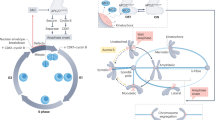
Abstract
Faithful chromosome segregation during mitosis is essential for genome integrity and is mediated by the bi-oriented attachment of replicated chromosomes to spindle microtubules through kinetochores. Errors in kinetochore–microtubule (k–MT) attachment that could cause chromosome mis-segregation are frequent and are corrected by the dynamic turnover of k–MT attachments. Thus, regulating the rate of spindle microtubule attachment and detachment to kinetochores is crucial for mitotic fidelity and is frequently disrupted in cancer cells displaying chromosomal instability. A model based on homeostatic principles involving receptors, a core control network, effectors and feedback control may explain the precise regulation of k–MT attachment stability during mitotic progression to ensure error-free mitosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cimini, D. & Degrassi, F. Aneuploidy: a matter of bad connections. Trends Cell Biol. 15, 442–451 (2001).
Cimini, D. Merotelic kinetochore orientation, aneuploidy, and cancer. Biochim. Biophys. Acta. 1786, 32–40 (2008).
Weaver, B. A. & Cleveland, D. W. Aneuploidy: instigator and inhibitor of tumorigenesis. Cancer Res. 67, 10103–10105 (2006).
Siegel, J. J. & Amon, A. New insights into the troubles of aneuploidy. Annu. Rev. Cell Devel. Biol. 28, 189–214 (2012).
Duijf, P. H. & Benezra, R. The cancer biology of whole-chromosome instability. Oncogene 32, 4727–4736 (2013).
Cheeseman, I. The kinetochore. Cold Spring Harb. Perspect. Biol. 6, a015826 (2014).
Cimini, D., Moree, B., Canman, J. C. & Salmon, E. D. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J. Cell Sci. 116, 4213–4225 (2003).
Salmon, E. D., Cimini, D., Cameron, L. A. & DeLuca, J. G. Merotelic kinetochores in mammalian tissue cells. Phil. Trans. R. Soc. 360, 553–568 (2005).
Rieder, C. L. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int. Rev. Cytol. 79, 1–58 (1982).
Magidson, V. et al. The spatial arrangement of chromosomes during prometaphase facilitates spindle assembly. Cell 146, 555–567 (2011).
Maure, J. F. et al. The Ndc80 looop region facilitates formation of kinetochore attachment to the dynamic microtubule plus end. Curr. Biol. 21, 207–213 (2011).
Shrestha, R. L. & Draviam, V. M. Lateral to end-on conversion of chromosome-microtubule attachment requires kinesins CENP-E and MCAK. Curr. Biol. 23, 1514–1526 (2013).
Cai, S., O'Connell, C. B., Khodjakov, A. & Walczak, C. E. Chromosome congression in the absence of kinetochore fibers. Nat. Cell Biol. 11, 832–838 (2009).
Tanaka, K. et al. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature 434, 987–994 (2005).
Maiato, H., DeLuca, J., Salmon, E. D. & Earnshaw, W. C. The dynamic kinetochore-microtubule interface. J. Cell Sci. 117, 5461–5477 (2004).
Nicklas, R. B. & Ward, S. C. Elements of error correction in mitosis: microtubule capture, release, and tension. J. Cell Biol. 126, 1241–1253 (1994).
Li, S. & Nicklas, R. B. Mitotic forces control a cell-cycle checkpoint. Nature 373, 630–632 (1995).
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instabilities in human cancers. Nature 396, 643–649 (1998).
Thompson, S. L. & Compton, D. A. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 180, 665–672 (2008).
Thompson, S. L., Bakhoum, S. F. & Compton, D. A. Mechanisms of chromosomal instability. Curr. Biol. 20, R285–R295 (2010).
Zhai, Y., Kronebusch, P. J. & Borisy, G. G. Kinetochore microtubule dynamics and the metaphase-anaphase transition. J. Cell Biol. 131, 721–734 (1995).
Bakhoum, S. F., Genovese, G. & Compton, D. A. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr. Biol. 19, 1937–1942 (2009).
Bakhoum, S. F., Thompson, S. L., Manning, A. L. & Compton, D. A. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat. Cell Biol. 11, 27–35 (2009).
