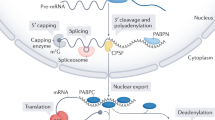
Key Points
-
RNA nucleotidyl transferases related to the canonical nuclear poly(A) polymerase modify various cytoplasmic RNAs in most eukaryotes, with the notable exception of Saccharomyces cerevisiae.
-
Regulated deadenylation is used in many biological contexts to generate cytoplasmic stores of stable, translationally inactive mRNAs. Non-canonical poly(A) polymerases restore such mRNAs to translational competence in a manner that is important for synaptic plasticity in the central nervous system.
-
Non-canonical poly(A) polymerases may also stabilize certain microRNAs, by adding single AMP residues to their 3′ ends.
-
Similarly, cytoplasmic RNA terminal uridylyl transferases add single UMP residues, or oligo(U) tails, to a range of microRNAs and their precursors. This results in the proper processing or turnover of the RNA substrate, according to its identity and the extent of its uridylation.
-
Cytoplasmic uridylyl transferases also modify mRNA 3′ ends in many eukaryotes, targeting the mRNAs for decapping and/or exonucleolytic decay.
-
The addition of non-templated nucleotides to RNA 3′ ends is consequently a widespread and multifaceted aspect of regulation of eukaryotic gene expression.
Abstract
The addition of poly(A) tails to eukaryotic nuclear mRNAs promotes their stability, export to the cytoplasm and translation. Subsequently, the balance between exonucleolytic deadenylation and selective re-establishment of translation-competent poly(A) tails by cytoplasmic poly(A) polymerases is essential for the appropriate regulation of gene expression from oocytes to neurons. In recent years, surprising roles for cytoplasmic poly(A) polymerase-related enzymes that add uridylyl, rather than adenylyl, residues to RNA 3′ ends have also emerged. These terminal uridylyl transferases promote the turnover of certain mRNAs but also modify microRNAs, their precursors and other small RNAs to modulate their stability or biological functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Aravind, L. & Koonin, E. V. DNA polymerase β-like nucleotidyltransferase superfamily: identification of three new families, classification and evolutionary history. Nucleic Acids Res. 27, 1609–1618 (1999).
Ingle, C. A. & Kushner, S. R. Development of an in vitro mRNA decay system for Escherichia coli: poly(A) polymerase I is necessary to trigger degradation. Proc. Natl Acad. Sci. USA 93, 12926–12931 (1996).
Schmidt, M. J. & Norbury, C. J. Polyadenylation and beyond: emerging roles for noncanonical poly(A) polymerases. Wiley Interdiscip. Rev. RNA 1, 142–151 (2010).
Kadaba, S. et al. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev. 18, 1227–1240 (2004).
Wyers, F. et al. Cryptic Pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121, 725–737 (2005).
LaCava, J. et al. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 121, 713–724 (2005).
Vanacova, S. et al. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol. 3, e189 (2005).
Lunde, B. M., Magler, I. & Meinhart, A. Crystal structures of the Cid1 poly (U) polymerase reveal the mechanism for UTP selectivity. Nucleic Acids Res. 40, 9815–9824 (2012).
Yates, L. A. et al. Structural basis for the activity of a cytoplasmic RNA terminal uridylyl transferase. Nature Struct. Mol. Biol. 19, 782–787 (2012).
Munoz-Tello, P., Gabus, C. & Thore, S. Functional implications from the Cid1 poly(U) polymerase crystal structure. Structure 20, 977–986 (2012).
Aphasizhev, R. & Aphasizheva, I. Uridine insertion/deletion editing in trypanosomes: a playground for RNA-guided information transfer. Wiley Interdiscip. Rev. RNA 2, 669–685 (2011).
Chang, J. H. & Tong, L. Mitochondrial poly(A) polymerase and polyadenylation. Biochim. Biophys. Acta 1819, 992–997 (2012).
Nagaike, T., Suzuki, T. & Ueda, T. Polyadenylation in mammalian mitochondria: insights from recent studies. Biochim. Biophys. Acta 1779, 266–269 (2008).
Marzluff, W. F., Wagner, E. J. & Duronio, R. J. Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. Nature Rev. Genet. 9, 843–854 (2008).
Parker, R. RNA degradation in Saccharomyces cerevisae. Genetics 191, 671–702 (2012).
