Key Points
-
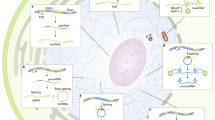
DNA- and RNA-binding proteins (DRBPs) constitute a significant fraction of cellular proteins and have important roles in cells. Their functions include control of transcription and translation, DNA repair, splicing, apoptosis and mediating stress responses.
-
Orthogonal binding of DNA and RNA provides an opportunity for competitive regulation of transcription by decoy RNAs, which can be mRNAs, tRNAs and long non-coding RNAs (lncRNAs). Simultaneous binding of DNA and RNA facilitates the assembly of RNA-tethered transcriptional complexes, allowing the recruitment of RNA-containing complexes to specific DNA loci.
-
Binding to both DNA and RNA enables DRBPs to integrate multiple signals into cellular signalling networks and allows improved gene targeting, finer control of gene expression and incorporation of metabolic states or stresses to modulate protein activity.
-
The structural features of DRBPs have been remarkably well conserved during evolution, indicating that dual nucleic acid binding confers selective advantages. DRBPs may have less stringent criteria for interacting with nucleic acids or may adjust their structure when DNA and RNA compete for the same DRBP interaction surface.
-
Nucleic acid sequence evolution has an important role in the development of DRBP function. Emerging studies of rapidly evolving lncRNAs suggest that RNA binding by proteins that were previously thought of as DNA-specific may be a widespread phenomenon.
Abstract
Proteins that bind both DNA and RNA typify the ability of a single gene product to perform multiple functions. Such DNA- and RNA-binding proteins (DRBPs) have unique functional characteristics that stem from their specific structural features; these developed early in evolution and are widely conserved. Proteins that bind RNA have typically been considered as functionally distinct from proteins that bind DNA and studied independently. This practice is becoming outdated, in partly owing to the discovery of long non-coding RNAs (lncRNAs) that target DNA-binding proteins. Consequently, DRBPs were found to regulate many cellular processes, including transcription, translation, gene silencing, microRNA biogenesis and telomere maintenance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kino, T., Hurt, D. E., Ichijo, T., Nader, N. & Chrousos, G. P. Noncoding RNA GAS5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 3, ra8 (2010). This paper shows that a widely expressed lncRNA was able to accumulate during stress and act as a decoy RNA to prevent steroid receptors from binding to their target DNA.
Ishmael, F. T. et al. The human glucocorticoid receptor as an RNA-binding protein: global analysis of glucocorticoid receptor-associated transcripts and identification of a target RNA motif. J. Immunol. 186, 1189–1198 (2011). This study shows that the GCR binds to mRNAs involved in inflammation to accelerate their degradation.
Davis, B. N., Hilyard, A. C., Nguyen, P. H., Lagna, G. & Hata, A. SMAD proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol. Cell 39, 373–384 (2010).
Shi, L. et al. Dynamic binding of Ku80, Ku70 and NF90 to the IL-2 promoter in vivo in activated T-cells. Nucleic Acids Res. 35, 2302–2310 (2007).
Shi, L., Godfrey, W. R., Lin, J., Zhao, G. & Kao, P. N. NF90 regulates inducible IL-2 gene expression in T cells. J. Exp. Med. 204, 971–977 (2007).
Xu, B. et al. Dax-1 and steroid receptor RNA activator (SRA) function as transcriptional coactivators for steroidogenic factor 1 in steroidogenesis. Mol. Cell. Biol. 29, 1719–1734 (2009).
Lanz, R. B. et al. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell 97, 17–27 (1999). This paper details the discovery of a lncRNA involved in coordinating steroid receptor-driven gene activation.
Hung, T. et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nature Genet. 43, 621–629 (2011). This study describes a high-throughput approach to identify lncRNAs that interfere with transcription factors to repress gene expression, suggesting potentially widespread roles for promoter lncRNAs in cell-growth control.
Rapicavoli, N. A. et al. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. eLife 2, e00762 (2013). This study shows that the expression of pseudogene lncRNAs is actively regulated and that these lncRNAs may interact directly with proteins to modulate cell signalling.
Lebruska, L. L. & Maher, L. J. 3rd. Selection and characterization of an RNA decoy for transcription factor NF-κB. Biochemistry 38, 3168–3174 (1999).
