Key Points
-
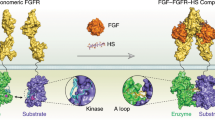
Receptor tyrosine kinases (RTKs) are transmembrane receptors that have intrinsic, cytoplasmic tyrosine kinase activity. These receptors are activated by ligand binding, which stabilizes a dimeric receptor configuration and facilitates trans phosphorylation of tyrosine (Tyr) residues in the cytoplasmic domain.
-
In addition to generating docking sites for downstream signalling proteins, Tyr phosphorylation stimulates receptor catalytic (tyrosine kinase) activity. For most RTKs, Tyr phosphorylation in the activation segment of the kinase domain is stimulatory for activity. And, for a subset of RTKs, Tyr phosphorylation in the juxtamembrane region of the receptor (between the transmembrane helix and the kinase domain) is also stimulatory.
-
Juxtamembrane autoinhibition, which is relieved by Tyr phosphorylation, has been shown biochemically to occur in Eph receptors (the receptors for ephrins), the platelet-derived growth factor (PDGF) receptor family, and in muscle-specific kinase (MUSK).
-
For Eph receptors and PDGF receptor family members, structural studies have revealed the mechanisms by which the unphosphorylated juxtamembrane region inhibits catalytic activity. In both cases, interactions between residues in the juxtamembrane region and the kinase domain prevent the kinase from adopting an active state, although the detailed mechanisms are distinct.
-
Point mutations and short in-frame deletions in the juxtamembrane regions of KIT (a PDGF receptor family member) and PDGF receptor-α that cause gastrointestinal stromal tumours disrupt the interactions between the juxtamembrane region and the kinase domain, which renders the receptors constitutively active.
-
Our ability to design small-molecule kinase inhibitors and activators should improve as a result of an increased structural understanding of juxtamembrane autoinhibition.
Abstract
Receptor tyrosine kinases are essential mediators of cell growth, differentiation, migration and metabolism. Accordingly, their catalytic activity is tightly regulated by several mechanisms including autoinhibition. Recent structural studies, together with biochemical experiments, are now unravelling the molecular mechanisms by which the juxtamembrane region (between the transmembrane helix and the cytoplasmic kinase domain) negatively regulates catalytic activity in various receptor tyrosine kinases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 103, 211–225 (2000).
Ullrich, A. & Schlessinger, J. Signal transduction by receptors with tyrosine kinase activity. Cell 61, 203–212 (1990).
Heldin, C. H. Dimerization of cell surface receptors in signal transduction. Cell 80, 213–223 (1995).
Pawson, T. Protein modules and signalling networks. Nature 373, 573–580 (1995).
Weiss, A. & Schlessinger, J. Switching signals on or off by receptor dimerization. Cell 94, 277–280 (1998).
Jiang, G. & Hunter, T. Receptor signaling: when dimerization is not enough. Curr. Biol. 9, R568–R571 (1999).
Sako, Y., Minoghchi, S. & Yanagida, T. Single-molecule imaging of EGFR signalling on the surface of living cells. Nature Cell Biol. 2, 168–172 (2000).
Moriki, T., Maruyama, H. & Maruyama, I. N. Activation of preformed EGF receptor dimers by ligand-induced rotation of the transmembrane domain. J. Mol. Biol. 311, 1011–1026 (2001). This paper provides evidence for inactive EGF receptor dimers on the cell surface and for the ligand-induced rotation of the transmembrane helices.
Burke, C. L. & Stern, D. F. Activation of Neu (ErbB-2) mediated by disulfide bond-induced dimerization reveals a receptor tyrosine kinase dimer interface. Mol. Cell. Biol. 18, 5371–5379 (1998).
Hays, J. L. & Watowich, S. J. Oligomerization-induced modulation of TPR-MET tyrosine kinase activity. J. Biol. Chem. 278, 27456–27463 (2003).
Baer, K. et al. Dimerization-induced activation of soluble insulin/IGF-1 receptor kinases: an alternative mechanism of activation. Biochemistry 40, 14268–14278 (2001).