Yamashita, A. et al. Concerted action of poly(A) nucleases and decapping enzyme in mammalian mRNA turnover. Nature Struct. Mol. Biol. 12, 1054–1063 (2005).
van Hoof, A., Frischmeyer, P. A., Dietz, H. C. & Parker, R. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science 295, 2262–2264 (2002).
Frischmeyer, P. A. et al. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science 295, 2258–2261 (2002).
Chen, C. Y. & Shyu, A. B. Rapid deadenylation triggered by a nonsense codon precedes decay of the RNA body in a mammalian cytoplasmic nonsense-mediated decay pathway. Mol. Cell. Biol. 23, 4805–4813 (2003).
Schoenberg, D. R. & Maquat, L. E. Regulation of cytoplasmic mRNA decay. Nature Rev. Genet. 13, 246–259 (2012).
Boeck, R. et al. The yeast Pan2 protein is required for poly(A)-binding protein-stimulated poly(A)-nuclease activity. J. Biol. Chem. 271, 432–438 (1996).
Tucker, M. et al. The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell 104, 377–386 (2001).
Chen, J., Chiang, Y. C. & Denis, C. L. CCR4, a 3′–5′ poly(A) RNA and ssDNA exonuclease, is the catalytic component of the cytoplasmic deadenylase. EMBO J. 21, 1414–1426 (2002).
Eulalio, A., Behm-Ansmant, I. & Izaurralde, E. P bodies: at the crossroads of post-transcriptional pathways. Nature Rev. Mol. Cell Biol. 8, 9–22 (2007).
Parker, R. & Sheth, U. P bodies and the control of mRNA translation and degradation. Mol. Cell 25, 635–646 (2007).
Kruk, J. A., Dutta, A., Fu, J., Gilmour, D. S. & Reese, J. C. The multifunctional Ccr4–Not complex directly promotes transcription elongation. Genes Dev. 25, 581–593 (2011).
Gao, M., Wilusz, C. J., Peltz, S. W. & Wilusz, J. A novel mRNA-decapping activity in HeLa cytoplasmic extracts is regulated by AU-rich elements. EMBO J. 20, 1134–1143 (2001).
Vasudevan, S. & Peltz, S. W. Regulated ARE-mediated mRNA decay in Saccharomyces cerevisiae. Mol. Cell 7, 1191–1200 (2001).
Mukherjee, D. et al. The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J. 21, 165–174 (2002).
Chritton, J. J. & Wickens, M. A role for the poly(A)-binding protein Pab1p in PUF protein-mediated repression. J. Biol. Chem. 286, 33268–33278 (2011).
Goldstrohm, A. C., Hook, B. A., Seay, D. J. & Wickens, M. PUF proteins bind Pop2p to regulate messenger RNAs. Nature Struct. Mol. Biol. 13, 533–539 (2006).
Beilharz, T. H. et al. microRNA-mediated messenger RNA deadenylation contributes to translational repression in mammalian cells. PLoS ONE 4, e6783 (2009).
Eulalio, A. et al. Deadenylation is a widespread effect of miRNA regulation. RNA 15, 21–32 (2009).
Braun, J. E., Huntzinger, E., Fauser, M. & Izaurralde, E. GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol. Cell 44, 120–133 (2011).
Zekri, L., Kuzuoglu-Ozturk, D. & Izaurralde, E. GW182 proteins cause PABP dissociation from silenced miRNA targets in the absence of deadenylation. EMBO J. 32, 1052–1065 (2013).
Goldstrohm, A. C. & Wickens, M. Multifunctional deadenylase complexes diversify mRNA control. Nature Rev. Mol. Cell Biol. 9, 337–344 (2008).
Lee, J. E. et al. The PARN deadenylase targets a discrete set of mRNAs for decay and regulates cell motility in mouse myoblasts. PLoS Genet. 8, e1002901 (2012).
Coller, J. & Parker, R. Eukaryotic mRNA decapping. Annu. Rev. Biochem. 73, 861–890 (2004).
Tharun, S. & Parker, R. Targeting an mRNA for decapping: displacement of translation factors and association of the Lsm1p–7p complex on deadenylated yeast mRNAs. Mol. Cell 8, 1075–1083 (2001).