Duss, O. et al. Structural basis of the non-coding RNA RsmZ acting as a protein sponge. Nature 509, 588–592 (2014). This paper shows how a bacterial lncRNA acts as a 'sponge' to sequester and release proteins involved in translation.
Binns, D. et al. QuickGO: a web-based tool for gene ontology searching. Bioinformatics 25, 3045–3046 (2009).
Hu, S. et al. Profiling the human protein-DNA interactome reveals ERK2 as a transcriptional repressor of interferon signaling. Cell 139, 610–622 (2009). This paper details the use of a combined bioinformatics- and protein microarray-based strategy to systematically characterize the human protein–dsDNA interactome.
Castello, A. et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406 (2012). This study identifies the mRNA interactome by using high-throughput covalent capture techniques coupled with proteomic-based detection strategies.
Kadmiel, M. & Cidlowski, J. A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 34, 518–530 (2013).
Reddy, T. E. et al. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res. 19, 2163–2171 (2009).
Clark, A. R. & Belvisi, M. G. Maps and legends: The quest for dissociated ligands of the glucocorticoid receptor. Pharmacol. Ther. 134, 54–67 (2012).
Kanai, A., Tani, H., Torimura, M. & Akimitsu, N. The RNA degradation pathway regulates the function of GAS5 a non-coding RNA in mammalian cells. PLoS ONE 8, e55684 (2013).
Fukunaga, J. et al. The Runt domain of AML1 (RUNX1) binds a sequence-conserved RNA motif that mimics a DNA element. RNA 19, 927–936 (2013).
Gros, C. et al. DNA methylation inhibitors in cancer: recent and future approaches. Biochimie 94, 2280–2296 (2012).
Holz-Schietinger, C. & Reich, N. O. RNA modulation of the human DNA methyltransferase 3A. Nucleic Acids Res. 40, 8550–8557 (2012).
Di Ruscio, A. et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature 503, 371–376 (2013). This paper describes how RNA transcripts are able to regulate local DNA methylation by interacting with DNA methyltransferases.
Cattaneo, A., Biocca, S., Corvaja, N. & Calissano, P. Nuclear localization of a lactic dehydrogenase with single-stranded DNA-binding properties. Exp. Cell Res. 161, 130–140 (1985).
Calissano, P., Volontè, C., Biocca, S. & Cattaneo, A. Synthesis and content of a DNA-binding protein with lactic dehydrogenase activity are reduced by nerve growth factor in the neoplastic cell line PC12. Exp. Cell Res. 161, 117–129 (1985).
Pioli, P. A., Hamilton, B. J., Connolly, J. E., Brewer, G. & Rigby, W. F. Lactate dehydrogenase is an AU-rich element-binding protein that directly interacts with AUF1. J. Biol. Chem. 277, 35738–35745 (2002).
Demarse, N. A. et al. Direct binding of glyceraldehyde 3-phosphate dehydrogenase to telomeric DNA protects telomeres against chemotherapy-induced rapid degradation. J. Mol. Biol. 394, 789–803 (2009).
Singh, R. & Green, M. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science 259, 365–368 (1993). This study identifies GAPDH as a tRNA-binding protein, demonstrating in principle how tRNA levels may control metabolism and how metabolites may influence protein synthesis.
Nagy, E. & Rigby, W. F. C. Glyceraldehyde-3-phosphate dehydrogenase selectively binds AU-rich RNA in the NAD+-binding region (Rossmann fold). J. Biol. Chem. 270, 2755–2763 (1995).
Ray, R. & Miller, D. M. Cloning and characterization of a human c-Myc promoter-binding protein. Mol. Cell. Biol. 11, 2154–2161 (1991).
Hernandez-Perez, L. et al. α-enolase binds to RNA. Biochimie 93, 1520–1528 (2011).
Fletcher, L., Rider, C. C. & Taylor, C. B. Enolase isoenzymes. III. Chromatographic and immunological characteristics of rat brain enolase. Biochim. Biophys. Acta 452, 245–252 (1976).
Kato, K. et al. Immunoassay of human muscle enolase subunit in serum: a novel marker antigen for muscle diseases. Clin. Chim. Acta 131, 75–85 (1983).
