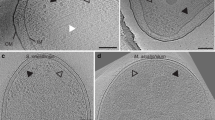
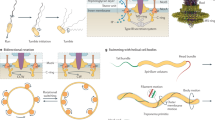
Key Points
-
Bacterial chemotaxis is the biasing of movement towards environments that contain higher concentrations of beneficial, or lower concentrations of toxic, chemicals. The signalling pathway that is involved has long been viewed as a paradigm of histidine–aspartate-phosphorelay signalling, and is one of the most well-understood physiological processes in biology.
-
The pathway is composed of chemoreceptors, the histidine protein kinase chemotaxis protein (Che)A and two diffusable response regulators (CheY and CheB). CheY controls flagellar motor switching, whereas CheB controls chemoreceptor adaptation.
-
The chemoreceptors and other proteins of the chemotaxis signalling pathway localize to specific regions of the cell as large higher-order arrays. This is thought to allow sensitivity and gain — cells can respond to a change of just a few molecules over background concentrations that can vary over five orders of magnitude.
-
Biochemical, cellular-concentration and molecular-structure data for the various components of this pathway are available. These data have allowed various mathematical and computational models to be generated and tested.
-
Many bacterial species have several chemosensory pathways, as well as further components that might be expressed under particular environmental conditions to allow bacteria to tune their responses to a specific environment.
-
Chemotaxis is thought to be involved in pathogenicity, symbiosis, biofilm formation and stability, and in maintaining bacteria in their optimal environmental niche. The correct interplay between chemotaxis and other sensing systems is essential for bacterial survival in a changing environment.
Abstract
Bacteria must be able to respond to a changing environment, and one way to respond is to move. The transduction of sensory signals alters the concentration of small phosphorylated response regulators that bind to the rotary flagellar motor and cause switching. This simple pathway has provided a paradigm for sensory systems in general. However, the increasing number of sequenced bacterial genomes shows that although the central sensory mechanism seems to be common to all bacteria, there is added complexity in a wide range of species.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
West, A. H. & Stock, A. M. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26, 369–376 (2001). A good review of the biochemical and structural aspects of chemotaxis and other two-component signalling systems.
Maeda, T., Wurglermurphy, S. M. & Saito, H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature 369, 242–245 (1994).
Nagahashi, S. et al. Isolation of CaSLN1 and CaNIK1, the genes for osmosensing histidine kinase homologues, from the pathogenic fungus Candida albicans. Microbiology 144, 425–432 (1998).
Schuster, S. C., Noegel, A. A., Oehme, F., Gerisch, G. & Simon, M. I. The hybrid histidine kinase DokA is part of the osmotic response system of Dictyostelium. EMBO J. 15, 3880–3889 (1996).
Wilkinson, J. Q., Lanahan, M. B., Yen, H. C., Giovannoni, J. J. & Klee, H. J. An ethylene-inducible component of signal transduction encoded by never-ripe. Science 270, 1807–1809 (1995).
Ashby, M. K. Survey of the number of two-component response regulator genes in the complete and annotated genome sequences of prokaryotes. FEMS Microbiol. Lett. 231, 277–281 (2004).
Stock, A. M., Robinson, V. L. & Goudreau, P. N. Two-component signal transduction. Annu. Rev. Biochem. 69, 183–215 (2000).
Inouye, M. & Dutta, R. Histidine Kinases in Signal Transduction (Academic Press, London, UK, 2003).
Potter, C. A. et al. Expression, purification and characterisation of full-length histidine protein kinase RegB from Rhodobacter sphaeroides. J. Mol. Biol. 320, 201–213 (2002).
Armitage, J. P. Bacterial tactic responses. Adv. Microb. Physiol. 41, 229–289 (1999).
Bren, A. & Eisenbach, M. How signals are heard during bacterial chemotaxis: protein–protein interactions in sensory signal propagation. J. Bacteriol. 182, 6865–6873 (2000).
Faguy, D. M. & Jarrell, K. F. A twisted tale: the origin and evolution of motility and chemotaxis in prokaryotes. Microbiology 145, 279–281 (1999).
Schnitzer, M. J., Block, S. M., Berg, H. C. & Purcell, E. M. Biology of the Chemotactic Response (Armitage, J. P. & Lackie, J. M. eds) 15–34 (Cambridge Univ. Press, UK, 1990).
Thar, R. & Kühl, M. Bacteria are not too small for spatial sensing of chemical gradients: an experimental evidence. Proc. Natl Acad. Sci. USA 100, 5748–5753 (2003).