Kabeche, L. & Compton, D. A. Cyclin A regulates kinetochore microtubules to promote faithful chromosome segregation. Nature 502, 110–113 (2013).
Stolz, A. et al. The CHIF2-BRCA1 tumor suppressor pathway ensures chromosomal stability in human somatic cells. Nat. Cell Biol. 12, 492–499 (2010).
Bakhoum, S. F., Danilova, O. V., Kaur, P., Levy, N. B. & Compton, D. A. Chromosomal instability substantiates poor prognosis in patients with diffuse large B-cell lymphoma. Clin. Cancer Res. 17, 7704–7711 (2011).
Hecht, B. K. et al. Cytogenetics of malignant gliomas: I. The autosomes with reference to rearrangements. Cancer Genet. Cytogenet. 84, 1–8 (1995).
Bakhoum, S. F. & Compton, D. A. Kinetochores and disease: keeping microtubule dynamics in check! Curr. Opin. Cell Biol. 24, 64–70 (2012).
Carmena, M., Wheelock, M., Funabiki, H. & Earnshaw, W. C. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell. Biol. 13, 789–803 (2012).
Vader, G., Medema, R. H. & Lens, S. M. The chromosomal passenger complex: guiding Aurora B through mitosis. J. Cell Biol. 173, 833–837 (2006).
Lampson, M. A. & Cheeseman, I. M. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 21, 133–140 (2011).
Cimini, D., Wan, X., Hirel, C. B. & Salmon, E. D. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr. Biol. 16, 1711–1718 (2006).
Shannon, K. B. & Salmon, E. D. Chromosome dynamics: new light on Aurora B kinase function. Curr. Biol. 12, R458–R460 (2002).
Liu, D., Vader, G., Vromans, M. J., Lampson, M. A. & Lens, S. M. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science 323, 1350–1353 (2009).
Wang, E., Ballister, E. R. & Lampson, M. A. Aurora B dynamics at centromeres create a diffusion-based phosphorylation gradient. J. Cell Biol. 194, 539–549 (2011).
Welburn, J. P. et al. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol. Cell 38, 383–392 (2010).
Campbell, C. S. & Desai, A. Tension sensing by Aurora B kinase is independent of survivin-based centromere localization. Nature 497, 118–121 (2013).
DeLuca, K. F., Lens, S. M. & DeLuca, J. G. Temporal changes in Hec1 phosphorylation control kinetochore-microtubule attachment stability during mitosis. J. Cell Sci. 124, 622–634 (2011).
Skibbens, R. V., Skeen, V. P. & Salmon, E. D. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: a push-pull mechanism. J. Cell Biol. 122, 859–875 (1993).
Jaqaman, K. et al. Kinetochore alignment within the metaphase plate is regulated by centromere stiffness and microtubule depolymerases. J. Cell Biol. 188, 665–679 (2010).
Lara-Gonzalez, P., Westhorpe, F. G. & Taylor, S. S. The spindle assembly checkpoint. Curr. Biol. 22, R966–R980 (2012).
Bruinsma, W., Baaijmakers, J. A. & Medema, R. H. Switching Polo-like kinase-1 on and off in time and space. Trends Biochem. Sci. 37, 534–542 (2012).
Maffini, S. et al. Motor-independent targeting of CLASPs to kinetochores by CENP-E promotes microtubule turnover and poleward flux. Curr. Biol. 19, 1566–1572 (2009).
Maia, A. R. et al. Cdk1 and Plk1 mediate a CLASP2 phospho-switch that stabilizes kinetochore-microtubule attachments. J. Cell Biol. 199, 285–301 (2012).
Manning, A. L., Bakhoum, S. F., Maffini, S., Correia-Melo, C. Maiato, H. & Compton, D. A. CLASP1, astrin and Kif2b form a molecular switch that regulates kinetochore-microtubule dynamics to promote mitotic progression and fidelity. EMBO J. 29, 3531–3543 (2010).
DeLuca, J. G. et al. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127, 969–982 (2006).
Cheeseman, I. M., Chappie, J. S., Wilson-Kubalek, E. M. & Desai, A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127, 983–997 (2006).
