Key Points
-
Although for a long time necrosis was considered to be a purely accidental cell death subroutine, multiple lines of evidence now show that necrotic cell death can be regulated, both in its occurrence and in its course. The term 'necroptosis' was introduced by Yuan's research group in 2005 to indicate 'programmed' (as opposed to 'accidental') necrosis.
-
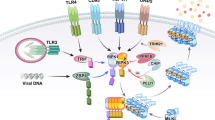
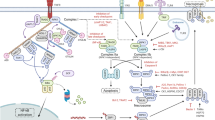
The best characterized signal transduction cascade leading to necroptosis is initiated by ligand-bound tumour necrosis factor (TNF) receptor 1 (TNFR1), which allows for the assembly of a cytoplasmic supramolecular complex — TNFR complex I — that includes (among other proteins) TNFR-associated death domain (TRADD), cellular inhibitor of apoptosis 1 (cIAP1), cIAP2 and receptor-interacting protein kinase 1 (RIP1; also known as RIPK1).
-
In complex I, RIP1 can be ubiquitylated by cIAPs and deubiquitylated by cylindromatosis (CYLD) and A20 (also known as TNFAIP3). Whereas ubiquitylated RIP1 promotes the activation of the nuclear factor κB (NF-κB) system, deubiquitylated RIP1 functions as a cell death-inducing kinase.
-
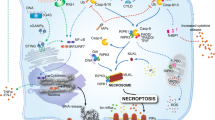
On TNFR1 internalization, the so-called TNFR complex II is formed, which contains TRADD, FAS-associated protein with a death domain (FADD) and caspase 8. Normally, caspase 8 becomes activated in complex II, thereby igniting a pro-apoptotic caspase cascade. When caspase activation is prevented, however, RIP1 physically and functionally interacts with RIP3 (also known as RIPK3), thereby generating a necroptosis-inducing complex known as the necrosome.
-
Necroptosis can also be ignited by pathogen recognition receptors, including Toll-like receptors, NOD-like receptors and retinoic acid-inducible gene I-like receptors, as well as in response to DNA damage, presumably by a poly(ADP-ribose) polymerase-1 (PARP1)-dependent signalling pathway.
-
Although the underlying molecular mechanisms remain obscure, reactive oxygen species (ROS), bioenergetic metabolic cascades and the release of cytotoxic factors from lysosomes and mitochondria all contribute to the execution of necroptosis.
-
Regulated necrosis has been seen in multiple, evolutionarily distant model organisms, including yeast, nematodes, fruit flies, rodents, primates and human cells, corroborating the notion that necroptosis may represent a phylogenetically conserved mechanism for programmed cell death.
-
Numerous in vivo studies indicate that the inhibition of necroptosis (by genetic means or by RIP1-targeting agents called necrostatins) can confer consistent cytoprotection, suggesting that necroptosis may constitute a promising target for drug development.
Abstract
For a long time, apoptosis was considered the sole form of programmed cell death during development, homeostasis and disease, whereas necrosis was regarded as an unregulated and uncontrollable process. Evidence now reveals that necrosis can also occur in a regulated manner. The initiation of programmed necrosis, 'necroptosis', by death receptors (such as tumour necrosis factor receptor 1) requires the kinase activity of receptor-interacting protein 1 (RIP1; also known as RIPK1) and RIP3 (also known as RIPK3), and its execution involves the active disintegration of mitochondrial, lysosomal and plasma membranes. Necroptosis participates in the pathogenesis of diseases, including ischaemic injury, neurodegeneration and viral infection, thereby representing an attractive target for the avoidance of unwarranted cell death.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lockshin, R. A. & Williams, C. M. Programmed cell death — II. Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. J. Insect Physiol. 10, 643–649 (1964).
Kerr, J. F., Wyllie, A. H. & Currie, A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 (1972).
Lettre, G. & Hengartner, M. O. Developmental apoptosis in, C. elegans: a complex CEDnario. Nature Rev. Mol. Cell Biol. 7, 97–108 (2006).
Schweichel, J. U. & Merker, H. J. The morphology of various types of cell death in prenatal tissues. Teratology 7, 253–266 (1973).
Kroemer, G. et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16, 3–11 (2009). This article provides up-to-date guidelines for the use of cell death-related terminology in scientific publications, as provided by the Nomenclature Committee on Cell Death, an organization composed of reputed researchers in the field of cell death worldwide.
Laster, S. M., Wood, J. G. & Gooding, L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J. Immunol. 141, 2629–2634 (1988).
Degterev, A. et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature Chem. Biol. 1, 112–119 (2005).
Hsu, H., Huang, J., Shu, H. B., Baichwal, V. & Goeddel, D. V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 (1996).
Holler, N. et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature Immunol. 1, 489–495 (2000). The authors discovered that, in some cell types, FAS can trigger non-apoptotic cell death that is independent of caspases but dependent on the adaptor protein FADD and the presence and enzymatic activity of the protein kinase RIP1. This milestone paper is the first report of RIP1-dependent necroptosis.
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137, 1100–1111 (2009).
