Key Points
-
Processive DNA polymerases that replicate chromosomes interact with a ring-shaped clamp that encircles DNA and slides along the duplex.
-
All sliding clamps (Escherichia coli β-clamp, T4 bacteriophage gp45, archaeal and eukaryotic proliferating cell nuclear antigen (PCNA)) form similar planar ring structures with a central channel of sufficient width to encircle duplex DNA.
-
In all the clamps, the two faces of the ring are structurally distinct and polymerases and clamp loaders compete for binding to the same face. Therefore, the clamp loader must depart from the clamp for the DNA polymerase to function.
-
The sliding clamp is loaded onto DNA by a clamp-loader complex driven by ATP. Clamp loaders are circular heteropentameric complexes that have been conserved throughout evolution and their subunits are members of the AAA+ (ATPases associated with a variety of cellular activities) family of ATPases.
-
The ATP-binding sites of clamp loaders are located at subunit interfaces. Although clamp loaders are very similar in structure and function, the specifics of ATP use varies among different replication systems.
-
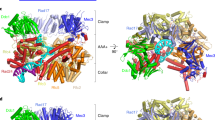
Insights into the way clamp loaders bind to DNA, recognize a primer–template junction, and open the clamp have been revealed by recent structural and biochemical studies. The findings of these studies indicate that the clamp assumes a right-handed spiral configuration when it opens, and it docks onto the helical surface of the clamp loader.
Abstract
Sliding clamps are ring-shaped proteins that tether DNA polymerases to DNA, which enables the rapid and processive synthesis of both leading and lagging strands at the replication fork. The clamp-loading machinery must repeatedly load sliding-clamp factors onto primed sites at the replication fork. Recent structural and biochemical analyses provide unique insights into how these clamp-loading ATPase machines function to load clamps onto the DNA. Moreover, these studies highlight the evolutionary conservation of the clamp-loading process in the three domains of life.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Johnson, A. & O'Donnell, M. Cellular DNA replicases: components and dynamics at the replication fork. Annu. Rev. Biochem. 74, 283–315 (2005).
Waga, S. & Stillman, B. The DNA replication fork in eukaryotic cells. Annu. Rev. Biochem. 67, 721–751 (1998).
Warbrick, E. The puzzle of PCNA's many partners. Bioessays 22, 997–1006 (2000).
Miyata, T. et al. Open clamp structure in the clamp-loading complex visualized by electron microscopic image analysis. Proc. Natl Acad. Sci. USA 102, 13795–13800 (2005). The EM structure of an archaeal RFC–PCNA–DNA complex in the presence of ATPγS shows the clamp in an open state. The open PCNA has a lock-washer conformation and fits onto the AAA+ surface of RFC. Density that probably corresponds to DNA is observed in the centre of PCNA and in the central chamber of RFC. The complex might represent an intermediate in which PCNA is kept open before ATP hydrolysis by RFC.
Bowman, G. D., O'Donnell, M. & Kuriyan, J. Structural analysis of a eukaryotic sliding DNA clamp–clamp loader complex. Nature 429, 724–730 (2004). The S. cerevisiae RFC–PCNA–ATPγS structure indicates a mechanism for DNA engagement by the AAA+ clamp-loader assembly. The ATPase domains of RFC are arranged in a right-handed spiral that complements the structure of dsDNA modelled inside. The PCNA lies below the RFC spiral in a closed conformation, which indicates that ring closure might precede hydrolysis of ATP.
Jeruzalmi, D., O'Donnell, M. & Kuriyan, J. Crystal structure of the processivity clamp loader gamma (γ) complex of E. coli DNA polymerase III. Cell 106, 429–441 (2001).
Jeruzalmi, D. et al. Mechanism of processivity clamp opening by the δ subunit wrench of the clamp loader complex of E. coli DNA polymerase III. Cell 106, 417–428 (2001).
Trakselis, M. A. & Benkovic, S. J. Intricacies in ATP-dependent clamp loading: variations across replication systems. Structure 9, 999–1004 (2001).
Kong, X. P., Onrust, R., O'Donnell, M. & Kuriyan, J. Three-dimensional structure of the β subunit of E. coli DNA polymerase III holoenzyme: a sliding DNA clamp. Cell 69, 425–437 (1992).