Anderson, S. L., Minard, K. I. & McAlister-Henn, L. Allosteric inhibition of NAD+-specific isocitrate dehydrogenase by a mitochondrial mRNA. Biochemistry 39, 5623–5629 (2000).
Hentze, M. W. & Preiss, T. The REM phase of gene regulation. Trends Biochem. Sci. 35, 423–426 (2010).
Auphan, N., DiDonato, J. A., Rosette, C., Helmberg, A. & Karin, M. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science 270, 286–290 (1995).
Surjit, M. et al. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell 145, 224–241 (2011).
Jonat, C. et al. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell 62, 1189–1204 (1990).
Dhawan, L., Liu, B., Blaxall, B. C. & Taubman, M. B. A novel role for the glucocorticoid receptor in the regulation of monocyte chemoattractant protein-1 mRNA stability. J. Biol. Chem. 282, 10146–10152 (2007).
Yang-Yen, H.-F. et al. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein–protein interaction. Cell 62, 1205–1215 (1990).
Ray, A. & Prefontaine, K. E. Physical association and functional antagonism between the p65 subunit of transcription factor NF-κB and the glucocorticoid receptor. Proc. Natl Acad. Sci. USA 91, 752–756 (1994).
Filipowicz, W., Bhattacharyya, S. N. & Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nature Rev. Genet. 2008, 102–114 (2008).
Kim, V. N., Han, J. & Siomi, M. C. Biogenesis of small RNAs in animals. Nature Rev. Mol. Cell Biol. 10, 126–139 (2009).
Massagué, J. & Wotton, D. Transcriptional control by the TGF-β/SMAD signaling system. EMBO J. 19, 1745–1754 (2000).
Davis, B. N., Hilyard, A. C., Lagna, G. & Hata, A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454, 56–61 (2008).
Kumarswamy, R., Volkmann, I. & Thum, T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 8, 706–713 (2011).
Kao, P. N. et al. Cloning and expression of cyclosporin A- and FK506-sensitive nuclear factor of activated T-cells: NF45 and NF90. J. Biol. Chem. 269, 20691–20699 (1994).
Kuwano, Y. et al. NF90 selectively represses the translation of target mRNAs bearing an AU-rich signature motif. Nucleic Acids Res. 38, 225–238 (2009).
Shim, J., Lim, H., R. Yates Iii, J. & Karin, M. Nuclear export of NF90 is required for interleukin-2 mRNA stabilization. Mol. Cell 10, 1331–1344 (2002).
Sakamoto, S. et al. The NF90–NF45 complex functions as a negative regulator in the microRNA processing pathway. Mol. Cell. Biol. 29, 3754–3769 (2009).
Malek, T. R. & Castro, I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity 33, 153–165 (2010).
Dienz, O. & Rincon, M. The effects of IL-6 on CD4 T cell responses. Clim. Immunol. 130, 27–33 (2009).
Iliopoulos, D., Hirsch, H. A. & Struhl, K. An epigenetic switch involving NF-κB, lin28, let-7 microRNA, and IL6 links inflammation to cell transformation. Cell 139, 693–706 (2009).
Takamizawa, J. et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 64, 3753–3756 (2004).
Guo, N. L. et al. Confirmation of gene expression-based prediction of survival in non-small cell lung cancer. Clin. Cancer Res. 14, 8213–8220 (2008).
Ng, S.-Y., Bogu, G. K., Soh, B. S. & Stanton, L. W. The long noncoding RNA RMST interacts with SOX2 to regulate neurogenesis. Mol. Cell 51, 349–359 (2013).
Sarkar, A. & Hochedlinger, K. The Sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 12, 15–30 (2013).
Ng, S.-Y., Johnson, R. & Stanton, L. W. Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J. 31, 522–533 (2012). References 55 and 57 describe how the lncRNA RMST physically interacts with the transcription factor SOX2 to co-regulate a large pool of downstream genes implicated in neurogenesis.
Feng, J. et al. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 20, 1470–1484 (2006).
Bond, A. M. et al. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nature Neurosci. 12, 1020–1027 (2009).
Jeffery, L. & Nakielny, S. Components of the DNA methylation system of chromatin control are RNA-binding proteins. J. Biol. Chem. 279, 49479–49487 (2004).