Zhang, D. W. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009). References 10–12 independently uncovered the obligate role of RIP3 in necroptosis. By multiple experimental approaches, RIP3 was shown to functionally and physically interact with RIP1, leading to a mitochondrial metabolic burst that underlies necroptosis execution. The pathophysiological role of RIP3 in vivo was substantiated by animal models of viral infection and acute pancreatitis.
Vercammen, D. et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187, 1477–1485 (1998). The sensitivity of murine L929 fibrosarcoma cells to TNF-induced necrosis was dramatically increased by expression of a serpin-like viral protein (CrmA) and by pharmacological caspase inhibitors, showing for the first time a major role for caspases in the apoptotis–necrosis switch.
Hitomi, J. et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135, 1311–1323 (2008). The first time systems biology was applied to compare necroptosis to apoptosis in murine cells. Genome wide RNAi-based screens coupled to in silico and in vitro analyses allowed the delineation of a signalling network that regulates the molecular bifurcation between necroptosis and apoptosis.
Goossens, V., Stange, G., Moens, K., Pipeleers, D. & Grooten, J. Regulation of tumor necrosis factor-induced, mitochondria- and reactive oxygen species-dependent cell death by the electron flux through the electron transport chain complex I. Antioxid. Redox Signal. 1, 285–295 (1999).
Kim, Y. S., Morgan, M. J., Choksi, S. & Liu, Z. G. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol. Cell 26, 675–687 (2007).
Yazdanpanah, B. et al. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature 460, 1159–1163 (2009).
Goossens, V., Grooten, J. & Fiers, W. The oxidative metabolism of glutamine. A modulator of reactive oxygen intermediate-mediated cytotoxicity of tumor necrosis factor in L929 fibrosarcoma cells. J. Biol. Chem. 271, 192–196 (1996).
Zong, W. X., Ditsworth, D., Bauer, D. E., Wang, Z. Q. & Thompson, C. B. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 18, 1272–1282 (2004).
Nakagawa, T. et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434, 652–658 (2005).
Schinzel, A. C. et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl Acad. Sci. USA 102, 12005–12010 (2005). References 20 and 21 show that CYPD-deficient mice are protected against ischaemic insults, as compared to their wild-type littermates. Moreover, multiple types of primary cells isolated from Ppif−/− mice exhibit enhanced resistance to necrotic (but not apoptotic) stimuli. Together, these studies provide unequivocal evidence that CYPD regulates necroptosis in vivo.
Temkin, V., Huang, Q., Liu, H., Osada, H. & Pope, R. M. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol. Cell. Biol. 26, 2215–2225 (2006).
Boya, P. & Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 27, 6434–6451 (2008).
Vanden Berghe, T. et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 17, 922–930 (2010).
Kroemer, G., Galluzzi, L. & Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 87, 99–163 (2007). This review provides a comprehensive analysis of the mitochondrial pathway of cell death and of its multifaceted implications for human physiology and pathology.
Green, D. R. & Kroemer, G. Pharmacological manipulation of cell death: clinical applications in sight? J. Clin. Invest. 115, 2610–2617 (2005).
Rosenbaum, D. M. et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J. Neurosci. Res. 88, 1569–1576 (2010).
Vanlangenakker, N., Vanden Berghe, T., Krysko, D. V., Festjens, N. & Vandenabeele, P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr. Mol. Med. 8, 207–220 (2008).
Vercammen, D. et al. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J. Exp. Med. 188, 919–930 (1998).
Chan, F. K. et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J. Biol. Chem. 278, 51613–51621 (2003).
Hacker, H. & Karin, M. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, re13 (2006).
Wilson, N. S., Dixit, V. & Ashkenazi, A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nature Immunol. 10, 348–355 (2009).
Fiers, W. et al. TNF-induced intracellular signaling leading to gene induction or to cytotoxicity by necrosis or by apoptosis. J. Inflamm. 47, 67–75 (1995).
Martinon, F., Gaide, O., Petrilli, V., Mayor, A. & Tschopp, J. NALP inflammasomes: a central role in innate immunity. Semin. Immunopathol. 29, 213–229 (2007).
Kalai, M. et al. Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell Death Differ. 9, 981–994 (2002).
Ma, Y., Temkin, V., Liu, H. & Pope, R. M. NF-κB protects macrophages from lipopolysaccharide-induced cell death: the role of caspase 8 and receptor-interacting protein. J. Biol. Chem. 280, 41827–41834 (2005).
Francois, M., Le Cabec, V., Dupont, M. A., Sansonetti, P. J. & Maridonneau-Parini, I. Induction of necrosis in human neutrophils by Shigella flexneri requires type III secretion, IpaB and IpaC invasins, and actin polymerization. Infect. Immun. 68, 1289–1296 (2000).
Koterski, J. F., Nahvi, M., Venkatesan, M. M. & Haimovich, B. Virulent Shigella flexneri causes damage to mitochondria and triggers necrosis in infected human monocyte-derived macrophages. Infect. Immun. 73, 504–513 (2005).
Willingham, S. B. et al. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2, 147–159 (2007).