Moarefi, I., Jeruzalmi, D., Turner, J., O'Donnell, M. & Kuriyan, J. Crystal structure of the DNA polymerase processivity factor of T4 bacteriophage. J. Mol. Biol. 296, 1215–1223 (2000).
Gulbis, J. M., Kelman, Z., Hurwitz, J., O'Donnell, M. & Kuriyan, J. Structure of the C-terminal region of p21WAF1/CIP1 complexed with human PCNA. Cell 87, 297–306 (1996).
Krishna, T. S., Kong, X. P., Gary, S., Burgers, P. M. & Kuriyan, J. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell 79, 1233–1243 (1994).
Matsumiya, S., Ishino, Y. & Morikawa, K. Crystal structure of an archaeal DNA sliding clamp: proliferating cell nuclear antigen from Pyrococcus furiosus. Protein Sci. 10, 17–23 (2001).
Cullmann, G., Fien, K., Kobayashi, R. & Stillman, B. Characterization of the five replication factor C genes of Saccharomyces cerevisiae. Mol. Cell. Biol. 15, 4661–4671 (1995).
Kornberg, A. & Baker, T. A. DNA Replication 2nd edn (WH Freeman and Company, New York, 1992).
Pritchard, A. E., Dallmann, H. G., Glover, B. P. & McHenry, C. S. A novel assembly mechanism for the DNA polymerase III holoenzyme DnaX complex: association of δδ′ with DnaX4 forms DnaX3δδ′. EMBO J. 19, 6536–6545 (2000).
Flower, A. M. & McHenry, C. S. The γ subunit of DNA polymerase III holoenzyme of Escherichia coli is produced by ribosomal frameshifting. Proc. Natl Acad. Sci. USA 87, 3713–3717 (1990).
Tsuchihashi, Z. & Kornberg, A. Translational frameshifting generates the γ subunit of DNA polymerase III holoenzyme. Proc. Natl Acad. Sci. USA 87, 2516–2520 (1990).
Blinkowa, A. L. & Walker, J. R. Programmed ribosomal frameshifting generates the Escherichia coli DNA polymerase III γ subunit from within the τ subunit reading frame. Nucleic Acids Res. 18, 1725–1729 (1990).
Dallmann, H. G., Kim, S., Pritchard, A. E., Marians, K. J. & McHenry, C. S. Characterization of the unique C terminus of the Escherichia coli τ DnaX protein. Monomeric C-τ binds α and DnaB and can partially replace τ in reconstituted replication forks. J. Biol. Chem. 275, 15512–15519 (2000).
Gao, D. & McHenry, C. S. τ binds and organizes Escherichia coli replication through distinct domains. Partial proteolysis of terminally tagged τ to determine candidate domains and to assign domain V as the α binding domain. J. Biol. Chem. 276, 4433–4440 (2001).
Kim, S., Dallmann, H. G., McHenry, C. S. & Marians, K. J. Coupling of a replicative polymerase and helicase: a τ–DnaB interaction mediates rapid replication fork movement. Cell 84, 643–650 (1996). Shows that the E. coli replicase couples to the helicase through mutual connection to the τ-subunit of the clamp loader. The interaction leads to the dramatically increased speed of helicase-mediated unwinding of DNA.
Yuzhakov, A., Turner, J. & O'Donnell, M. Replisome assembly reveals the basis for asymmetric function in leading and lagging strand replication. Cell 86, 877–886 (1996).
Onrust, R. & O'Donnell, M. DNA polymerase III accessory proteins. II. Characterization of δ and δ′. J. Biol. Chem. 268, 11766–11772 (1993).
Pritchard, A. E., Dallmann, H. G. & McHenry, C. S. In vivo assembly of the τ-complex of the DNA polymerase III holoenzyme expressed from a five-gene artificial operon. Cleavage of the τ-complex to form a mixed γ–τ-complex by the OmpT protease. J. Biol. Chem. 271, 10291–10298 (1996).
Gulbis, J. M. et al. Crystal structure of the χ:π sub-assembly of the Escherichia coli DNA polymerase clamp-loader complex. Eur. J. Biochem. 271, 439–449 (2004).
Glover, B. P. & McHenry, C. S. The χπ subunits of DNA polymerase III holoenzyme bind to single-stranded DNA-binding protein (SSB) and facilitate replication of an SSB-coated template. J. Biol. Chem. 273, 23476–23484 (1998).