Turner, L., Ryu, W. S. & Berg, H. C. Real-time imaging of fluorescent flagellar filaments. J. Bacteriol. 182, 2793–2801 (2000).
Armitage, J. P. & Schmitt, R. Bacterial chemotaxis: Rhodobacter sphaeroides and Sinorhizobium meliloti — variations on a theme? Microbiology 143, 3671–3682 (1997).
Adler, J. Chemoreceptors in bacteria. Science 166, 1588–1597 (1969).
Levin, M. D., Morton, F. C., Abouhamad, W. N., Bourret, R. B. & Bray, D. Origins of individual swimming behavior in bacteria. Biophys. J. 74, 175–181 (1998).
Alon, U., Surette, M. G., Barkai, N. & Leibler, S. Robustness in bacterial chemotaxis. Nature 397, 168–171 (1999). A mathematical consideration of the processes of adaptation and robustness in the bacterial chemotaxis pathway.
Kim, S. H., Wang, W. R. & Kim, K. K. Dynamic and clustering model of bacterial chemotaxis receptors: structural basis for signaling and high sensitivity. Proc. Natl Acad. Sci. USA 99, 11611–11615 (2002).
Sourjik, V. & Berg, H. C. Functional interactions between receptors in bacterial chemotaxis. Nature 428, 437–441 (2004).
Sourjik, V. & Berg, H. C. Receptor sensitivity in bacterial chemotaxis. Proc. Natl Acad. Sci. USA 99, 123–127 (2002). References 21 and 22 use fluorescence resonance energy transfer to assay the interactions between chemoreceptors in E. coli and between CheY–P and CheZ to explain sensitivity and gain in the chemotaxis pathway.
Kim, C., Jackson, M., Lux, R. & Khan, S. Determinants of chemotactic signal amplification in Escherichia coli. J. Mol. Biol. 307, 119–135 (2001).
Hess, J. F., Oosawa, K., Kaplan, N. & Simon, M. I. Phosphorylation of three proteins in the signalling pathway of bacterial chemotaxis. Cell 53, 79–87 (1988). An early report showing that the phosphorylation of chemotaxis proteins is a mechanism for signal transduction.
Anand, G. S., Goudreau, P. N. & Stock, A. M. Activation of methylesterase CheB: evidence of a dual role for the regulatory domain. Biochemistry 37, 14038–14047 (1998).
Welch, M., Oosawa, K., Aizawa, S. -I. & Eisenbach, M. Phosphorylation-dependent binding of a signal molecule to the flagellar switch of bacteria. Proc. Natl Acad. Sci. USA 90, 8787–8791 (1993).
Toker, A. S. & Macnab, R. M. Distinct regions of bacterial flagellar switch protein FliM interact with FliG, FliN and CheY. J. Mol. Biol. 273, 623–634 (1997).
McEvoy, M. M., Bren, A., Eisenbach, M. & Dahlquist, F. W. Identification of the binding interfaces on CheY for two of its targets, the phosphatase CheZ and the flagellar switch protein FliM. J. Mol. Biol. 289, 1423–1433 (1999).
Sourjik, V. & Berg, H. C. Binding of the Escherichia coli response regulator CheY to its target measured in vivo by fluorescence resonance energy transfer. Proc. Natl Acad. Sci. USA 99, 12669–12674 (2002).
Morgan, D. G., Baumgartner, J. B. & Hazelbauer, G. L. Proteins antigenically related to methyl-accepting chemotaxis proteins of Escherichia coli detected in a wide range of bacterial species. J. Bacteriol. 175, 133–140 (1993).
Falke, J. J. & Hazelbauer, G. L. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem. Sci. 26, 257–265 (2001).
Yeh, J. I. et al. High resolution structures of the ligand binding domain of the wild type aspartate receptor. J. Mol. Biol. 262, 186–201 (1996).
Kim, K. K., Yokota, H. & Kim, S. H. Four-helical-bundle structure of the cytoplasmic domain of a serine chemotaxis receptor. Nature 400, 787–792 (1999).
Milburn, M. V. et al. Structural changes in a transmembrane receptor — crystal structures of the ligand domain of aspartate chemotaxis receptor with and without aspartate. Biochemistry 31, 2192 (1992).
Mowbray, S. L. & Koshland, D. E. Jr. Additive and independent responses to a single receptor: aspartate and maltose stimuli on the Tar protein. Cell 50, 171–180 (1987).