Zaytsev, A. V., Sundin, L. J., DeLuca, K. F., Grishchuk, E. L. & DeLuca, J. G. Accurate phosphoregulation of kinetochore-microtubule affinity requires unconstrained molecular interactions. J. Cell Biol. 206, 45–59 (2014).
Ahonen, L. F. et al. Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr. Biol. 15, 1078–1089 (2005).
Lenart, P. et al. The small-molecule inhibitor BI2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol. 17, 304–315 (2007).
Elowe, S., Hummer, S., Uldschmid, A., Li, X. & Nigg, E. A. Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes Dev. 21, 2205–2219 (2007).
Liu, D., Davydenko, O. & Lampson, M. A. Polo-like kinase 1 regulates kinetocohore-microtubule dynamics and spindle checkpoint silencing. J. Cell Biol. 198, 491–499 (2012).
Lampson, M. A. & Kapoor, M. The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nat. Cell Biol. 7, 93–98 (2005).
Manning, A. L. et al. The kinesin-13 proteins Kif2a, Kif2b, and Kif2c/MCAK have distinct roles during mitosis in human cells. Mol. Biol. Cell 18, 2970–2979 (2007).
Hood, E. A., Kettenbach, A. N., Gerber, S. A. & Compton, D. A. Plk1 regulates the kinesin-13 protein Kif2b to promote faithful chromosome segregation. Mol. Biol. Cell 23, 2264–2274 (2012).
Carmena, M. et al. The chromosomal passenger complex activated Polo kinase at centromeres. PLoS Biol. 10, e1001250 (2012).
Kruse, T. et al. Direct binding between BubR1 and B56-PP2A phosphatase complexes regulate mitotic progression. J. Cell Sci. 126, 1086–1092 (2013).
Foley, E. A., Maldonado, M. & Kapoor, T. M. Formation of stable attachments between kinetochores and microtubules depends on the B56-PP2A phosphatase. Nat. Cell Biol. 13, 1265–1271 (2011).
Knowlton, A. L., Lan, W. & Stukenberg, P. T. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr. Biol. 16, 1705–1710 (2006).
Chu, Y. et al. Aurora B kinase activation requires survivin priming phosphorylation by PLK1. J. Mol. Cell. Biol. 3, 260–267 (2011).
Salimian, K. J. et al. Feedback control in sensing chromosome biorientation by the Aurora B kinase. Curr. Biol. 21, 1158–1165 (2011).
Goto, H. et al. Complex formation of Plk1 and INCENP required for metaphase-anaphase transition. Nature Cell Biol. 8, 180–187 (2006).
Trinkle-Mulcahy, L. et al. Time-lapse imaging reveals dynamic relocalization of PP1γ throughout the mammalian cell cycle. Mol. Biol. Cell 14, 107–117 (2003).
De Wulf, P., Montani, F. & Visintin, R. Protein phosphatases take the mitotic stage. Curr. Opin. Cell Biol. 21, 806–815 (2009).
Liu, D. et al. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J. Cell Biol. 188, 809–820 (2010).
Parry, D. H., Hickson, G. R., O'Farrell, P. H. Cyclin B destruction triggers changes in kinetochore behavior essential for successful anaphase. Curr. Biol. 13, 647–653 (2003).
Soni, D. V., Sramkoski, R. M., Lam, M., Stegan, T. & Jacobberger, J. W. Cyclin B1 is rate limiting but not essential for mitotic entry and progression in mammalian somatic cells. Cell Cycle 7, 1285–1300 (2008).
Vazquez-Novelle, M. D. et al. Cdk1 inactivation terminates mitotic checkpoint surveillance and stabilizes kinetochore attachments in anaphase. Curr. Biol. 24, 638–645 (2014).
Porter, I. M. et al. Bod1, a novel kinetochore protein required for chromosome biorientation. J. Cell Biol. 179, 187–197 (2007).
Porter, I. M., Schleicher, K., Porter, M. & Swedlow, J. R. Bod1 regulates protein phosphatase 2A at mitotic kinetochores. Nat. Commun. 4, 2677 (2013).
Collin, P., Nashchenkina, O., Walker, R. & Pines, J. The spindle assembly checkpoint works like a rheostat rather than a toggle switch. Nat. Cell Biol. 15, 1378–1385 (2013).