Yuzhakov, A., Kelman, Z. & O'Donnell, M. Trading places on DNA — a three-point switch underlies primer handoff from primase to the replicative DNA polymerase. Cell 96, 153–163 (1999).
Jeruzalmi, D. The opened processivity clamp slides into view. Proc. Natl Acad. Sci. USA 102, 14939–14940 (2005).
Lenzen, C. U., Steinmann, D., Whiteheart, S. W. & Weis, W. I. Crystal structure of the hexamerization domain of N-ethylmaleimide-sensitive fusion protein. Cell 94, 525–536 (1998).
Yu, R. C., Hanson, P. I., Jahn, R. & Brunger, A. T. Structure of the ATP-dependent oligomerization domain of N-ethylmaleimide sensitive factor complexed with ATP. Nature Struct. Biol. 5, 803–811 (1998).
Turner, J., Hingorani, M. M., Kelman, Z. & O'Donnell, M. The internal workings of a DNA polymerase clamp-loading machine. EMBO J. 18, 771–783 (1999).
Naktinis, V., Onrust, R., Fang, L. & O'Donnell, M. Assembly of a chromosomal replication machine: two DNA polymerases, a clamp loader, and sliding clamps in one holoenzyme particle. II. Intermediate complex between the clamp loader and its clamp. J. Biol. Chem. 270, 13358–13365 (1995).
Bertram, J. G. et al. Molecular mechanism and energetics of clamp assembly in Escherichia coli. The role of ATP hydrolysis when γ complex loads β on DNA. J. Biol. Chem. 275, 28413–28420 (2000).
Ason, B. et al. A model for Escherichia coli DNA polymerase III holoenzyme assembly at primer/template ends. DNA triggers a change in binding specificity of the γ complex clamp loader. J. Biol. Chem. 275, 3006–3015 (2000).
Ason, B. et al. Mechanism of loading the Escherichia coli DNA polymerase III β sliding clamp on DNA. Bona fide primer/templates preferentially trigger the γ complex to hydrolyze ATP and load the clamp. J. Biol. Chem. 278, 10033–10040 (2003).
Indiani, C. & O'Donnell, M. Mechanism of the δ wrench in opening the β sliding clamp. J. Biol. Chem. 278, 40272–40281 (2003).
Kazmirski, S. L., Podobnik, M., Weitze, T. F., O'Donnell, M. & Kuriyan, J. Structural analysis of the inactive state of the Escherichia coli DNA polymerase clamp-loader complex. Proc. Natl Acad. Sci. USA 101, 16750–16755 (2004).
Tsuchihashi, Z. & Kornberg, A. ATP interactions of the τ and γ subunits of DNA polymerase III holoenzyme of Escherichia coli. J. Biol. Chem. 264, 17790–17795 (1989).
Johnson, A. & O'Donnell, M. Ordered ATP hydrolysis in the γ complex clamp loader AAA+ machine. J. Biol. Chem. 278, 14406–14413 (2003).
Snyder, A. K., Williams, C. R., Johnson, A., O'Donnell, M. & Bloom, L. B. Mechanism of loading the Escherichia coli DNA polymerase III sliding clamp: II. Uncoupling the β and DNA binding activities of the γ complex. J. Biol. Chem. 279, 4386–4393 (2004).
Williams, C. R., Snyder, A. K., Kuzmic, P., O'Donnell, M. & Bloom, L. B. Mechanism of loading the Escherichia coli DNA polymerase III sliding clamp: I. Two distinct activities for individual ATP sites in the γ complex. J. Biol. Chem. 279, 4376–4385 (2004).
Glover, B. P. & McHenry, C. S. The DNA polymerase III holoenzyme: an asymmetric dimeric replicative complex with leading and lagging strand polymerases. Cell 105, 925–934 (2001). This study shows, by using ATPγS, that the two polymerases in the E. coli holoenzyme are functionally distinct. The structurally asymmetric clamp loader to which they are both attached probably imposes the unique properties.
McHenry, C. S. Chromosomal replicases as asymmetric dimers: studies of subunit arrangement and functional consequences. Mol. Microbiol. 49, 1157–1165 (2003).
Kim, S., Dallmann, H. G., McHenry, C. S. & Marians, K. J. τ protects β in the leading-strand polymerase complex at the replication fork. J. Biol. Chem. 271, 4315–4318 (1996).
von Hippel, P. H. The recombination–replication interface. Trends Biochem. Sci. 25, 155 (2000).