Beel, B. D. & Hazelbauer, G. L. Substitutions in the periplasmic domain of low-abundance chemoreceptor Trg that induce or reduce transmembrane signaling: kinase activation and context effects. J. Bacteriol. 183, 671–679 (2001).
Isaac, B., Gallagher, G. J., Balazs, Y. S. & Thompson, L. K. Site-directed rotational resonance solid-state NMR distance measurements probe structure and mechanism in the transmembrane domain of the serine bacterial chemoreceptor. Biochemistry 41, 3025–3036 (2002).
Murphy, O. J., Kovacs, F. A., Sicard, E. L. & Thompson, L. K. Site-directed solid-state NMR measurement of a ligand-induced conformational change in the serine bacterial chemoreceptor. Biochemistry 40, 1358–1366 (2001).
Ottemann, K. M., Xiao, W., Shin, Y. K. & Koshland, D. E. Jr. A piston model for transmembrane signaling of the aspartate receptor. Science 285, 1751–1754 (1999).
Ames, P. & Parkinson, J. S. Transmembrane signaling by bacterial chemoreceptors: E. coli transducers with locked signal output. Cell 55, 817–826 (1988).
Surette, M. G. & Stock, J. B. Role of α-helical coiled-coil interactions in receptor dimerization, signaling, and adaptation during bacterial chemotaxis. J. Biol. Chem. 271, 17966–17973 (1996).
Storch, K. F., Rudolph, J. & Oesterhelt, D. Car: a cytoplasmic sensor responsible for arginine chemotaxis in the archaeon Halobacterium salinarum. EMBO J. 18, 1146–1158 (1999).
Wadhams, G. H. et al. TlpC, a novel chemotaxis protein in Rhodobacter sphaeroides, localizes to a discrete region in the cytoplasm. Mol. Microbiol. 46, 1211–1221 (2002).
Nishiyama, S., Maruyama, I. N., Homma, M. & Kawagishi, I. Inversion of thermosensing property of the bacterial receptor Tar by mutations in the second transmembrane region. J. Mol. Biol. 286, 1275–1284 (1999).
Appleman, J. A., Chen, L. L. & Stewart, V. Probing conservation of HAMP linker structure and signal transduction mechanism through analysis of hybrid sensor kinases. J. Bacteriol. 185, 4872–4882 (2003).
Aravind, L. & Ponting, C. P. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol. Lett. 176, 111–116 (1999).
Weis, R. M. & Koshland, D. E. Jr. Reversible receptor methylation is essential for normal chemotaxis of Escherichia coli in gradients of aspartic acid. Proc. Natl Acad. Sci. USA 85, 83–87 (1988).
Kehry, M. R., Bond, M. W., Hunkapiller, M. W. & Dahlquist, F. W. Enzymatic deamidation of methyl-accepting chemotaxis proteins in Escherichia coli catalyzed by the cheB gene product. Proc. Natl Acad. Sci. USA 80, 3599–3603 (1983).
Wu, J. G., Li, J. Y., Li, G. Y., Long, D. G. & Weis, R. M. The receptor binding site for the methyltransferase of bacterial chemotaxis is distinct from the sites of methylation. Biochemistry 35, 4984–4993 (1996).
Barnakov, A. N., Barnakova, L. A. & Hazelbauer, G. L. Comparison in vitro of a high- and a low-abundance chemoreceptor of Escherichia coli: similar kinase activation but different methyl-accepting activities. J. Bacteriol. 180, 6713–6718 (1998).
Le Moual, H., Quang, T. & Koshland, D. E. Jr. Methylation of the Escherichia coli chemotaxis receptors: intra- and interdimer mechanisms. Biochemistry 36, 13441–13448 (1997).
Maddock, J. R. & Shapiro, L. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science 259, 1717–1723 (1993). The use of immunogold electron microscopy to show for the first time that chemoreceptors cluster at the poles of bacterial cells.
Wadhams, G. H., Martin, A. C. & Armitage, J. P. Identification and localization of a methyl-accepting chemotaxis protein in Rhodobacter sphaeroides. Mol. Microbiol. 36, 1222–1233 (2000).
Thomason, P. A., Wolanin, P. M. & Stock, J. B. Signal transduction: receptor clusters as information processing arrays. Curr. Biol. 12, R399–R401 (2002).
Sourjik, V. & Berg, H. C. Localization of components of the chemotaxis machinery of Escherichia coli using fluorescent protein fusions. Mol. Microbiol. 37, 740–751 (2000).