Chowdhury, A., Mukhopadhyay, J. & Tharun, S. The decapping activator Lsm1p–7p–Pat1p complex has the intrinsic ability to distinguish between oligoadenylated and polyadenylated RNAs. RNA 13, 998–1016 (2007).
Tharun, S. et al. Yeast Sm-like proteins function in mRNA decapping and decay. Nature 404, 515–518 (2000).
Lykke-Andersen, S., Brodersen, D. E. & Jensen, T. H. Origins and activities of the eukaryotic exosome. J. Cell Sci. 122, 1487–1494 (2009).
Rissland, O. S. & Norbury, C. J. Decapping is preceded by 3′ uridylation in a novel pathway of bulk mRNA turnover. Nature Struct. Mol. Biol. 16, 616–624 (2009).
Hu, W., Sweet, T. J., Chamnongpol, S., Baker, K. E. & Coller, J. Co-translational mRNA decay in Saccharomyces cerevisiae. Nature 461, 225–229 (2009).
McGrew, L. L., Dworkin-Rastl, E., Dworkin, M. B. & Richter, J. D. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 3, 803–815 (1989). The first indication that cytoplasmic polyadenylation is an important regulator of mRNA function and that it is governed by a cis -acting cytoplasmic polyadenylation element.
Weill, L., Belloc, E., Bava, F. A. & Mendez, R. Translational control by changes in poly(A) tail length: recycling mRNAs. Nature Struct. Mol. Biol. 19, 577–585 (2012).
Groisman, I. et al. CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell 103, 435–447 (2000).
Eliscovich, C., Peset, I., Vernos, I. & Mendez, R. Spindle-localized CPE-mediated translation controls meiotic chromosome segregation. Nature Cell Biol. 10, 858–865 (2008).
Kim, K. W., Wilson, T. L. & Kimble, J. GLD-2/RNP-8 cytoplasmic poly(A) polymerase is a broad-spectrum regulator of the oogenesis program. Proc. Natl Acad. Sci. USA 107, 17445–17450 (2010).
Schmid, M., Kuchler, B. & Eckmann, C. R. Two conserved regulatory cytoplasmic poly(A) polymerases, GLD-4 and GLD-2, regulate meiotic progression in C. elegans. Genes Dev. 23, 824–836 (2009).
Wang, L., Eckmann, C. R., Kadyk, L. C., Wickens, M. & Kimble, J. A regulatory cytoplasmic poly(A) polymerase in Caenorhabditis elegans. Nature 419, 312–316 (2002).
Kwak, J. E. et al. GLD2 poly(A) polymerase is required for long-term memory. Proc. Natl Acad. Sci. USA 105, 14644–14649 (2008).
Benoit, P., Papin, C., Kwak, J. E., Wickens, M. & Simonelig, M. PAP- and GLD-2-type poly(A) polymerases are required sequentially in cytoplasmic polyadenylation and oogenesis in Drosophila. Development 135, 1969–1979 (2008).
Barnard, D. C., Ryan, K., Manley, J. L. & Richter, J. D. Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell 119, 641–651 (2004).
Lin, C. L., Evans, V., Shen, S., Xing, Y. & Richter, J. D. The nuclear experience of CPEB: implications for RNA processing and translational control. RNA 16, 338–348 (2010).
Bava, F. A. et al. CPEB1 coordinates alternative 3′-UTR formation with translational regulation. Nature 495, 121–125 (2013).
Kim, J. H. & Richter, J. D. Opposing polymerase–deadenylase activities regulate cytoplasmic polyadenylation. Mol. Cell 24, 173–183 (2006).
Stebbins-Boaz, B., Cao, Q., de Moor, C. H., Mendez, R. & Richter, J. D. Maskin is a CPEB-associated factor that transiently interacts with elF-4E. Mol. Cell 4, 1017–1027 (1999).
Morris, J. Z., Hong, A. & Lilly, M. A. & Lehmann, R. twin, a CCR4 homolog, regulates cyclin poly(A) tail length to permit Drosophila oogenesis. Development 132, 1165–1174 (2005).
Novoa, I., Gallego, J., Ferreira, P. G. & Mendez, R. Mitotic cell-cycle progression is regulated by CPEB1 and CPEB4-dependent translational control. Nature Cell Biol. 12, 447–456 (2010).
