Abstract
The paradigm states that cytoplasmic actin operates as filaments and nuclear actin is mainly monomeric, acting as a scaffold in transcription complexes. However, why should a powerful function of actin, namely polymerization, not be used in the nucleus? Recent progress in the field forces us to rethink this issue, as many actin filament assembly proteins have been linked to nuclear functions and new experimental approaches have provided the first direct visualizations of polymerized nuclear actin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schoenenberger, C. A. et al. Conformation-specific antibodies reveal distinct actin structures in the nucleus and the cytoplasm. J. Struct. Biol. 152, 157–168 (2005).
Scheer, U., Hinssen, H., Franke, W. W. & Jockusch, B. M. Microinjection of actin-binding proteins and actin antibodies demonstrates involvement of nuclear actin in transcription of lampbrush chromosomes. Cell 39, 111–122 (1984).
Egly, J. M., Miyamoto, N. G., Moncollin, V. & Chambon, P. Is actin a transcription initiation factor for RNA polymerase B? EMBO J. 3, 2363–2371 (1984).
Percipalle, P. Co-transcriptional nuclear actin dynamics. Nucleus 4, 43–52 (2013).
Miyamoto, K. & Gurdon, J. B. Transcriptional regulation and nuclear reprogramming: roles of nuclear actin and actin-binding proteins. Cell. Mol. Life Sci. 70, 3289–3302 (2012).
de Lanerolle, P. & Serebryannyy, L. Nuclear actin and myosins: life without filaments. Nature Cell Biol. 13, 1282–1288 (2011).
Vartiainen, M. K., Guettler, S., Larijani, B. & Treisman, R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316, 1749–1752 (2007).
Obrdlik, A. et al. The histone acetyltransferase PCAF associates with actin and hnRNP U for RNA polymerase II transcription. Mol. Cell. Biol. 28, 6342–6357 (2008).
Percipalle, P. et al. Nuclear actin is associated with a specific subset of hnRNP A/B-type proteins. Nucleic Acid. Res. 30, 1725–1734 (2002).
Percipalle, P. et al. An actin-ribonucleoprotein interaction is involved in transcription by RNA polymerase II. Proc. Nat. Acad. Sci. USA 100, 6475–6480 (2003).
Qi, T. et al. G-actin participates in RNA polymerase II-dependent transcription elongation by recruiting positive transcription elongation factor b (P-TEFb). J. Biol. Chem. 286, 15171–15181 (2011).
Fenn, S. et al. Structural biochemistry of nuclear actin-related proteins 4 and 8 reveals their interaction with actin. EMBO J. 30, 2153–2166 (2011).
Kapoor, P., Chen, M., Winkler, D. D., Luger, K. & Shen, X. Evidence for monomeric actin function in INO80 chromatin remodeling. Nature Struct. Mol. Biol. 20, 426–432 (2013).
McDonald, D., Carrero, G., Andrin, C., de Vries, G. & Hendzel, M. J. Nucleoplasmic β-actin exists in a dynamic equilibrium between low-mobility polymeric species and rapidly diffusing populations. J. Cell Biol. 172, 541–552 (2006).
Ye, J., Zhao, J., Hoffmann-Rohrer, U. & Grummt, I. Nuclear myosin I acts in concert with polymeric actin to drive RNA polymerase I transcription. Genes Dev. 22, 322–330 (2008).
Hu, Q. et al. Enhancing nuclear receptor-induced transcription requires nuclear motor and LSD1-dependent gene networking in interchromatin granules. Proc. Natl Acad. Sci. USA 105, 19199–19204 (2008).
Dundr, M. et al. Actin-dependent intranuclear repositioning of an active gene locus in vivo. J. Cell Biol. 179, 1095–1103 (2007).
Chuang, C. H. et al. Long-range directional movement of an interphase chromosome site. Curr. Biol. 16, 825–831 (2006).
Muratani, M. et al. Metabolic-energy-dependent movement of PML bodies within the mammalian cell nucleus. Nature Cell Biol. 4, 106–110 (2002).
Sarshad, A. et al. Nuclear myosin 1c facilitates the chromatin modifications required to activate rRNA gene transcription and cell cycle progression. PLoS Genet. 9, e1003397 (2013).
Huang, W. et al. Coronin 2A mediates actin-dependent de-repression of inflammatory response genes. Nature 470, 414–418 (2011).
Wu, X. et al. Regulation of RNA-polymerase II-dependent transcription by N-WASP and its nuclear-binding partners. Nature Cell Biol. 8, 756–763 (2006).
Yoo, Y., Wu, X. & Guan, J. L. A novel role of the actin-nucleating Arp2/3 complex in the regulation of RNA polymerase II-dependent transcription. J. Biol. Chem. 282, 7616–7623 (2007).
Obrdlik, A. & Percipalle, P. The F-actin severing protein cofilin-1 is required for RNA polymerase II transcription elongation Nucleus 2, 72–79 (2011).
Bohnsack, M. T., Stuven, T., Kuhn, C., Cordes, V. C. & Gorlich, D. A selective block of nuclear actin export stabilizes the giant nuclei of Xenopus oocytes. Nature Cell Biol. 8, 257–263 (2006).