Martin, A. C., Wadhams, G. H. & Armitage, J. P. The roles of the multiple CheW and CheA homologues in chemotaxis and in chemoreceptor localization in Rhodobacter sphaeroides. Mol. Microbiol. 40, 1261–1272 (2001).
Homma, M., Shiomi, D., Homma, M. & Kawagishi, I. Attractant binding alters arrangement of chemoreceptor dimers within its cluster at a cell pole. Proc. Natl Acad. Sci. USA 101, 3462–3467 (2004).
Studdert, C. A. & Parkinson, J. S. Crosslinking snapshots of bacterial chemoreceptor squads. Proc. Natl Acad. Sci. USA 101, 2117–2122 (2004).
Wolanin, P. M. & Stock, J. B. Bacterial chemosensing: cooperative molecular logic. Curr. Biol. 14, R486–R487 (2004).
Bray, D., Levin, M. D. & Morton, F. C. Receptor clustering as a cellular mechanism to control sensitivity. Nature 393, 85–88 (1998). One of the first papers to propose that chemoreceptor clustering could explain the sensitivity and gain in the chemotaxis pathway.
Levit, M. N., Grebe, T. W. & Stock, J. B. Organization of the receptor-kinase signaling array that regulates Escherichia coli chemotaxis. J. Biol. Chem. 277, 36748–36754 (2002).
Lamanna, A. C. et al. Conserved amplification of chemotactic responses through chemoreceptor interactions. J. Bacteriol. 184, 4981–4987 (2002).
Ames, P., Studdert, C. A., Reiser, R. H. & Parkinson, J. S. Collaborative signaling by mixed chemoreceptor teams in Escherichia coli. Proc. Natl Acad. Sci. USA 99, 7060–7065 (2002).
Li, M. & Hazelbauer, G. L. Cellular stoichiometries of the components of the chemotaxis signaling complex. J. Bacteriol 186, 3687–3694 (2004). A quantitative western-blot analysis of chemotaxis proteins in cells that were grown under different growth conditions, which showed that although the absolute numbers of the signalling components vary, the stoichiometry between them remains relatively constant.
Shimizu, T. S. et al. Molecular model of a lattice of signalling proteins involved in bacterial chemotaxis. Nature Cell Biol. 2, 792–796 (2000).
Rebbapragada, A. et al. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and transduce oxygen, redox, and energy signals for Escherichia coli behavior. Proc. Natl Acad. Sci. USA 94, 10541–10546 (1997).
Bibikov, S. I., Barnes, L. A., Gitin, Y. & Parkinson, J. S. Domain organization and flavin adenine dinucleotide-binding determinants in the aerotaxis signal transducer Aer of Escherichia coli. Proc. Natl Acad. Sci. USA 97, 5830–5835 (2000).
Fu, R., Wall, J. D. & Voordouw, G. DcrA, a c-type heme-containing methyl-accepting chemotaxis protein from Desulfovibrio vulgaris Hildenborough, senses the oxygen concentration or redox potential of the environment. J. Bacteriol. 176, 344–350 (1994).
Hou, S. et al. Myoglobin-like aerotaxis transducers in Archaea and bacteria. Nature 403, 540–544 (2000).
Lux, R. et al. Elucidation of a PTS-carbohydrate chemotactic signal pathway in Escherichia coli using a time-resolved behavioral assay. Mol. Biol. Cell 10, 1133–1146 (1999).
Boukhvalova, M., VanBruggen, R. & Stewart, R. C. CheA kinase and chemoreceptor interaction surfaces on CheW. J. Biol. Chem. 277, 23596–23603 (2002).
Griswold, I. J. et al. The solution structure and interactions of CheW from Thermotoga maritima. Nature Struct. Biol. 9, 121–125 (2002).
Shah, D. S. et al. Identification of a fourth cheY gene in Rhodobacter sphaeroides and interspecies interaction within the bacterial chemotaxis signal transduction pathway. Mol. Microbiol. 35, 101–112 (2000).
Hamblin, P. A., Bourne, N. A. & Armitage, J. P. Characterization of the chemotaxis protein CheW from Rhodobacter sphaeroides and its effect on the behaviour of Escherichia coli. Mol. Microbiol. 24, 41–51 (1997).