Udagawa, T. et al. Bidirectional control of mRNA translation and synaptic plasticity by the cytoplasmic polyadenylation complex. Mol. Cell 47, 253–266 (2012). Shows that hundreds of mRNAs are subject to translational regulation by cytoplasmic polyadenylation in mammalian neurons and provides strong evidence for the involvement of this process in synaptic plasticity.
Wu, L. et al. CPEB-mediated cytoplasmic polyadenylation and the regulation of experience-dependent translation of α-CaMKII mRNA at synapses. Neuron 21, 1129–1139 (1998).
Groisman, I. et al. Control of cellular senescence by CPEB. Genes Dev. 20, 2701–2712 (2006).
Burns, D. M., D'Ambrogio, A., Nottrott, S. & Richter, J. D. CPEB and two poly(A) polymerases control miR-122 stability and p53 mRNA translation. Nature 473, 105–108 (2011).
Lubas, M. et al. Interaction profiling identifies the human nuclear exosome targeting complex. Mol. Cell 43, 624–637 (2011).
Shcherbik, N., Wang, M., Lapik, Y. R. Srivastava, L. & Pestov, D. G. Polyadenylation and degradation of incomplete RNA polymerase I transcripts in mammalian cells. EMBO Rep. 11, 106–111 (2010).
Berndt, H. et al. Maturation of mammalian H/ACA box snoRNAs: PAPD5-dependent adenylation and PARN-dependent trimming. RNA 18, 958–972 (2012).
Nakanishi, T. et al. Possible role of mouse poly(A) polymerase mGLD-2 during oocyte maturation. Dev. Biol. 289, 115–126 (2006).
Nakanishi, T. et al. Disruption of mouse poly(A) polymerase mGLD-2 does not alter polyadenylation status in oocytes and somatic cells. Biochem. Biophys. Res. Commun. 364, 14–19 (2007).
Tay, J. & Richter, J. D. Germ cell differentiation and synaptonemal complex formation are disrupted in CPEB knockout mice. Dev. Cell 1, 201–213 (2001).
Berger-Sweeney, J., Zearfoss, N. R. & Richter, J. D. Reduced extinction of hippocampal-dependent memories in CPEB knockout mice. Learn. Mem. 13, 4–7 (2006).
Huang, Y. S., Kan, M. C., Lin, C. L. & Richter, J. D. CPEB3 and CPEB4 in neurons: analysis of RNA-binding specificity and translational control of AMPA receptor GluR2 mRNA. EMBO J. 25, 4865–4876 (2006).
Kojima, S., Sher-Chen, E. L. & Green, C. B. Circadian control of mRNA polyadenylation dynamics regulates rhythmic protein expression. Genes Dev. 26, 2724–2736 (2012). Shows that a substantial number of mouse liver mRNAs undergo circadian variation in poly(A) tail length, possibly in part as a result of cytoplasmic polyadenylation–deadenylation cycles.
Wang, Y. et al. Rhythmic expression of Nocturnin mRNA in multiple tissues of the mouse. BMC Dev. Biol. 1, 9 (2001).
Ibrahim, F., Rohr, J., Jeong, W. J., Hesson, J. & Cerutti, H. Untemplated oligoadenylation promotes degradation of RISC-cleaved transcripts. Science 314, 1893 (2006).
Katoh, T. et al. Selective stabilization of mammalian microRNAs by 3′ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes Dev. 23, 433–438 (2009). Shows that miR-122 is monoadenylated, and hence stabilized, by the same enzyme that mediates cytoplasmic polyadenylation of mRNAs.
D'Ambrogio, A., Gu, W., Udagawa, T., Mello, C. C. & Richter, J. D. Specific miRNA stabilization by Gld2-catalyzed monoadenylation. Cell Rep. 2, 1537–1545 (2012).
Kwak, J. E., Wang, L., Ballantyne, S., Kimble, J. & Wickens, M. Mammalian GLD-2 homologs are poly(A) polymerases. Proc. Natl Acad. Sci. USA 101, 4407–4412 (2004).
Shen, B. & Goodman, H. M. Uridine addition after microRNA-directed cleavage. Science 306, 997 (2004).
Rissland, O. S. & Norbury, C. J. The Cid1 poly(U) polymerase. Biochim. Biophys. Acta 1779, 286–294 (2008).