Wassenegger, M., Heimes, S., Riedel, L. & Sänger, H. L. RNA-directed de novo methylation of genomic sequences in plants. Cell 76, 567–576 (1994).
Schmitz, K. M., Mayer, C., Postepska, A. & Grummt, I. Interaction of noncoding RNA with the rDNA promoter mediates recruitment of DNMT3b and silencing of rRNA genes. Genes Dev. 24, 2264–2269 (2010).
Morris, K. V., Chan, S. W., Jacobsen, S. E. & Looney, D. J. Small interfering RNA-induced transcriptional gene silencing in human cells. Science 305, 1289–1292 (2004).
Sun, B. K., Deaton, A. M. & Lee, J. T. A transient heterochromatic state in Xist preempts X inactivation choice without RNA stabilization. Mol. Cell 21, 617–628 (2006).
Zazopoulos, E., Lalli, E., Stocco, D. M. & Sassone-Corsi, P. DNA binding and transcriptional repression by DAX-1 blocks steroidogenesis. Nature 390, 311–315 (1997).
Kabe, Y. et al. The role of human MBF1 as a transcriptional coactivator. J. Biol. Chem. 274, 34196–34202 (1999).
Xu, B. & Koenig, R. J. An RNA-binding domain in the thyroid hormone receptor enhances transcriptional activation. J. Biol. Chem. 279, 33051–33056 (2004).
Zhao, X. et al. Regulation of nuclear receptor activity by a pseudouridine synthase through posttranscriptional modification of steroid receptor RNA activator. Mol. Cell 15, 549–558 (2004).
Watanabe, M. et al. A subfamily of RNA-binding DEAD-box proteins acts as an estrogen receptor α coactivator through the N-terminal activation domain (AF-1) with an RNA coactivator, SRA. EMBO J. 20, 1341–1352 (2001).
Deblois, G. & Giguere, V. Ligand-independent coactivation of ERα AF-1 by steroid receptor RNA activator (SRA) via MAPK activation. J. Steroid Biochem. Mol. Biol. 85, 123–131 (2003).
Poon, M. M. & Chen, L. Retinoic acid-gated sequence-specific translational control by RARα. Proc. Natl Acad. Sci. USA 105, 20303–20308 (2008).
van Steensel, B., Smogorzewska, A. & de Lange, T. TRF2 protects human telomeres from end-to-end fusions. Cell 92, 401–413 (1998).
Court, R., Chapman, L., Fairall, L. & Rhodes, D. How the human telomeric proteins TRF1 and TRF2 recognize telomeric DNA: a view from high-resolution crystal structures. EMBO Rep. 6, 39–45 (2005).
Chesnokov, I. N. in International Review of Cytolology. Vol. 256 (ed. Kwang, W. J.) 69–109 (Academic Press, 2007).
Deng, Z., Dheekollu, J., Broccoli, D., Dutta, A. & Lieberman, P. M. The origin recognition complex localizes to telomere repeats and prevents telomere-circle formation. Curr. Biol. 17, 1989–1995 (2007).
Deng, Z., Norseen, J., Wiedmer, A., Riethman, H. & Lieberman, P. M. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol. Cell 35, 403–413 (2009).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protoc. 4, 44–57 (2009).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 (2009).
Maris, C., Dominguez, C. & Allain, F. H. T. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 272, 2118–2131 (2005).
Fiset, S. & Chabot, B. hnRNP A1 may interact simultaneously with telomeric DNA and the human telomerase RNA in vitro. Nucleic Acids Res. 29, 2268–2275 (2001).
Bahadur, R. P., Zacharias, M. & Janin, J. Dissecting protein–RNA recognition sites. Nucleic Acids Res. 36, 2705–2716 (2008).
Allers, J. & Shamoo, Y. Structure-based analysis of protein–RNA interactions using the program ENTANGLE. J. Mol. Biol. 311, 75–86 (2001).
Buratti, E. et al. Nuclear factor TDP43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 20, 1774–1784 (2001).
Kawahara, Y. & Mieda-Sato, A. TDP43 promotes microRNA biogenesis as a component of the Drosha and Dicer complexes. Proc. Natl Acad. Sci. USA 109, 3347–3352 (2012).
