Glossary
- Walker A and Walker B motifs
-
A pair of nucleotide-binding motifs that is commonly found in most, if not all, nucleotide-binding proteins.
- Coiled-coil motif
-
A rod-like structural motif found in many proteins that is formed by two long α-helices twisted around each other. Parallel arrangements of the two helices are much more common than antiparallel arrangements.
- Signature motif
-
(C motif). An amino-acid-sequence motif that is highly conserved among the ABC-ATPase superfamily, which includes ABC transporters, Rad50 and SMC proteins. This motif is not required for ATP binding but is essential for its hydrolysis.
- ABC transporters
-
A large family of transmembrane ATPases that mediate the active translocation of a diverse range of small molecules in and out of cells and organelles. The functional unit of an ABC transporter is composed of two transmembrane domains and a pair of ATP-binding cassette (ABC) domains.
- Rad50
-
The ATPase core subunit of the MRN (Mre11–Rad50–Nbs1) complex that has a crucial role in double-strand break repair. Rad50 shares many structural similarities with SMC proteins, including its ATP-binding head domains and its antiparallel coiled coils.
- Transition-state mutation
-
A specific point mutation in the Walker B motif that stabilizes the engagement of two ABC domains by suppressing the hydrolysis of ATP molecules that are sandwiched between them.
- Zinc-hook domain
-
A folding domain that is created at one end (apex) of the antiparallel coiled-coil arm of Rad50. A zinc ion bridges two hook domains and mediates their dimerization.
- Atomic force microscopy
-
A scanning-microscopy technique that allows imaging of the surface of a sample at atomic resolution by measuring repulsive forces between a probing tip and the sample. It is possible to collect a series of time-resolved images under aqueous and physiological conditions.
- Kleisins
-
A conserved family of proteins that directly interact with SMC protein dimers. Members of this family include the Scc1 subunit of cohesin, the CAP-H subunit of condensin and the ScpA subunit of the bacterial SMC complex.
- Type I topoisomerase
-
A type of DNA topoisomerase that changes the topology of DNA by nicking and rejoining one strand of the DNA double helix.
- Type II topoisomerase
-
A type of DNA topoisomerase that changes the topology of DNA by breaking and rejoining both strands of the DNA double helix.
- Electron spectroscopic imaging
-
An electron-microscopy technique that provides both structural and analytical information on the basis of energy losses of an electron beam. The mapping of phosphorus allows the visualization of the path of DNA within a nucleoprotein complex. The technique is also used to measure the mass of the complex and to determine the stoichiometric relationship of protein and DNA components within the complex.
- Magnetic tweezers
-
An experimental set-up that allows nanomanipulation of a single DNA molecule tethered to a paramagnetic bead. By measuring end-to-end extension of the DNA molecule to which a fixed force is applied, one can monitor the process of DNA compaction and decompaction in real time.
- HEAT repeat
-
A ∼30 amino-acid-repeat motif that is found in a number of proteins with diverse functions. It was named after four proteins in which the repeat was originally detected (huntingtin, elongation factor 3, the regulatory A subunit of protein phosphatase 2A and TOR1).
- Separase
-
A cysteine protease that promotes sister-chromatid separation at the onset of anaphase by cleaving the Scc1 subunit of cohesin.
- Prereplication complex
-
A protein complex that assembles at replication origins from late mitosis through to the G1 phase. The assembly of this complex is a prerequisite for the initiation of DNA replication in S phase.
Rights and permissions
About this article
Cite this article
Hirano, T. At the heart of the chromosome: SMC proteins in action. Nat Rev Mol Cell Biol 7, 311–322 (2006). https://doi.org/10.1038/nrm1909
Issue Date:
DOI: https://doi.org/10.1038/nrm1909
This article is cited by
-
NCAPG2 promotes prostate cancer malignancy and stemness via STAT3/c-MYC signaling
Journal of Translational Medicine (2024)
-
The SMC5/6 complex subunit MMS21 regulates stem cell proliferation in rice
Plant Cell Reports (2023)
-
Revealing biophysical properties of KfrA-type proteins as a novel class of cytoskeletal, coiled-coil plasmid-encoded proteins
BMC Microbiology (2021)
-
Aurora B kinase: a potential drug target for cancer therapy
Journal of Cancer Research and Clinical Oncology (2021)
-
Therapeutic role of recurrent ESR1-CCDC170 gene fusions in breast cancer endocrine resistance
Breast Cancer Research (2020)