Kwak, J. E. & Wickens, M. A family of poly(U) polymerases. RNA 13, 860–867 (2007). Finds that ZCCHC6 and ZCCHC11 behave as poly(U) polymerases in RNA tethering assays in X. laevis oocytes.
Rissland, O. S., Mikulasova, A. & Norbury, C. J. Efficient RNA polyuridylation by noncanonical poly(A) polymerases. Mol. Cell. Biol. 27, 3612–3624 (2007). Describes the uridylation of polyadenylated mRNAs in fission yeast, identifies Cid1 as the corresponding cytoplasmic TUT and shows that human ZCCHC6 has a similar activity.
Li, J., Yang, Z., Yu, B., Liu, J. & Chen, X. Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr. Biol. 15, 1501–1507 (2005).
Ibrahim, F. et al. Uridylation of mature miRNAs and siRNAs by the MUT68 nucleotidyltransferase promotes their degradation in Chlamydomonas. Proc. Natl Acad. Sci. USA 107, 3906–3911 (2010).
Jones, M. R. et al. Zcchc11 uridylates mature miRNAs to enhance neonatal IGF-1 expression, growth, and survival. PLoS Genet. 8, e1003105 (2012).
Jones, M. R. et al. Zcchc11-dependent uridylation of microRNA directs cytokine expression. Nature Cell Biol. 11, 1157–1163 (2009).
Heo, I. et al. Mono-uridylation of pre-microRNA as a key step in the biogenesis of group II let-7 microRNAs. Cell 151, 521–532 (2012).
Newman, M. A., Mani, V. & Hammond, S. M. Deep sequencing of microRNA precursors reveals extensive 3′ end modification. RNA 17, 1795–1803 (2011).
Hagan, J. P., Piskounova, E. & Gregory, R. I. Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nature Struct. Mol. Biol. 16, 1021–1025 (2009).
Heo, I. et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138, 696–708 (2009).
Lehrbach, N. J. et al. LIN-28 and the poly(U) polymerase PUP-2 regulate let-7 microRNA processing in Caenorhabditis elegans. Nature Struct. Mol. Biol. 16, 1016–1027 (2009).
Chang, H. M., Triboulet, R., Thornton, J. E. & Gregory, R. I. A role for the Perlman syndrome exonuclease Dis3l2 in the Lin28–let-7 pathway. Nature 497, 244–248 (2013).
Thornton, J. E., Chang, H. M., Piskounova, E. & Gregory, R. I. Lin28-mediated control of let-7 microRNA expression by alternative TUTases Zcchc11 (TUT4) and Zcchc6 (TUT7). RNA 18, 1875–1885 (2012).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Morozov, I. Y. et al. mRNA 3′ tagging is induced by nonsense-mediated decay and promotes ribosome dissociation. Mol. Cell. Biol. 32, 2585–2595 (2012).
Morozov, I. Y., Jones, M. G., Razak, A. A., Rigden, D. J. & Caddick, M. X. CUCU modification of mRNA promotes decapping and transcript degradation in Aspergillus nidulans. Mol. Cell. Biol. 30, 460–469 (2010).
Malecki, M. et al. The exoribonuclease Dis3L2 defines a novel eukaryotic RNA degradation pathway. EMBO J. 32, 1842–1854 (2013).
Mullen, T. E. & Marzluff, W. F. Degradation of histone mRNA requires oligouridylation followed by decapping and simultaneous degradation of the mRNA both 5′ to 3′ and 3′ to 5′. Genes Dev. 22, 50–65 (2008). Describes for the first time the uridylation of mRNAs in mammalian cells and implicates this modification in histone mRNA turnover.
Meeks-Wagner, D. & Hartwell, L. H. Normal stoichiometry of histone dimer sets is necessary for high fidelity of mitotic chromosome transmission. Cell 44, 43–52 (1986).
Schmidt, M. J., West, S. & Norbury, C. J. The human cytoplasmic RNA terminal U-transferase ZCCHC11 targets histone mRNAs for degradation. RNA 17, 39–44 (2011).
Hoefig, K. P. et al. Eri1 degrades the stem–loop of oligouridylated histone mRNAs to induce replication-dependent decay. Nature Struct. Mol. Biol. 20, 73–81 (2013). Shows that the 3′–5′ exoribonuclease ERI1 can initiate histone mRNA turnover following its recruitment to uridylated mRNA substrates by the LSM1–LSM7 complex.