Lukavsky, P. J. et al. Molecular basis of UG-rich RNA recognition by the human splicing factor TDP43. Nature Struct. Mol. Biol. 20, 1443–1449 (2013).
Kuo, P. H., Doudeva, L. G., Wang, Y. T., Shen, C. K. J. & Yuan, H. S. Structural insights into TDP43 in nucleic-acid binding and domain interactions. Nucleic Acids Res. 37, 1799–1808 (2009).
Deo, R. C., Bonanno, J. B., Sonenberg, N. & Burley, S. K. Recognition of polyadenylate RNA by the poly(A)-binding protein. Cell 98, 835–845 (1999).
Hayden, M. S. & Ghosh, S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 26, 203–234 (2012).
Wurster, S. E. & Maher, L. J. Selection and characterization of anti-NF-κB p65 RNA aptamers. RNA 14, 1037–1047 (2008).
Huang, D. B. et al. Crystal structure of NF-κB (p50)2 complexed to a high-affinity RNA aptamer. Proc. Natl Acad. Sci. USA 100, 9268–9273 (2003).
Müller, C. W., Rey, F. A., Sodeoka, M., Verdine, G. L. & Harrison, S. C. Structure of the NF-κB p50 homodimer bound to DNA. Nature 373, 311–317 (1995).
Lu, X.-J. & Olson, W. K. 3DNA: a versatile, integrated software system for the analysis, rebuilding and visualization of three-dimensional nucleic-acid structures. Nature Protoc. 3, 1213–1227 (2008).
Barrick, J. E., Sudarsan, N., Weinberg, Z., Ruzzo, W. L. & Breaker, R. R. 6S RNA is a widespread regulator of eubacterial RNA polymerase that resembles an open promoter. RNA 11, 774–784 (2005).
Schwartz, T., Rould, M. A., Lowenhaupt, K., Herbert, A. & Rich, A. Crystal structure of the Zα domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA. Science 284, 1841–1845 (1999).
Placido, D., Brown, B. A., Lowenhaupt, K., Rich, A. & Athanasiadis, A. A left-handed RNA double helix bound by the Zα domain of the RNA-editing enzyme ADAR1. Structure 15, 395–404 (2007).
Goll, M. G. et al. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science 311, 395–398 (2006). This study shows that the DNA methylase DNMT2 evolved the ability to methylate tRNA.
Didier, D. K., Schiffenbauer, J., Woulfe, S. L., Zacheis, M. & Schwartz, B. D. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc. Natl Acad. Sci. USA 85, 7322–7326 (1988).
Stickeler, E. et al. The RNA binding protein YB-1 binds A/C-rich exon enhancers and stimulates splicing of the CD44 alternative exon v4. EMBO J. 20, 3821–3830 (2001).
Skabkina, O. V., Lyabin, D. N., Skabkin, M. A. & Ovchinnikov, L. P. YB-1 autoregulates translation of its own mRNA at or prior to the step of 40S ribosomal subunit joining. Mol. Cell. Biol. 25, 3317–3323 (2005).
Evdokimova, V. et al. The major mRNA-associated protein YB-1 is a potent 5′ cap-dependent mRNA stabilizer. EMBO J. 20, 5491–5502 (2001).
Marenstein, D. R. et al. Stimulation of human endonuclease III by Y box-binding protein 1 (DNA-binding protein B). Interaction between a base excision repair enzyme and a transcription factor. J. Biol. Chem. 276, 21242–21249 (2001).
de Souza-Pinto, N. C. et al. Novel DNA mismatch-repair activity involving YB-1 in human mitochondria. DNA Repair 8, 704–719 (2009).
Ise, T. et al. Transcription factor Y-box binding protein 1 binds preferentially to cisplatin-modified DNA and interacts with proliferating cell nuclear antigen. Cancer Res. 59, 342–346 (1999).
Stein, U. et al. Hyperthermia-induced nuclear translocation of transcription factor YB-1 leads to enhanced expression of multidrug resistance-related ABC transporters. J. Biol. Chem. 276, 28562–28569 (2001).
Koike, K. et al. Nuclear translocation of the Y-box binding protein by ultraviolet irradiation. FEBS Lett. 417, 390–394 (1997).
Yamanaka, K., Fang, L. & Inouye, M. The CspA family in Escherichia coli: multiple gene duplication for stress adaptation. Mol. Microbiol. 27, 247–255 (1998).