DiFiore, B. & Pines, J. How cyclin A destruction escapes the spindle assembly checkpoint. J. Cell Biol. 190, 501–509 (2010).
den Elzen, N. & Pines, J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 153, 121–136 (2001).
Musacchio, A. & Salmon, E. D. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell. Biol. 8, 379–393 (2007).
Maresca, T. J. & Salmon, E. D. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J. Cell Biol. 184, 373–381 (2009).
Uchida, K. S. K. et al. Kinetochore stretching inactivates the spindle assembly checkpoint. J. Cell Biol. 184, 383–390 (2009).
Foley, E. A. & Kapoor, T. M. Microtubule attachment and spindle assembly checkpoint signaling at the kinetochore. Nat. Rev. Mol. Cell. Biol. 14, 25–37 (2013).
Kettenbach, A. N. et al. Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Sci. Signal. 4, rs5 (2011).
Indjeian, V. B. & Murray, A. W. Budding yeast mitotic chromosomes have an intrinsic bias to biorient on the spindle. Curr. Biol. 17, 1837–1846 (2007).
Loncarek, J. et al. The centromere geometry essential for keeping mitosis error free is controlled by spindle forces. Nature 450, 745–749 (2007).
Salic, A., Waters, J. C. & Mitchison, T. J. Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118, 567–578 (2004).
Logarinho, E., Resende, T., Torres, C. & Bousbaa, H. The human spindle assembly checkpoint protein Bub3 is required for the establishment of efficient kinetochore-microtubule attachments. Mol. Biol. Cell. 19, 1798–1813 (2008).
Kabeche, L. & Compton, D. A. Checkpoint-independent stabilization of kinetochore-microtubule attachments by Mad2 in human cells. Curr. Biol. 22, 638–644 (2012).
Amaro, A. C. et al. Molecular control of kinetochore-microtubule dynamics and chromosome oscillations. Nature Cell Biol. 12, 319–329 (2010).
McHedlishvili, N. et al. Kinetochores accelerate centrosome separation to ensure faithful chromosome segregation. J. Cell Sci. 125, 906–918 (2012).
Ye, F. et al. HURP regulates chromosome congression by modulating kinesin Kif18A function. Curr. Biol. 21, 1584–1591 (2011).
Wang, X. et al. Mitotic regulator SKAP forms a link between kinetochore core complex KMN and dynamic spindle microtubules. J. Biol. Chem. 287, 39380–39390 (2012).
DeLuca, J. G. et al. Hec1 and Nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol. Biol. Cell. 16, 519–531 (2005).
Ishii, S., Kurasawa, Y., Wong, J. & Yu-Lee, L. Y. Histone deacetylase 3 localizes to the mitotic spindle and is required for kinetochore-microtubule attachment. Proc. Natl Acad. Sci. USA 105, 4179–4184 (2008).
Gaitanos, T. N. et al. Stable kinetochore-microtubule interactions depend on the Ska complex and its new component Ska3/C13Orf3. EMBO J. 28, 1442–1452 (2009).
Acknowledgements
The authors thank members of the laboratory for their input and feedback on the manuscript. This work was supported by grants from the US National Institutes of Health (GM51542 to D.A.C and GM008704 to L.K.) and the American Cancer Society (PF-12-103-01-CCG to K.M.G.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Godek, K., Kabeche, L. & Compton, D. Regulation of kinetochore–microtubule attachments through homeostatic control during mitosis. Nat Rev Mol Cell Biol 16, 57–64 (2015). https://doi.org/10.1038/nrm3916
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3916
This article is cited by
-
NET1 is a critical regulator of spindle assembly and actin dynamics in mouse oocytes
Reproductive Biology and Endocrinology (2024)
-
PAK2 is essential for chromosome alignment in metaphase I oocytes
Cell Death & Disease (2023)
-
The cell cycle, cancer development and therapy
Molecular Biology Reports (2022)
-
Spatially and temporally defined lysosomal leakage facilitates mitotic chromosome segregation
Nature Communications (2020)
-
Microtubule end conversion mediated by motors and diffusing proteins with no intrinsic microtubule end-binding activity
Nature Communications (2019)