Song, M. G. & Kiledjian, M. 3′ terminal oligo U-tract-mediated stimulation of decapping. RNA 13, 2356–2365 (2007).
Acknowledgements
The author apologises to those whose work could not be cited directly owing to space constraints and is grateful to D. Scott for comments on the manuscript. Work in the author's laboratory is supported by the Breast Cancer Campaign and Cancer Research UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- DNA polymerase-β
-
(Pol β). A non-processive eukaryotic DNA polymerase that acts in the base excision DNA repair pathway to fill in single-strand DNA gaps.
- Poly(A) polymerases
-
(PAPs). Nucleotidyl transferases that covalently add multiple AMP moieties from ATP to the 3′-OH group of an RNA substrate.
- Exosome
-
In this context, a multisubunit ribonuclease complex with associated endonuclease and 3′–5′ exonuclease activities (note that the same term is used in other contexts to denote a membrane-bound secretory vesicle generated by exocytosis). The catalytic subunit of the cytoplasmic exosome is Rrp44 (also known as Dis3) in yeast and DIS3L in human cells.
- Terminal uridylyl transferases
-
(TUTs). Nucleotidyl transferases that covalently add one or more UMP moieties from UTP to the 3′-OH group of an RNA substrate.
- Nonsense-mediated decay
-
(NMD). A quality control pathway by which mRNAs containing premature termination codons are targeted for destruction. A substantial proportion of fully functional mRNAs are also targeted for degradation by NMD factors.
- MicroRNAs
-
(miRNAs). Short (∼22 nucleotides) single-stranded cytoplasmic RNAs, processed from highly structured primary transcripts via sequential cleavage by the RNase III enzymes Drosha and Dicer. When loaded onto proteins of the Argonaute family, miRNAs negatively regulate target mRNAs to which they are at least partially complementary.
- Sm-like proteins
-
A widely conserved family of RNA-binding proteins that are structurally related to the Sm proteins. LSM1–LSM7 form a seven-membered ring, initially identified through its association with the U6 small nuclear RNA.
- Meiotic maturation
-
The hormonally triggered resumption of meiosis in a metazoan oocyte, resulting in the formation of an egg.
- Synaptic plasticity
-
The phenomenon, thought to be fundamental to memory and learning, by which synaptic connections between neurons can become potentiated or attenuated as a result of particular patterns of stimulation.
- Long-term potentiation
-
A form of synaptic plasticity in which long-term synaptic strength is increased in response to repeated neuronal stimulation.
- PIWI-interacting RNAs
-
Short, non-coding RNAs that are expressed in animal germ cells and bound by PIWI proteins of the Argonaute family. They act principally to inhibit the expression of retrotransposons.
- siRNAs
-
Short, non-coding RNAs that negatively regulate target gene expression when bound to Argonaute proteins, these approximately 21 base pair duplex RNAs with two-nucleotide 3′ overhangs are generated by Dicer cleavage of double-stranded RNA.
- Circularized rapid amplification of cDNA ends
-
(cRACE). A technique involving circularization of RNA molecules using RNA ligase, subsequent reverse transcription and PCR amplification of the junction corresponding to the two ends of the RNA molecule. It allows the description of the two ends of an RNA molecule without requiring prior knowledge of their precise positions or sequences.
Rights and permissions
About this article
Cite this article
Norbury, C. Cytoplasmic RNA: a case of the tail wagging the dog. Nat Rev Mol Cell Biol 14, 643–653 (2013). https://doi.org/10.1038/nrm3645
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3645
This article is cited by
-
Membrane linked RNA glycosylation as new trend to envision epi-transcriptome epoch
Cancer Gene Therapy (2023)
-
A tale of non-canonical tails: gene regulation by post-transcriptional RNA tailing
Nature Reviews Molecular Cell Biology (2020)
-
A programmed wave of uridylation-primed mRNA degradation is essential for meiotic progression and mammalian spermatogenesis
Cell Research (2019)
-
Terminal uridylyltransferases target RNA viruses as part of the innate immune system
Nature Structural & Molecular Biology (2018)
-
CNOT6 regulates a novel pattern of mRNA deadenylation during oocyte meiotic maturation
Scientific Reports (2018)