Gall, J. G. Exporting actin. Nature Cell Biol. 8, 205–207 (2006).
Samwer, M. et al. The nuclear F-actin interactome of Xenopus oocytes reveals an actin-bundling kinesin that is essential for meiotic cytokinesis. EMBO J. 32, 1886–1902 (2013).
Miyamoto, K., Pasque, V., Jullien, J. & Gurdon, J. B. Nuclear actin polymerization is required for transcriptional reprogramming of Oct4 by oocytes. Genes Dev. 25, 946–958 (2011).
Lenart, P. et al. A contractile nuclear actin network drives chromosome congression in oocytes. Nature 436, 812–818 (2005).
Winder, S. J. et al. Utrophin actin binding domain: analysis of actin binding and cellular targeting. J. Cell Sci. 108, 63–71 (1995).
Burkel, B. M., von Dassow, G. & Bement, W. M. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell. Motil. Cytoskeleton 64, 822–832 (2007).
Schuh, M. An actin-dependent mechanism for long-range vesicle transport. Nature Cell Biol. 13, 1431–1436 (2011).
Belin, B. J., Cimini, B. A., Blackburn, E. H. & Mullins, R. D. Visualization of actin filaments and monomers in somatic cell nuclei. Mol. Biol. Cell 24, 982–994 (2013).
Baarlink, C., Wang, H. & Grosse, R. Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science 340, 864–867 (2013).
Chesarone, M. A., DuPage, A. G. & Goode, B. L. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nature Rev. Mol. Cell Biol. 11, 62–74 (2010).
Vartiainen, M. K. Nuclear actin dynamics — from form to function. FEBS Lett. 582, 2033–2040 (2008).
Weston, L., Coutts, A. S. & La Thangue, N. B. Actin nucleators in the nucleus: an emerging theme. J. Cell Sci. 125, 3519–3527 (2012).
Archer, S. K., Claudianos, C. & Campbell, H. D. Evolution of the gelsolin family of actin-binding proteins as novel transcriptional coactivators. Bioessays 27, 388–396 (2005).
Dopie, J., Skarp, K. P., Kaisa Rajakyla, E., Tanhuanpaa, K. & Vartiainen, M. K. Active maintenance of nuclear actin by importin 9 supports transcription. Proc. Natl Acad. Sci. USA 109, 544–552 (2012).
Johnson, M. A., Sharma, M., Mok, M. T. & Henderson, B. R. Stimulation of in vivo nuclear transport dynamics of actin and its co-factors IQGAP1 and Rac1 in response to DNA replication stress. Biochim. Biophys. Acta 1833, 2334–2347 (2013).
Mejat, A. & Misteli, T. LINC complexes in health and disease. Nucleus 1, 40–52 (2010).
Simon, D. N., Zastrow, M. S. & Wilson, K. L. Direct actin binding to A- and B-type lamin tails and actin filament bundling by the lamin A tail. Nucleus 1, 264–272 (2010).
Holaska, J. M., Kowalski, A. K. & Wilson, K. L. Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLoS Biol. 2, E231 (2004).
Ho, C. Y., Jaalouk, D. E., Vartiainen, M. K. & Lammerding, J. Lamin A/C and emerin regulate MKL1–SRF activity by modulating actin dynamics. Nature 497, 507–511 (2013).
Guettler, S., Vartiainen, M. K., Miralles, F., Larijani, B. & Treisman, R. RPEL motifs link the serum response factor cofactor MAL but not myocardin to Rho signaling via actin binding. Mol. Cell. Biol. 28, 732–742 (2008).
Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nature Methods 5, 605–607 (2008).
Acknowledgements
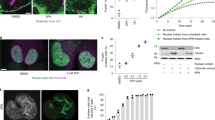
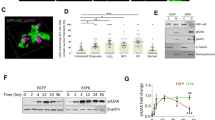
The authors thank members of their laboratories for discussions and C. Baarlink for the image in figure 1. The work in the laboratory of M.K.V is funded by the European Research Council (ERC) Starting grant, Academy of Finland and Sigrid Juselius foundation. Work in the laboratory of R.G. is partly funded by the Deutsche Forschnungsgemeinschaft (GR 2111/2 and SFB 593).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Grosse, R., Vartiainen, M. To be or not to be assembled: progressing into nuclear actin filaments. Nat Rev Mol Cell Biol 14, 693–697 (2013). https://doi.org/10.1038/nrm3681
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3681
This article is cited by
-
A glance on the role of actin in osteogenic and adipogenic differentiation of mesenchymal stem cells
Stem Cell Research & Therapy (2020)
-
GPCR-induced calcium transients trigger nuclear actin assembly for chromatin dynamics
Nature Communications (2019)
-
The SWI/SNF complex is a mechanoregulated inhibitor of YAP and TAZ
Nature (2018)
-
Breakdancing on actin
Nature Reviews Molecular Cell Biology (2018)
-
The dendritic spine morphogenic effects of repeated cocaine use occur through the regulation of serum response factor signaling
Molecular Psychiatry (2018)