Nossal, N. G. Protein–protein interactions at a DNA replication fork: bacteriophage T4 as a model. FASEB J. 6, 871–878 (1992).
Sexton, D. J., Berdis, A. J. & Benkovic, S. J. Assembly and disassembly of DNA polymerase holoenzyme. Curr. Opin. Chem. Biol. 1, 316–322 (1997).
Shamoo, Y. & Steitz, T. A. Building a replisome from interacting pieces: sliding clamp complexed to a peptide from DNA polymerase and a polymerase editing complex. Cell 99, 155–166 (1999).
Jarvis, T. C., Paul, L. S. & von Hippel, P. H. Structural and enzymatic studies of the T4 DNA replication system. I. Physical characterization of the polymerase accessory protein complex. J. Biol. Chem. 264, 12709–12716 (1989).
Millar, D., Trakselis, M. A. & Benkovic, S. J. On the solution structure of the T4 sliding clamp (gp45). Biochemistry 43, 12723–12727 (2004). FRET analysis is used to show that the T4 clamp exists in an asymmetric open state in solution.
Jarvis, T. C., Paul, L. S., Hockensmith, J. W. & von Hippel, P. H. Structural and enzymatic studies of the T4 DNA replication system. II. ATPase properties of the polymerase accessory protein complex. J. Biol. Chem. 264, 12717–12729 (1989).
Young, M. C., Weitzel, S. E. & von Hippel, P. H. The kinetic mechanism of formation of the bacteriophage T4 DNA polymerase sliding clamp. J. Mol. Biol. 264, 440–452 (1996).
Rush, J. et al. The 44P subunit of the T4 DNA polymerase accessory protein complex catalyzes ATP hydrolysis. J. Biol. Chem. 264, 10943–10953 (1989).
Berdis, A. J. & Benkovic, S. J. Role of adenosine 5′-triphosphate hydrolysis in the assembly of the bacteriophage T4 DNA replication holoenzyme complex. Biochemistry 35, 9253–9265 (1996).
Alley, S. C., Abel-Santos, E. & Benkovic, S. J. Tracking sliding clamp opening and closing during bacteriophage T4 DNA polymerase holoenzyme assembly. Biochemistry 39, 3076–3090 (2000).
Latham, G. J., Pietroni, P., Dong, F., Young, M. C. & von Hippel, P. H. Fluorescence monitoring of T4 polymerase holoenzyme accessory protein interactions during loading of the sliding clamp onto the template–primer junction. J. Mol. Biol. 264, 426–439 (1996).
Sexton, D. J., Carver, T. E., Berdis, A. J. & Benkovic, S. J. Protein–protein and protein–DNA interactions at the bacteriophage T4 DNA replication fork. Characterization of a fluorescently labeled DNA polymerase sliding clamp. J. Biol. Chem. 271, 28045–28051 (1996).
Sexton, D. J., Kaboord, B. F., Berdis, A. J., Carver, T. E. & Benkovic, S. J. Dissecting the order of bacteriophage T4 DNA polymerase holoenzyme assembly. Biochemistry 37, 7749–7756 (1998).
Trakselis, M. A., Alley, S. C., Abel-Santos, E. & Benkovic, S. J. Creating a dynamic picture of the sliding clamp during T4 DNA polymerase holoenzyme assembly by using fluorescence resonance energy transfer. Proc. Natl Acad. Sci. USA 98, 8368–8375 (2001).
Trakselis, M. A., Berdis, A. J. & Benkovic, S. J. Examination of the role of the clamp-loader and ATP hydrolysis in the formation of the bacteriophage T4 polymerase holoenzyme. J. Mol. Biol. 326, 435–451 (2003). This study shows that the T4 clamp loader loads the clamp onto DNA through the sequential hydrolysis of two ATP before and two ATP after the addition of DNA. The final holoenzyme complex is formed upon departure of the clamp loader from this complex.
Pietroni, P., Young, M. C., Latham, G. J. & von Hippel, P. H. Structural analyses of gp45 sliding clamp interactions during assembly of the bacteriophage T4 DNA polymerase holoenzyme. I. Conformational changes within the gp44/62–gp45–ATP complex during clamp loading. J. Biol. Chem. 272, 31666–31676 (1997).