Hubbard, S. R. & Till, J. H. Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 69, 373–398 (2000).
Johnson, L. N., Noble, M. E. M. & Owen, D. J. Active and inactive protein kinases: structural basis for regulation. Cell 85, 149–158 (1996).
Hubbard, S. R. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 16, 5572–5581 (1997).
Gotoh, N., Tojo, A., Hino, M., Yazaki, Y. & Shibuya, M. A highly conserved tyrosine residue at codon 845 within the kinase domain is not required for the transforming activity of human epidermal growth factor receptor. Biochem. Biophys. Res. Commun. 186, 768–774 (1992).
Sherrill, J. M. Insufficiency of self-phosphorylation for the activation of epidermal growth factor receptor. Biochem. 36, 5677–5684 (1997).
Stamos, J., Sliwkowski, M. X. & Eigenbrot, C. Structure of the EGF receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J. Biol. Chem. 277, 46265–46272 (2002).
Shewchuk, L. M. et al. Structure of the Tie2 RTK domain: self-inhibition by the nucleotide binding loop, activation loop, and C-terminal tail. Structure Fold. Des. 8, 1105–1113 (2000).
Niu, X. L., Peters, K. G. & Kontos, C. D. Deletion of the carboxy-terminus of Tie2 enhances kinase activity, signaling, and function: evidence for an autoinhibitory mechanism. J. Biol. Chem. 277, 31768–31773 (2002).
Bellus, G. A. et al. A recurrent mutation in the tyrosine kinase domain of fibroblast growth factor receptor 3 causes hypochondroplasia. Nature Genet. 10, 357–359 (1995).
Tavormina, P. L. et al. Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nature Genet. 9, 321–328 (1995).
Tonks, N. K. & Neel, B. G. From form to function: signaling by protein tyrosine phosphatases. Cell 87, 365–368 (1996).
Hunter, T., Ling, N. & Cooper, J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature 311, 480–483 (1984).
Gandino, L., Di Renzo, M. F., Giordano, S., Bussolino, F. & Comoglio, P. M. Protein kinase-c activation inhibits tyrosine phosphorylation of the c-met protein. Oncogene 5, 721–725 (1990).
Holder, N. & Klein, R. Eph receptors and ephrins: effectors of morphogenesis. Development 126, 2033–2044 (1999).
Dodelet, V. C. & Pasquale, E. B. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene 19, 5614–5619 (2000).
Binns, K. L., Taylor, P. P., Sicheri, F., Pawson, T. & Holland, S. J. Phosphorylation of tyrosine residues in the kinase domain and juxtamembrane region regulates the biological and catalytic activities of Eph receptors. Mol. Cell. Biol. 20, 4791–4805 (2000).
White, M. F. et al. Mutation of the insulin receptor at tyrosine 960 inhibits signal transmission but does not affect its tyrosine kinase activity. Cell 54, 641–649 (1988).
Wybenga-Groot, L. E. et al. Structural basis for autoinhibition of the Ephb2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell 106, 745–757 (2001). The first crystal structure to reveal how the juxtamembrane region of a receptor tyrosine kinase interacts with the kinase domain to suppress catalytic activity.
Li, S., Covino, N. D., Stein, E. G., Till, J. H. & Hubbard, S. R. Structural and biochemical evidence for an autoinhibitory role for tyrosine 984 in the juxtamembrane region of the insulin receptor. J. Biol. Chem. 278, 26007–26014 (2003). This work reveals the regulatory role of a non-phosphorylatable Tyr residue in the juxtamembrane region of the insulin receptor and of other receptor tyrosine kinases.
Jennings, C. G., Dyer, S. M. & Burden, S. J. Muscle-specific trk-related receptor with a kringle domain defines a distinct class of receptor tyrosine kinases. Proc. Natl Acad. Sci. USA 90, 2895–2899 (1993).
DeChiara, T. M. et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 85, 501–512 (1996).