Etchegaray, J. P., Jones, P. G. & Inouye, M. Differential thermoregulation of two highly homologous cold-shock genes, cspA and cspB, of Escherichia coli. Genes Cells 1, 171–178 (1996).
Wang, N., Yamanaka, K. & Inouye, M. CspI, the ninth member of the CspA family of Escherichia coli, is induced upon cold shock. J. Bacteriol. 181, 1603–1609 (1999).
Nakashima, K., Kanamaru, K., Mizuno, T. & Horikoshi, K. A novel member of the cspA family of genes that is induced by cold shock in Escherichia coli. J. Bacteriol. 178, 2994–2997 (1996).
Goldstein, J., Pollitt, N. S. & Inouye, M. Major cold shock protein of Escherichia coli. Proc. Natl Acad. Sci. USA 87, 283–287 (1990).
Xia, B., Ke, H. & Inouye, M. Acquirement of cold sensitivity by quadruple deletion of the cspA family and its suppression by PNPase S1 domain in Escherichia coli. Mol. Microbiol. 40, 179–188 (2001).
Yamanaka, K. & Inouye, M. Growth-phase-dependent expression of cspD, encoding a member of the CspA family in Escherichia coli. J. Bacteriol. 179, 5126–5130 (1997).
Phadtare, S. & Inouye, M. Role of CspC and CspE in regulation of expression of RpoS and UspA, the stress response proteins in Escherichia coli. J. Bacteriol. 183, 1205–1214 (2001).
Jiang, W., Hou, Y. & Inouye, M. CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J. Biol. Chem. 272, 196–202 (1997).
Phadtare, S. & Inouye, M. Sequence-selective interactions with RNA by CspB, CspC and CspE, members of the CspA family of Escherichia coli. Mol. Endocrinol. 33, 1004–1014 (1999).
Hanna, M. M. & Liu, K. Nascent RNA in transcription complexes interacts with CspE, a small protein in E. coli implicated in chromatin condensation. J. Mol. Biol. 282, 227–239 (1998).
Bae, W., Xia, B., Inouye, M. & Severinov, K. Escherichia coli CspA-family RNA chaperones are transcription antiterminators. Proc. Natl Acad. Sci. USA 97, 7784–7789 (2000).
Yamanaka, K., Zheng, W., Crooke, E., Wang, Y. H. & Inouye, M. CspD, a novel DNA replication inhibitor induced during the stationary phase in Escherichia coli. Mol. Microbiol. 39, 1572–1584 (2001).
Chen, C. S. et al. A proteome ChIP approach reveals new DNA damage recognition activities in Escherichia coli. Nature Methods 5, 69–74 (2008).
Hu, K. H. et al. Overproduction of three genes leads to camphor resistance and chromosome condensation in Escherichia coli. Genetics 143, 1521–1532 (1996).
Karlson, D. & Imai, R. Conservation of the cold shock domain protein family in plants. Plant Physiol. 131, 12–15 (2003).
Karlson, D., Nakaminami, K., Toyomasu, T. & Imai, R. A cold-regulated nucleic acid-binding protein of winter wheat shares a domain with bacterial cold shock proteins. J. Biol. Chem. 277, 35248–35256 (2002).
Nakaminami, K., Karlson, D. T. & Imai, R. Functional conservation of cold shock domains in bacteria and higher plants. Proc. Natl Acad. Sci. USA 103, 10122–10127 (2006).
Imai, R., Kim, M. H., Sasaki, K., Sato, S. & Sonoda, Y. Plant and Microbe Adaptations to Cold in a Changing World (Springer, 2013).
Juntawong, P., Sorenson, R. & Bailey-Serres, J. Cold shock protein 1 chaperones mRNAs during translation in Arabidopsis thaliana. Plant J. 74, 1016–1028 (2013).
Kim, J. S. et al. Cold shock domain proteins and glycine-rich RNA-binding proteins from Arabidopsis thaliana can promote the cold adaptation process in Escherichia coli. Nucleic Acids Res. 35, 506–516 (2007).
Triqueneaux, G., Velten, M., Franzon, P., Dautry, F. & Jacquemin-Sablon, H. RNA binding specificity of Unr, a protein with five cold shock domains. Nucleic Acids Res. 27, 1926–1934 (1999).