Morrison, T. B. & Parkinson, J. S. A fragment liberated from the Escherichia coli CheA kinase that blocks stimulatory, but not inhibitory, chemoreceptor signaling. J. Bacteriol. 179, 5543–5550 (1997).
Mourey, L. et al. Crystal structure of the CheA histidine phosphotransfer domain that mediates response regulator phosphorylation in bacterial chemotaxis. J. Biol. Chem. 276, 31074–31082 (2001).
Bilwes, A. M., Alex, L. A., Crane, B. R. & Simon, M. I. Structure of CheA, a signal-transducing histidine kinase. Cell 96, 131–141 (1999).
Bourret, R. B., Davagnino, J. & Simon, M. I. The carboxy-terminal portion of the CheA kinase mediates regulation of autophosphorylation by transducer and CheW. J. Bacteriol. 175, 2097–2101 (1993).
Levit, M. N., Liu, Y. & Stock, J. B. Mechanism of CheA protein kinase activation in receptor signaling complexes. Biochemistry 38, 6651–6658 (1999).
Li, J. Y., Swanson, R. V., Simon, M. I. & Weis, R. M. The response regulators CheB and CheY exhibit competitive binding to the kinase CheA. Biochemistry 34, 14626–14636 (1995).
Welch, M., Chinardet, N., Mourey, L., Birck, C. & Samama, J. P. Structure of the CheY-binding domain of histidine kinase CheA in complex with CheY. Nature Struct. Biol. 5, 25–29 (1998).
Stewart, R. C., Jahreis, K. & Parkinson, J. S. Rapid phosphotransfer to CheY from a CheA protein lacking the CheY-binding domain. Biochemistry 39, 13157–13165 (2000).
Hess, J. F., Bourret, R. B. & Simon, M. I. Histidine phosphorylation and phosphoryl group transfer in bacterial chemotaxis. Nature 336, 139–143 (1988).
Halkides, C. J. et al. The 1.9 Å resolution crystal structure of phosphono-CheY, an analogue of the active form of the response regulator, CheY. Biochemistry 39, 5280–5286 (2000).
Lee, S. Y. et al. Crystal structure of activated CheY — comparison with other activated receiver domains. J. Biol. Chem. 276, 16425–16431 (2001).
Cho, H. S. et al. NMR structure of activated CheY. J. Mol. Biol. 297, 543–551 (2000).
Bren, A. & Eisenbach, M. The N terminus of the flagellar switch protein, FliM, is the binding domain for the chemotactic response regulator, CheY. J. Mol. Biol. 278, 507–514 (1998).
Sagi, Y., Khan, S. & Eisenbach, M. Binding of the chemotaxis response regulator CheY to the isolated, intact switch complex of the bacterial flagellar motor — lack of cooperativity. J. Biol. Chem. 278, 25867–25871 (2003). Showed that CheY–P binds to the switch component of the bacterial flagellar motor in a non-cooperative manner, which indicates that any amplification that occurs at the motor occurs after CheY–P binding.
Lee, S. Y. et al. Crystal structure of an activated response regulator bound to its target. Nature Struct. Biol. 8, 52–56 (2001).
Da Re, S. S., Deville-Bonne, D., Tolstykh, T., Veron, M. & Stock, J. B. Kinetics of CheY phosphorylation by small molecule phosphodonors. FEBS Lett. 457, 323–326 (1999).
Barak, R. & Eisenbach, M. Acetylation of the response regulator, CheY, is involved in bacterial chemotaxis. Mol. Microbiol. 40, 731–743 (2001).
Blat, Y. & Eisenbach, M. Oligomerization of the phosphatase CheZ upon interaction with the phosphorylated form of CheY — the signal protein of bacterial chemotaxis. J. Biol. Chem. 271, 1226–1231 (1996).
Blat, Y. & Eisenbach, M. Mutants with defective phosphatase activity show no phosphorylation-dependent oligomerization of CheZ. The phosphatase of bacterial chemotaxis. J. Biol. Chem. 271, 1232–1236 (1996).
Zhao, R., Collins, E. J., Bourret, R. B. & Silversmith, R. E. Structure and catalytic mechanism of the E. coli chemotaxis phosphatase CheZ. Nature Struct. Biol. 9, 570–575 (2002).
Sourjik, V. & Schmitt, R. Phosphotransfer between CheA, CheY1, and CheY2 in the chemotaxis signal transduction chain of Rhizobium meliloti. Biochemistry 37, 2327–2335 (1998). Identified an alternative signal-termination mechanism, which uses a phosphate sink in a bacterial species that lacks CheZ.