Trakselis, M. A., Roccasecca, R. M., Yang, J., Valentine, A. M. & Benkovic, S. J. Dissociative properties of the proteins within the bacteriophage T4 replisome. J. Biol. Chem. 278, 49839–49849 (2003).
Capson, T. L., Benkovic, S. J. & Nossal, N. G. Protein–DNA cross-linking demonstrates stepwise ATP-dependent assembly of T4 DNA polymerase and its accessory proteins on the primer–template. Cell 65, 249–258 (1991).
Hockensmith, J. W., Kubasek, W. L., Evertsz, E. M., Mesner, L. D. & von Hippel, P. H. Laser cross-linking of proteins to nucleic acids. II. Interactions of the bacteriophage T4 DNA replication polymerase accessory proteins complex with DNA. J. Biol. Chem. 268, 15721–15730 (1993).
Alley, S. C. et al. Building a replisome solution structure by elucidation of protein–protein interactions in the bacteriophage T4 DNA polymerase holoenzyme. J. Biol. Chem. 276, 39340–39349 (2001).
Berdis, A. J. & Benkovic, S. J. Mechanism of bacteriophage T4 DNA holoenzyme assembly: the 44/62 protein acts as a molecular motor. Biochemistry 36, 2733–2743 (1997).
Latham, G. J., Bacheller, D. J., Pietroni, P. & von Hippel, P. H. Structural analyses of gp45 sliding clamp interactions during assembly of the bacteriophage T4 DNA polymerase holoenzyme. III. The gp43 DNA polymerase binds to the same face of the sliding clamp as the clamp loader. J. Biol. Chem. 272, 31685–31692 (1997).
Latham, G. J., Bacheller, D. J., Pietroni, P. & von Hippel, P. H. Structural analyses of gp45 sliding clamp interactions during assembly of the bacteriophage T4 DNA polymerase holoenzyme. II. The gp44/62 clamp loader interacts with a single defined face of the sliding clamp ring. J. Biol. Chem. 272, 31677–31684 (1997).
Reddy, M. K., Weitzel, S. E. & von Hippel, P. H. Assembly of a functional replication complex without ATP hydrolysis: a direct interaction of bacteriophage T4 gp45 with T4 DNA polymerase. Proc. Natl Acad. Sci. USA 90, 3211–3215 (1993).
Garg, P. & Burgers, P. M. DNA polymerases that propagate the eukaryotic DNA replication fork. Crit. Rev. Biochem. Mol. Biol. 40, 115–128 (2005).
Hubscher, U., Maga, G. & Spadari, S. Eukaryotic DNA polymerases. Annu. Rev. Biochem. 71, 133–163 (2002).
Mossi, R., Jonsson, Z. O., Allen, B. L., Hardin, S. H. & Hubscher, U. Replication factor C interacts with the C-terminal side of proliferating cell nuclear antigen. J. Biol. Chem. 272, 1769–1776 (1997).
Oku, T. et al. Functional sites of human PCNA which interact with p21Cip1/Waf1, DNA polymerase δ and replication factor C. Genes Cells 3, 357–369 (1998).
Uhlmann, F., Gibbs, E., Cai, J., O'Donnell, M. & Hurwitz, J. Identification of regions within the four small subunits of human replication factor C required for complex formation and DNA replication. J. Biol. Chem. 272, 10065–10071 (1997).
Bunz, F., Kobayashi, R. & Stillman, B. cDNAs encoding the large subunit of human replication factor C. Proc. Natl Acad. Sci. USA 90, 11014–11018 (1993).
Gomes, X. V. & Burgers, P. M. ATP utilization by yeast replication factor C. I. ATP-mediated interaction with DNA and with proliferating cell nuclear antigen. J. Biol. Chem. 276, 34768–34775 (2001).
Gomes, X. V., Gary, S. L. & Burgers, P. M. Overproduction in Escherichia coli and characterization of yeast replication factor C lacking the ligase homology domain. J. Biol. Chem. 275, 14541–14549 (2000).
Green, C. M., Erdjument-Bromage, H., Tempst, P. & Lowndes, N. F. A novel Rad24 checkpoint protein complex closely related to replication factor C. Curr. Biol. 10, 39–42 (2000).