Herbst, R. & Burden, S. J. The juxtamembrane region of MuSK has a critical role in agrin-mediated signaling. EMBO J. 19, 67–77 (2000).
Till, J. H. et al. Crystal structure of the MuSK tyrosine kinase: insights into receptor autoregulation. Structure 10, 1187–1196 (2002). This paper shows that MUSK kinase activity is suppressed by the activation segment by a mechanism similar to that used by the insulin receptor, and that the MUSK juxtamembrane region is disordered.
Huse, M., Chen, Y. G., Massague, J. & Kuriyan, J. Crystal structure of the cytoplasmic domain of the type I TGFβ receptor in complex with FKBP12. Cell 96, 425–436 (1999).
Hirota, S. et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279, 577–580 (1998).
Heinrich, M. C. et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299, 708–710 (2003).
Nakao, M. et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 10, 1911–1918 (1996).
Mori, S. et al. Identification of two juxtamembrane autophosphorylation sites in the PDGFβ-receptor; involvement in the interaction with Src family tyrosine kinases. EMBO J. 12, 2257–2264 (1993).
Baxter, R. M., Secrist, J. P., Vaillancourt, R. R. & Kazlauskas, A. Full activation of the platelet-derived growth factor β-receptor kinase involves multiple events. J. Biol. Chem. 273, 17050–17055 (1998).
Griffith, J. et al. The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol. Cell 13, 169–178 (2004). This study provides the structural basis by which the juxtamembrane region of FLT3, and of other PDGF receptor family members, interacts with the kinase domain to suppress catalytic activity.
Irusta, P. M. & DiMaio, D. A single amino acid substitution in a WW-like domain of diverse members of the PDGF receptor subfamily of tyrosine kinases causes constitutive receptor activation. EMBO J. 17, 6912–6923 (1998).
Irusta, P. M. et al. Definition of an inhibitory juxtamembrane WW-like domain in the platelet-derived growth factor beta receptor. J. Biol. Chem. 277, 38627–38634 (2002). This paper describes an extensive mutagenesis study of the juxtamembrane region of PDGF receptor-β.
Macias, M. J., Wiesner, S. & Sudol, M. WW and SH3 domains, two different scaffolds to recognize proline-rich ligands. FEBS Lett. 513, 30–37 (2002).
Chan, P. M., Ilangumaran, S., La Rose, J., Chakrabartty, A. & Rottapel, R. Autoinhibition of the kit receptor tyrosine kinase by the cytosolic juxtamembrane region. Mol. Cell. Biol. 23, 3067–3078 (2003). This work provides evidence for an autonomously folded domain in the juxtamembrane region of KIT a result that is not supported by the FLT3 crystal structure.
Mol, C. D. et al. Structure of a c-Kit product complex reveals the basis for kinase transactivation. J. Biol. Chem. 278, 31461–31464 (2003). This study reveals the structure of the activated KIT kinase domain, in which the juxtamembrane region is Tyr phosphorylated.
Rajagopalan, M., Neidigh, J. L. & McClain, D. A. Amino acid sequences Gly-Pro-Leu-Tyr and Asn-Pro-Glu-Tyr in the submembranous domain of the insulin receptor are required for normal endocytosis. J. Biol. Chem. 266, 23068–23073 (1991).
Gustafson, T. A., He, W., Craparo, A., Schaub, C. D. & O'Neill, T. J. Phosphotyrosine-dependent interaction of SHC and insulin receptor substrate 1 with the NPEY motif of the insulin receptor via a novel non-SH2 domain. Mol. Cell. Biol. 15, 2500–2508 (1995).
Backer, J. M., Kahn, C. R., Cahill, D. A., Ullrich, A. & White, M. F. Receptor-mediated internalization of insulin requires a 12-amino acid sequence in the juxtamembrane region of the insulin receptor β-subunit. J. Biol. Chem. 265, 16450–16454 (1990).
Mohammadi, M., Schlessinger, J. & Hubbard, S. R. Structure of the FGF receptor tyrosine kinase domain reveals a novel autoinhibitory mechanism. Cell 86, 577–587 (1996).