Karatan, E., Saulmon, M. M., Bunn, M. W. & Ordal, G. W. Phosphorylation of the response regulator CheV is required for adaptation to attractants during Bacillus subtilis chemotaxis. J. Biol. Chem. 276, 43618–43626 (2001).
Pittman, M. S., Goodwin, M. & Kelly, D. J. Chemotaxis in the human gastric pathogen Helicobacter pylori: different roles for CheW and the three CheV paralogues, and evidence for CheV2 phosphorylation. Microbiology 147, 2493–2504 (2001).
Jiang, Z. Y. & Bauer, C. E. Analysis of a chemotaxis operon from Rhodospirillum centenum. J. Bacteriol. 179, 5712–5719 (1997).
Porter, S. L. & Armitage, J. P. Phosphotransfer in Rhodobacter sphaeroides chemotaxis. J. Mol. Biol. 324, 35–45 (2002). Showed that different CheA proteins from R. sphaeroides differentially phosphorylate specific RRs.
Springer, W. R. & Koshland, D. E. Jr. Identification of a protein methyltransferase as the cheR gene product in the bacterial sensing system. Proc. Natl Acad. Sci. USA 74, 533–537 (1977).
Kehry, M. R. & Dahlquist, F. W. Adaptation in bacterial chemotaxis: CheB-dependent modification permits additional methylations of sensory transducing proteins. Cell 29, 761–772 (1982).
Kehry, M. R., Doak, T. G. & Dahlquist, F. W. Sensory adaptation in bacterial chemotaxis — regulation of demethylation. J. Bacteriol. 163, 983–990 (1985).
Djordjevic, S. & Stock, A. M. Chemotaxis receptor recognition by protein methyltransferase CheR. Nature Struct. Biol. 5, 446–450 (1998).
Djordjevic, S. & Stock, A. M. Crystal structure of the chemotaxis receptor methyltransferase CheR suggests a conserved structural motif for binding S-adenosylmethionine. Structure 5, 545–558 (1997).
Shiomi, D., Zhulin, I. B., Homma, M. & Kawagishi, I. Dual recognition of the bacterial chemoreceptor by chemotaxis-specific domains of the CheR methyltransferase. J. Biol. Chem. 277, 42325–42333 (2002).
Djordjevic, S., Goudreau, P. N., Xu, Q., Stock, A. M. & West, A. H. Structural basis for methylesterase CheB regulation by a phosphorylation-activated domain. Proc. Natl Acad. Sci. USA 95, 1381–1386 (1998).
Anand, G. S. & Stock, A. M. Kinetic basis for the stimulatory effect of phosphorylation on the methylesterase activity of CheB. Biochemistry 41, 6752–6760 (2002).
Levit, M. N., Liu, Y. & Stock, J. B. Stimulus response coupling in bacterial chemotaxis: receptor dimers in signalling arrays. Mol. Microbiol. 30, 459–466 (1998).
Szurmant, H. & Ordal, G. W. Diversity in chemotaxis mechanisms among the bacteria and Archaea. Microbiol. Mol. Biol. Rev. 68, 301–319 (2004).
Bischoff, D. S., Bourret, R. B., Kirsch, M. L. & Ordal, G. W. Purification and characterization of Bacillus subtilis CheY. Biochemistry 32, 9256–9261 (1993).
Zimmer, M. A., Tiu, J., Collins, M. A. & Ordal, G. W. Selective methylation changes on the Bacillus subtilis chemotaxis receptor McpB promote adaptation. J. Biol. Chemistry 275, 24264–24272 (2000).
Nordmann, B. et al. Identification of volatile forms of methyl groups released by Halobacterium salinarium. J. Biol. Chem. 269, 16449–16454 (1994).
Thoelke, M. S., Kirby, J. R. & Ordal, G. W. Novel methyl transfer during chemotaxis in Bacillus subtilis. Biochemistry 28, 5585–5589 (1989).
Kirby, J. R., Kristich, C. J., Feinberg, S. L. & Ordal, G. W. Methanol production during chemotaxis to amino acids in Bacillus subtilis. Mol. Microbiol. 24, 869–878 (1997).
Kirsch, M. L., Peters, P. D., Hanlon, D. W., Kirby, J. R. & Ordal, G. W. Chemotactic methylesterase promotes adaptation to high concentrations of attractant in Bacillus subtilis. J. Biol. Chem. 268, 18610–18616 (1993).