Lindsey-Boltz, L. A., Bermudez, V. P., Hurwitz, J. & Sancar, A. Purification and characterization of human DNA damage checkpoint Rad complexes. Proc. Natl Acad. Sci. USA 98, 11236–11241 (2001).
Bellaoui, M. et al. Elg1 forms an alternative RFC complex important for DNA replication and genome integrity. EMBO J. 22, 4304–4313 (2003).
Ben-Aroya, S., Koren, A., Liefshitz, B., Steinlauf, R. & Kupiec, M. ELG1, a yeast gene required for genome stability, forms a complex related to replication factor C. Proc. Natl Acad. Sci. USA 100, 9906–9911 (2003).
Kanellis, P., Agyei, R. & Durocher, D. Elg1 forms an alternative PCNA-interacting RFC complex required to maintain genome stability. Curr. Biol. 13, 1583–1595 (2003).
Ellison, V. & Stillman, B. Biochemical characterization of DNA damage checkpoint complexes: clamp loader and clamp complexes with specificity for 5′ recessed DNA. PLoS Biol. 1, E33 (2003).
Zou, L., Liu, D. & Elledge, S. J. Replication protein A-mediated recruitment and activation of Rad17 complexes. Proc. Natl Acad. Sci. USA 100, 13827–13832 (2003).
Sancar, A., Lindsey-Boltz, L. A., Unsal-Kacmaz, K. & Linn, S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 73, 39–85 (2004).
Majka, J. & Burgers, P. M. The PCNA–RFC families of DNA clamps and clamp loaders. Prog. Nucleic Acid Res. Mol. Biol. 78, 227–260 (2004).
Bylund, G. O. & Burgers, P. M. Replication protein A-directed unloading of PCNA by the Ctf18 cohesion establishment complex. Mol. Cell. Biol. 25, 5445–5455 (2005). This study shows that the Ctf18–RFC complex is extremely efficient in removing PCNA from DNA, which indicates that it might recycle clamps.
Yao, N. Y., Johnson, A., Bowman, G., Kuriyan, J. & O'Donnell, M. Mechanism of PCNA clamp opening by RFC. J. Biol. Chem. 281, 17528–17539 (2006).
Fotedar, R. et al. A conserved domain of the large subunit of replication factor C binds PCNA and acts like a dominant negative inhibitor of DNA replication in mammalian cells. EMBO J. 15, 4423–4433 (1996).
Burgers, P. M. Saccharomyces cerevisiae replication factor C. II. Formation and activity of complexes with the proliferating cell nuclear antigen and with DNA polymerases δ and ε. J. Biol. Chem. 266, 22698–22706 (1991).
Hingorani, M. M. & O'Donnell, M. ATP binding to the Escherichia coli clamp loader powers opening of the ring-shaped clamp of DNA polymerase III holoenzyme. J. Biol. Chem. 273, 24550–24563 (1998).
Lee, S. H. & Hurwitz, J. Mechanism of elongation of primed DNA by DNA polymerase δ, proliferating cell nuclear antigen, and activator 1. Proc. Natl Acad. Sci. USA 87, 5672–5676 (1990).
Tsurimoto, T. & Stillman, B. Replication factors required for SV40 DNA replication in vitro. I. DNA structure-specific recognition of a primer–template junction by eukaryotic DNA polymerases and their accessory proteins. J. Biol. Chem. 266, 1950–1960 (1991).
Gomes, X. V., Schmidt, S. L. & Burgers, P. M. ATP utilization by yeast replication factor C. II. Multiple stepwise ATP binding events are required to load proliferating cell nuclear antigen onto primed DNA. J. Biol. Chem. 276, 34776–34783 (2001).
Schmidt, S. L., Gomes, X. V. & Burgers, P. M. ATP utilization by yeast replication factor C. III. The ATP-binding domains of Rfc2, Rfc3, and Rfc4 are essential for DNA recognition and clamp loading. J. Biol. Chem. 276, 34784–34791 (2001).
Kazmirski, S. L., Zhao, Y., Bowman, G. D., O'Donnell, M. & Kuriyan, J. Out-of-plane motions in open sliding clamps: molecular dynamics simulations of eukaryotic and archaeal proliferating cell nuclear antigen. Proc. Natl Acad. Sci. USA 102, 13801–13806 (2005).