McTigue, M. A. et al. Crystal structure of the kinase domain of human vascular endothelial growth factor receptor 2: a key enzyme in angiogenesis. Structure Fold. Des. 7, 319–330 (1999).
Blume-Jensen, P. & Hunter, T. Oncogenic kinase signalling. Nature 411, 355–365 (2001).
Schindler, T. et al. Structural mechanism for STI-571 inhibition of Abelson tyrosine kinase. Science 289, 1938–1942 (2000).
Hubbard, S. R. Protein tyrosine kinases: autoregulation and small-molecule inhibition. Curr. Opin. Struct. Biol. 12, 735–741 (2002).
Hubbard, S. R., Wei, L., Ellis, L. & Hendrickson, W. A. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature 372, 746–754 (1994).
Mol, C. D. et al. Structural basis for the autoinhibition and STI-571 Inhibition of c-Kit tyrosine kinase. J. Biol. Chem. 29 April 2004 (doi:10.1074/jbc.M403319200).
Acknowledgements
I acknowledge research support from the National Institutes of Health and the American Diabetes Association.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Protein Data Bank
phosphorylated insulin receptor tyrosine kinase domain
unphosphorylated insulin receptor tyrosine kinase domain
unphosphorylated MUSK cytoplasmic domain
Swiss-Prot
FURTHER INFORMATION
Glossary
- PROTEIN TYROSINE KINASE
-
An enzyme that transfers the γ-phosphate of ATP to tyrosine residues in protein substrates.
- RECEPTOR TYROSINE KINASE
-
(RTK). A cell-surface receptor that has an intracellular protein tyrosine kinase domain.
- TRANS PHOSPHORYLATION
-
The transfer of a phosphate group by a protein kinase to a residue in a different kinase molecule.
- CIS PHOSPHORYLATION
-
The transfer of a phosphate group by a protein kinase to a residue in the same kinase molecule.
- SRC HOMOLOGY-2 DOMAIN
-
(SH2 domain). An ∼100-residue domain that binds to phosphorylated tyrosine sequences in proteins.
- ACTIVATION SEGMENT/LOOP
-
A 20–25-residue segment in a protein kinase that functions to modulate kinase activity.
- BASAL-LEVEL ACTIVITY
-
The catalytic activity of a protein kinase that has not been activated, for example, by ligand-mediated phosphorylation.
- AUTOINHIBITION
-
The suppression of protein activity owing to interactions within the protein.
- JUXTAMEMBRANE REGION
-
The polypeptide segment in a receptor that connects the transmembrane helix to the kinase domain.
- AUTOPHOSPHORYLATION
-
The transfer of a phosphate group by a protein kinase either to a residue in the same kinase molecule (cis) or to a residue in a different kinase molecule (trans) but of the same type.
- DISORDERED
-
In a crystal structure, this describes a polypeptide segment that does not adopt a preferred conformation, but rather multiple conformations.
- PROTEIN SERINE/THREONINE KINASE
-
An enzyme that transfers the γ-phosphate of ATP to serine or threonine residues in protein substrates.
Rights and permissions
About this article
Cite this article
Hubbard, S. Juxtamembrane autoinhibition in receptor tyrosine kinases. Nat Rev Mol Cell Biol 5, 464–471 (2004). https://doi.org/10.1038/nrm1399
Issue Date:
DOI: https://doi.org/10.1038/nrm1399
This article is cited by
-
Molecular basis of VEGFR1 autoinhibition at the plasma membrane
Nature Communications (2024)
-
Promoter swapping of truncated PDGFRB drives Ph-like acute lymphoblastic leukemia
npj Precision Oncology (2023)
-
The combined action of the intracellular regions regulates FGFR2 kinase activity
Communications Biology (2023)
-
A stepwise activation model for the insulin receptor
Experimental & Molecular Medicine (2023)
-
Advances in protein glycosylation and its role in tissue repair and regeneration
Glycoconjugate Journal (2023)