Rosario, M. M. & Ordal, G. W. CheC and CheD interact to regulate methylation of Bacillus subtilis methyl-accepting chemotaxis proteins. Mol. Microbiol. 21, 511–518 (1996).
Szurmant, H., Muff, T. J. & Ordal, G. W. Bacillus subtilis CheC and FliY are members of a novel class of CheY–P-hydrolyzing proteins in the chemotactic signal transduction cascade. J. Biol. Chem. 279, 21787–21792 (2004). Identified roles for extra chemotaxis proteins in B. subtilis.
Porter, S. L., Warren, A. V., Martin, A. C. & Armitage, J. P. The third chemotaxis locus of Rhodobacter sphaeroides is essential for chemotaxis. Mol. Microbiol. 46, 1081–1094 (2002).
Wadhams, G. H., Warren, A. V., Martin, A. C. & Armitage, J. P. Targeting of two signal transduction pathways to different regions of the bacterial cell. Mol. Microbiol. 50, 763–770 (2003). Showed for the first time that the components of two chemotaxis pathways are physically separated within a bacterial cell.
Porter, S. L. & Armitage, J. P. Chemotaxis in Rhodobacter sphaeroides requires an atypical histidine protein kinase. J. Biol. Chem. 12 Oct 2004 (doi:10.1074/jbc.M408855200).
O'Toole, R. et al. The chemotactic response of Vibrio anguillarum to fish intestinal mucus is mediated by a combination of multiple mucus components. J. Bacteriol. 181, 4308–4317 (1999).
Kim, H. & Farrand, S. K. Opine catabolic loci from Agrobacterium plasmids confer chemotaxis to their cognate substrates. Mol. Plant Microbe Interact. 11, 131–143 (1998).
Zhu, J. & Mekalanos, J. J. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 5, 647–656 (2003).
Butler, S. M. & Camilli, A. Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc. Natl Acad. Sci. USA 101, 5018–5023 (2004).
Pandya, S., Iyer, P., Gaitonde, V., Parekh, T. & Desai, A. Chemotaxis of Rhizobium SP.S2 towards Cajanus cajan root exudate and its major components. Curr. Microbiol. 38, 205–209 (1999).
Millikan, D. S. & Ruby, E. G. FlrA, a σ54-dependent transcriptional activator in Vibrio fischeri, is required for motility and symbiotic light-organ colonization. J. Bacteriol. 185, 3547–3557 (2003).
Stoodley, P., Sauer, K., Davies, D. G. & Costerton, J. W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56, 187–209 (2002).
Costerton, J. W. Anaerobic biofilm infections in cystic fibrosis. Mol. Cell 10, 699–700 (2002).
Taga, M. E. & Bassler, B. L. Chemical communication among bacteria. Proc. Natl Acad. Sci. USA 100, 14549–14554 (2003).
Berg, H. C. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72, 19–54 (2003). A comprehensive review of the mechanism of rotation of the bacterial flagellar motor.
Atsumi, T., McCarter, L. & Imae, Y. Polar and lateral flagellar motors of marine Vibrio are driven by different ion-motive forces. Nature 355, 182–184 (1992).
Mattick, J. S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56, 289–314 (2002).
Kaiser, D. Coupling cell movement to multicellular development in Myxobacteria. Nature Rev. Microbiol. 1, 45–54 (2003).
McBride, M. J. Bacterial gliding motility: multiple mechanisms for cell movement over surfaces. Annu. Rev. Microbiol. 55, 49–75 (2001).
Wolgemuth, C. W., Igoshin, O. & Oster, G. The motility of mollicutes. Biophys. J. 85, 828–842 (2003).
Armitage, J. P., Pitta, T. P., Vigeant, M. A., Packer, H. L. & Ford, R. M. Transformations in flagellar structure of Rhodobacter sphaeroides and possible relationship to changes in swimming speed. J. Bacteriol. 181, 4825–4833 (1999).
Macnab, R. M. How bacteria assemble flagella. Annu. Rev. Microbiol. 57, 77–100 (2003). A review of the process of bacterial flagella assembly.
Hueck, C. J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62, 379–433 (1998).
Oster, G. & Wang, H. Rotary protein motors. Trends Cell Biol. 13, 114–121 (2003).
Shi, W., Kohler, T. & Zusman, D. R. Chemotaxis plays a role in the social behaviour of Myxococcus xanthus. Mol. Microbiol. 9, 601–611 (1993).