Zhuang, Z., Yoder, B. L., Burgers, P. M. & Benkovic, S. J. The structure of a ring-opened proliferating cell nuclear antigen–replication factor C complex revealed by fluorescence energy transfer. Proc. Natl Acad. Sci. USA 3, 2546–2551 (2006).
Goedken, E. R., Kazmirski, S. L., Bowman, G. D., O'Donnell, M. & Kuriyan, J. Mapping the interaction of DNA with the Escherichia coli DNA polymerase clamp loader complex. Nature Struct. Mol. Biol. 12, 183–190 (2005).
Podust, L. M., Podust, V. N., Sogo, J. M. & Hubscher, U. Mammalian DNA polymerase auxiliary proteins: analysis of replication factor C-catalyzed proliferating cell nuclear antigen loading onto circular double-stranded DNA. Mol. Cell. Biol. 15, 3072–3081 (1995).
Woese, C. R. & Fox, G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc. Natl Acad. Sci. USA 74, 5088–5090 (1977).
Olsen, G. J. & Woese, C. R. Archaeal genomics: an overview. Cell 89, 991–994 (1997).
Edgell, D. R. & Doolittle, W. F. Archaea and the origin(s) of DNA replication proteins. Cell 89, 995–998 (1997).
Leipe, D. D., Aravind, L. & Koonin, E. V. Did DNA replication evolve twice independently? Nucleic Acids Res. 27, 3389–3401 (1999).
Chapados, B. R. et al. Structural basis for FEN-1 substrate specificity and PCNA-mediated activation in DNA replication and repair. Cell 116, 39–50 (2004).
Cann, I. K. et al. Functional interactions of a homolog of proliferating cell nuclear antigen with DNA polymerases in archaea. J. Bacteriol. 181, 6591–6599 (1999).
Dionne, I., Nookala, R. K., Jackson, S. P., Doherty, A. J. & Bell, S. D. A heterotrimeric PCNA in the hyperthermophilic archaeon Sulfolobus solfataricus. Mol. Cell 11, 275–282 (2003).
Cann, I. K. & Ishino, Y. Archaeal DNA replication: identifying the pieces to solve a puzzle. Genetics 152, 1249–1267 (1999).
Cann, I. K. et al. Biochemical analysis of replication factor C from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 183, 2614–2623 (2001).
Pisani, F. M., De Felice, M., Carpentieri, F. & Rossi, M. Biochemical characterization of a clamp-loader complex homologous to eukaryotic replication factor C from the hyperthermophilic archaeon Sulfolobus solfataricus. J. Mol. Biol. 301, 61–73 (2000).
Seybert, A., Scott, D. J., Scaife, S., Singleton, M. R. & Wigley, D. B. Biochemical characterisation of the clamp/clamp loader proteins from the euryarchaeon Archaeoglobus fulgidus. Nucleic Acids Res. 30, 4329–4338 (2002).
Kelman, Z. & Hurwitz, J. A unique organization of the protein subunits of the DNA polymerase clamp loader in the archaeon Methanobacterium thermoautotrophicum δH. J. Biol. Chem. 275, 7327–7336 (2000).
Matsumiya, S., Ishino, S., Ishino, Y. & Morikawa, K. Physical interaction between proliferating cell nuclear antigen and replication factor C from Pyrococcus furiosus. Genes Cells 7, 911–922 (2002).
Oyama, T., Ishino, Y., Cann, I. K., Ishino, S. & Morikawa, K. Atomic structure of the clamp loader small subunit from Pyrococcus furiosus. Mol. Cell 8, 455–463 (2001).
Seybert, A., Singleton, M. R., Cook, N., Hall, D. R. & Wigley, D. B. Communication between subunits within an archaeal clamp-loader complex. EMBO J. 25, 2209–2218 (2006). The crystal structure of the hexameric complex formed by the RFC-s from A. fulgidus shows a conformational change that is associated with nucleotide binding. Mutational analysis shows distinct regulatory roles during clamp loading that distinguish the large and the small subunits in the RFC complex.
Mayanagi, K., Miyata, T., Oyama, T., Ishino, Y. & Morikawa, K. Three-dimensional electron microscopy of the clamp loader small subunit from Pyrococcus furiosus. J. Struct. Biol. 134, 35–45 (2001).
Podobnik, M., Weitze, T. F., O'Donnell, M. & Kuriyan, J. Nucleotide-induced conformational changes in an isolated Escherichia coli DNA polymerase III clamp loader subunit. Structure 11, 253–263 (2003).