Eliseeva, I. A., Kim, E. R., Guryanov, S. G., Ovchinnikov, L. P. & Lyabin, D. N. Y-box-binding protein 1 (YB-1) and its functions. Biochem. 76, 1402–1433 (2012).
Mihailovich, M., Militti, C., Gabaldón, T. & Gebauer, F. Eukaryotic cold shock domain proteins: highly versatile regulators of gene expression. BioEssays 32, 109–118 (2010).
Hermann, A., Schmitt, S. & Jeltsch, A. The human Dnmt2 has residual DNA-(cytosine-C5) methyltransferase activity. J. Biol. Chem. 278, 31717–31721 (2003).
Lyko, F., Jurkowski, T. P. & Jeltsch, A. On the evolutionary origin of eukaryotic DNA methyltransferases and Dnmt2. PLoS ONE 6, e28104 (2011).
Tuorto, F. et al. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nature Struct. Mol. Biol. 19, 900–905 (2012).
Pavlopoulou, A. & Kossida, S. Phylogenetic analysis of the eukaryotic RNA (cytosine-5)-methyltransferases. Genomics 93, 350–357 (2009).
Sakita-Suto, S. et al. Aurora-B regulates RNA methyltransferase NSUN2. Mol. Biol. Cell 18, 1107–1117 (2007).
Hussain, S. et al. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 4, 255–261 (2013).
Reiter, N. J., Maher, L. J. & Butcher, S. E. DNA mimicry by a high-affinity anti-NF-κB RNA aptamer. Nucleic Acids Res. 36, 1227–1236 (2008). This paper shows that the RNA aptamer developed in reference 10 mimics DNA to target the DNA-binding interface of NF-κB.
Ray, D. et al. A compendium of RNA-binding motifs for decoding gene regulation. Nature 499, 172–177 (2013).
Nakagawa, S., Gisselbrecht, S. S., Rogers, J. M., Hartl, D. L. & Bulyk, M. L. DNA-binding specificity changes in the evolution of forkhead transcription factors. Proc. Natl Acad. Sci. USA 110, 12349–12354 (2013).
Tompa, P. & Csermely, P. The role of structural disorder in the function of RNA and protein chaperones. FASEB J. 18, 1169–1175 (2004).
Volders, P. J. et al. LNCipedia: a database for annotated human lncRNA transcript sequences and structures. Nucleic Acids Res. 41, D246–D251 (2012).
Ali, M. & Vedeckis, W. The glucocorticoid receptor protein binds to transfer RNA. Science 235, 467–470 (1987).
Feldman, M., Kallos, J. & Hollander, V. P. RNA inhibits estrogen receptor binding to DNA. J. Biol. Chem. 256, 1145–1148 (1981).
Rossini, G. P. RNA-containing nuclear binding sites for glucocorticoid-receptor complexes. Biochem. Biophys. Res. Commun. 123, 78–83 (1984).
Necsulea, A. et al. The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature 505, 635–640 (2014). References 2, 10, 57, 143 and 144 reveal that RNA binding to transcription factors may be a widespread phenomenon.
Flicek, P. et al. Ensembl 2014. Nucleic Acids Res. 42, D749–D755 (2013).
Acknowledgements
W.H.H. is supported by American Heart Association predoctoral fellowship 13PRE16920012 and by a US National Institutes of Health (NIH) training grant to Emory University (5T32GM008602-14). Work in the laboratory of E.A.O. is supported by grant R01DK095750 from the NIH National Institute of Diabetes and Digestive and Kidney Diseases and by grant 14GRNT20460124 from the American Heart Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Human proteins demonstrated to bind DNA and RNA. (PDF 983 kb)
Related links
Related links
DATABASE
FURTHER INFORMATION
Glossary
- Rossmann fold
-
A common protein-folding pattern that contains the topology of a β-α-β fold. It is found in many nucleotide- binding proteins.
- Drosha
-
A nuclease of the RNase III family that, as part of a complex, cleaves primary microRNA (pri-miRNA) transcripts into precursor miRNAs (pre-miRNAs) in the nucleus.