Shi, W. Y., Yang, Z. M., Sun, H., Lancero, H. & Tong, L. M. Phenotypic analyses of frz and dif double mutants of Myxococcus xanthus. FEMS Microbiol. Lett. 192, 211–215 (2000).
Kirby, J. R. & Zusman, D. R. Chemosensory regulation of developmental gene expression in Myxococcus xanthus. Proc. Natl Acad. Sci. USA 100, 2008–2013 (2003). Provides an example of an operon that encodes chemotaxis-protein homologues that are not involved in the regulation of bacterial motility.
Koradi, R., Billeter, M. & Wuthrich, K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 14, 51–61 (1996).
Bray, D. Genomics: molecular prodigality. Science 299, 1189–1190 (2003).
Acknowledgements
We would like to thank the Biotechnology and Biological Sciences Research Council for funding the research on R. sphaeroides behaviour that has been carried out in our laboratory, and we apologise to the many authors whose work we have not been able to include because of space restraints.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
Interpro
Protein Data Bank
FURTHER INFORMATION
Glossary
- HAMP DOMAIN
-
('Histidine kinases, adenylyl cyclases, methyl-binding proteins and phosphatases' domain). A domain that is broadly conserved in histidine protein kinases, chemoreceptors and phosphatases. It contains two amphipathic helices, and is presumed to have a role in signal transduction.
- NODULE
-
A swelling on the roots of nitrogen-fixing plants that contains symbiotic nitrogen-fixing bacteria.
- QUORUM SENSING
-
The ability of bacteria to sense their own cell density by detecting the concentration of signalling molecules that have been released in their environment.
- PILI
-
Short, polymerized-protein projections that protrude from the bacterial cell and are used for surface attachment and twitching motility.
- PAS DOMAIN
-
(PER, ARNT, SIM domain). A domain that is involved in recognizing stimuli such as light, oxygen, redox potential, energy status and small ligands.
- HPt DOMAIN
-
(Histidine-containing phosphotransfer domain). These domains are ∼120 amino acids long and contain a histidine residue that can participate in phosphoryl-transfer reactions. They function as phosphoreceivers and phosphodonors, and can therefore be used to create multistep phosphorelay systems.
- HYPERFLAGELLATE
-
An increased number of flagella.
- GAIN
-
A parameter of chemotactic behaviour that describes the relationship between the stimulus and the response. It is defined as the fractional increase in anticlockwise motor bias divided by the fractional change in receptor occupancy.
- CHEMORECEPTOR
-
A sensory receptor that responds to chemical stimuli. The term covers transmembrane receptors (such as the methyl-accepting chemotaxis proteins) and cytoplasmic receptors (such as transducer-like proteins).
- METHYL-ACCEPTING CHEMOTAXIS PROTEIN
-
(MCP). A transmembrane chemoreceptor that shows methylation-dependent adaptation.
- FLAVIN ADENINE DINUCLEOTIDE
-
(FAD). FAD functions as a redox centre in many proteins.
- SRC-HOMOLOGY-3 DOMAIN
-
(SH3 domain). A 60-amino-acid domain that mediates the assembly of specific protein complexes through binding to proline-rich peptides. It is found in many proteins that are involved in signal transduction and membrane–cytoskeleton interactions.
- TRANSDUCER-LIKE PROTEIN
-
(Tlp). Tlp proteins are cytoplasmic chemoreceptors that have the cytoplasmic domain of methyl-accepting chemotaxis proteins and lack transmembrane domains.
Rights and permissions
About this article
Cite this article
Wadhams, G., Armitage, J. Making sense of it all: bacterial chemotaxis. Nat Rev Mol Cell Biol 5, 1024–1037 (2004). https://doi.org/10.1038/nrm1524
Issue Date:
DOI: https://doi.org/10.1038/nrm1524
This article is cited by
-
Bacterial c-di-GMP signaling gene affects mussel larval metamorphosis through outer membrane vesicles and lipopolysaccharides
npj Biofilms and Microbiomes (2024)
-
Harnessing synthetic active particles for physical reservoir computing
Nature Communications (2024)
-
Transcriptome-wide marker gene expression analysis of stress-responsive sulfate-reducing bacteria
Scientific Reports (2023)
-
Negative chemotaxis of Ligilactobacillus agilis BKN88 against gut-derived substances
Scientific Reports (2023)
-
Flagellar dynamics reveal fluctuations and kinetic limit in the Escherichia coli chemotaxis network
Scientific Reports (2023)