Seybert, A. & Wigley, D. B. Distinct roles for ATP binding and hydrolysis at individual subunits of an archaeal clamp loader. EMBO J. 23, 1360–1371 (2004).
Miyata, T. et al. The clamp-loading complex for processive DNA replication. Nature Struct. Mol. Biol. 11, 632–636 (2004).
Burnouf, D. Y. et al. Structural and biochemical analysis of sliding clamp–ligand interactions suggest a competition between replicative and translesion DNA polymerases. J. Mol. Biol. 335, 1187–1197 (2004).
Indiani, C., McInerney, P., Georgescu, R., Goodman, M. F. & O'Donnell, M. A sliding-clamp toolbelt binds high- and low-fidelity DNA polymerases simultaneously. Mol. Cell 19, 805–815 (2005).
Davey, M. J., Jeruzalmi, D., Kuriyan, J. & O'Donnell, M. Motors and switches: AAA+ machines within the replisome. Nature Rev. Mol. Cell. Biol. 3, 826–835 (2002).
Erzberger, J. P. & Berger, J. M. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu. Rev. Biophys. Biomol. Struct. 35, 93–114 (2006). This review describes critical features of the AAA+ domain, provides classifications of the AAA+ modules and discusses the versatility and adaptability of the hexameric AAA+ assembly.
Neuwald, A. F., Aravind, L., Spouge, J. L. & Koonin, E. V. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9, 27–43 (1999).
Neuwald, A. F. Evolutionary clues to eukaryotic DNA clamp-loading mechanisms: analysis of the functional constraints imposed on replication factor C AAA+ ATPases. Nucleic Acids Res. 33, 3614–3628 (2005).
Guenther, B., Onrust, R., Sali, A., O'Donnell, M. & Kuriyan, J. Crystal structure of the δ′ subunit of the clamp-loader complex of E. coli DNA polymerase III. Cell 91, 335–345 (1997).
Ahmadian, M. R., Stege, P., Scheffzek, K. & Wittinghofer, A. Confirmation of the arginine-finger hypothesis for the GAP-stimulated GTP-hydrolysis reaction of Ras. Nature Struct. Biol. 4, 686–689 (1997).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Replicase
-
A DNA polymerase with accessory subunits, including a clamp and clamp loader, that replicates chromosomal DNA.
- Protomers
-
Subunits from which a larger structure is built.
- AAA+ family of ATPases
-
(ATPases associated with a variety of cellular activities). A large family of functionally divergent proteins that have a region of homology around the ATP sites. They are often used to remodel other macromolecules.
- Primase
-
A specialized RNA polymerase that synthesizes short RNA primers to initiate DNA synthesis by DNA polymerases.
- SRC motif
-
A three-residue motif (serine–arginine–cysteine) that is conserved in clamp-loading subunits and that contains a catalytic arginine residue that is involved in ATP hydrolysis.
- FRET
-
(Fluorescence resonance energy transfer). The transfer of energy from a light-activated fluorophore to a second fluorophore that has a longer excitation wavelength. The efficiency of energy transfer depends on the distance between fluorophores.
- Walker A and B motifs
-
These motifs are found in most ATP-binding proteins. The Walker A region, also known as a P-loop, binds ATP, whereas the Walker B region binds Mg2+. Both are required for ATP hydrolysis.
Rights and permissions
About this article
Cite this article
Indiani, C., O'Donnell, M. The replication clamp-loading machine at work in the three domains of life. Nat Rev Mol Cell Biol 7, 751–761 (2006). https://doi.org/10.1038/nrm2022
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2022
This article is cited by
-
Biochemical Assessment of the Mutant Sliding β-Clamp on Stimulation of Endonuclease IV from Staphylococcus aureus
Indian Journal of Microbiology (2024)
-
MutS functions as a clamp loader by positioning MutL on the DNA during mismatch repair
Nature Communications (2022)
-
DNA is loaded through the 9-1-1 DNA checkpoint clamp in the opposite direction of the PCNA clamp
Nature Structural & Molecular Biology (2022)
-
Deep Analysis of Residue Constraints (DARC): identifying determinants of protein functional specificity
Scientific Reports (2020)
-
A proteomic portrait of dinoflagellate chromatin reveals abundant RNA-binding proteins
Chromosoma (2018)