- Primary miRNA
-
(Pri-miRNA). The primary transcripts of microRNA-encoding genes, which are stem–loop structures processed by the Drosha complex into precursor miRNAs (pre-miRNAs). Typical pri-miRNAs are hundreds of base pairs long and can contain several pre-miRNAs.
- Precursor miRNA
-
(Pre-miRNA). The product of Drosha-mediated cleavage of primary microRNAs (pri-miRNAs). Stem–loop-structured pre-miRNAs are exported to the cytoplasm and further processed by Dicer to fully mature miRNAs.
- MAD homology 1 domain
-
An evolutionarily conserved domain found in SMAD proteins. It contains four α-helices, six short β-strands and five loops, and it recognizes specific DNA sequences.
- Homeodomain
-
A protein structural domain that binds DNA and RNA, and that is found most commonly in transcription factors. It consists of a helix–turn–helix structure of 60 amino acids.
- GAR domain
-
A Gly- and Arg-rich motif that adopts a repeated β-turn structure. It is found most commonly in proteins that bind RNA.
- Zinc-finger domains
-
Structural motifs that are characterized by the coordination of one or more zinc ions. There are several different zinc-finger motifs, and each displays a different binding mode and structure.
- WW domains
-
Small protein motifs of 40 amino acids that mediate specific protein–protein interactions with short Pro-rich or Pro-containing motifs.
- Ftz-F1 domain
-
A protein domain first identified in the Fushi Tarazu factor 1 (FTZ-F1) nuclear receptor. It contains an evolutionarily conserved LXXLL motif that recognizes other LXXLL-related motifs.
- K-homology domain
-
A conserved protein domain that interacts with both RNA and DNA through a binding cleft formed between two α-helices, two β-sheets and the GXXG loop.
- Cold-shock domain
-
(CSD). A small (∼ 70 kDa) domain with high similarity to the ribonucleoprotein 1 RNA-binding motif. This domain is found in DNA- and RNA-binding proteins in bacteria, archaea and eukaryotes.
- Interfaces
-
The solvent-accessible portions of a protein that are capable of binding a ligand — including DNA and RNA — in a competitive or non-competitive manner.
- π-stacking interactions
-
Non-covalent interactions between two aromatic molecules owing to the attractive force originating from the opposing electrostatic potentials between two adjacent aromatic amino acid residues.
- Aptamers
-
Single-stranded DNA or RNA molecules that selectively bind small molecules, proteins and peptides with high affinity. Aptamers have dynamic tertiary structures, which contribute to the diversity of their binding targets.
- Z-DNA
-
DNA in the conformation of a left-handed double helix.
- Z-RNA
-
RNA in the conformation of a left-handed double helix.
- Y box
-
A DNA target sequence with the consensus CTGATTG, which is recognized and bound by certain proteins.
- RNA homopolymers
-
Sequences of ribonucleotides consisting of a single base; for example, CCCCCCCCC.
- Intrinsically disordered protein
-
A protein that does not have a well-ordered three-dimensional structure, such as proteins containing random coils or multiple domains connected with flexible linkers.
- Systematic evolution of ligands by exponential enrichment
-
(SELEX). A technique to identify ligand-binding-sequence specificity that is based on sequential rounds of binding of an oligonucleotide library to the ligand, followed by PCR amplification of the bound sequences.
Rights and permissions
About this article
Cite this article
Hudson, W., Ortlund, E. The structure, function and evolution of proteins that bind DNA and RNA. Nat Rev Mol Cell Biol 15, 749–760 (2014). https://doi.org/10.1038/nrm3884
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3884
This article is cited by
-
Assessment of the structural change of DNA by binding with a small molecule based on capillary sieving electrophoresis
Analytical Sciences (2024)
-
Dynamic RNA profiles in the small intestinal epithelia of cats after Toxoplasma gondii infection
Infectious Diseases of Poverty (2023)
-
ENO1 promotes liver carcinogenesis through YAP1-dependent arachidonic acid metabolism
Nature Chemical Biology (2023)
-
Long noncoding RNA LINC01594 inhibits the CELF6-mediated splicing of oncogenic CD44 variants to promote colorectal cancer metastasis
Cell Death & Disease (2023)
-
Structural and mechanistic insights into the DNA glycosylase AAG-mediated base excision in nucleosome
Cell Discovery (2023)