Chu, J. J. & Ng, M. L. The mechanism of cell death during West Nile virus infection is dependent on initial infectious dose. J. Gen. Virol. 84, 3305–3314 (2003).
Ray, C. A. & Pickup, D. J. The mode of death of pig kidney cells infected with cowpox virus is governed by the expression of the CrmA gene. Virology 217, 384–391 (1996).
Chan, F. K. et al. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 288, 2351–2354 (2000).
Micheau, O. & Tschopp, J. Induction of TNF receptor I- mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003). This paper elucidated the early biochemical events that are engaged on TNFR1 ligation and, in particular, showed the existence of two distinct signalling complexes, one at the plasma membrane that includes TNFR1, TRAF2 and RIP1, and one at an endocytic compartment that involves internalized TNFR1, TRADD, FADD and caspase 8.
Deveraux, Q. L. et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 17, 2215–2223 (1998).
Csomos, R. A., Brady, G. F. & Duckett, C. S. Enhanced cytoprotective effects of the inhibitor of apoptosis protein cellular IAP1 through stabilization with TRAF2. J. Biol. Chem. 284, 20531–20539 (2009).
Rothe, M., Pan, M. G., Henzel, W. J., Ayres, T. M. & Goeddel, D. V. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 83, 1243–1252 (1995).
Bertrand, M. J. et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 (2008).
Wong, W. W. et al. RIPK1 is not essential for TNFR1-induced activation of NF-κB. Cell Death Differ. 17, 482–487 (2010).
Ea, C. K., Deng, L., Xia, Z. P., Pineda, G. & Chen, Z. J. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22, 245–257 (2006).
Christofferson, D. E. & Yuan, J. Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 22, 263–268 (2010).
Ting, A. T., Pimentel-Muinos, F. X. & Seed, B. RIP mediates tumor necrosis factor receptor 1 activation of NF-κB but not Fas/APO-1-initiated apoptosis. EMBO J. 15, 6189–6196 (1996).
Shembade, N., Ma, A. & Harhaj, E. W. Inhibition of NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327, 1135–1139 (2010).
Enesa, K. et al. NF-κB suppression by the deubiquitinating enzyme Cezanne: a novel negative feedback loop in pro-inflammatory signaling. J. Biol. Chem. 283, 7036–7045 (2008).
Xu, G. et al. Ubiquitin-specific peptidase 21 inhibits tumor necrosis factor α-induced nuclear factor κB activation via binding to and deubiquitinating receptor-interacting protein 1. J. Biol. Chem. 285, 969–978 (2010).
Feng, S. et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal 19, 2056–2067 (2007).
Wang, L., Du, F. & Wang, X. TNF-α induces two distinct caspase-8 activation pathways. Cell 133, 693–703 (2008).
Lin, Y. et al. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J. Biol. Chem. 279, 10822–10828 (2004).
Ermolaeva, M. A. et al. Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nature Immunol. 9, 1037–1046 (2008).
Declercq, W., Vanden Berghe, T. & Vandenabeele, P. RIP kinases at the crossroads of cell death and survival. Cell 138, 229–232 (2009).
Vandenabeele, P., Vanden Berghe, T. & Festjens, N. Caspase inhibitors promote alternative cell death pathways. Sci. STKE 2006, pe44 (2006).
Sun, X., Yin, J., Starovasnik, M. A., Fairbrother, W. J. & Dixit, V. M. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J. Biol. Chem. 277, 9505–9511 (2002).
Kelliher, M. A. et al. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8, 297–303 (1998).
Degterev, A. et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nature Chem. Biol. 4, 313–321 (2008).
Teng, X. et al. Structure–activity relationship study of [1,2,3]thiadiazole necroptosis inhibitors. Bioorg. Med. Chem. Lett. 17, 6836–6840 (2007).
Vandenabeele, P., Declercq, W. & Berghe, T. V. Necrotic cell death and 'necrostatins': now we can control cellular explosion. Trends Biochem. Sci. 33, 352–355 (2008).
Upton, J. W., Kaiser, W. J. & Mocarski, E. S. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7, 302–313 (2010).
Soldani, C. & Scovassi, A. I. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis 7, 321–328 (2002).
Saelens, X. et al. Protein synthesis persists during necrotic cell death. J. Cell Biol. 168, 545–551 (2005).
Sun, X. M. et al. Caspase activation inhibits proteasome function during apoptosis. Mol. Cell 14, 81–93 (2004).
Leist, M., Single, B., Castoldi, A. F., Kuhnle, S. & Nicotera, P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J. Exp. Med. 185, 1481–1486 (1997).
Kraus, W. L. Transcriptional control by PARP-1: chromatin modulation, enhancer-binding, coregulation, and insulation. Curr. Opin. Cell Biol. 20, 294–302 (2008).
Los, M. et al. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell 13, 978–988 (2002).
Yu, S. W. et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297, 259–263 (2002).
Cao, G. et al. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J. Neurosci. 27, 9278–9293 (2007).
Moubarak, R. S. et al. Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol. Cell. Biol. 27, 4844–4862 (2007).
Slemmer, J. E. et al. Causal role of apoptosis-inducing factor for neuronal cell death following traumatic brain injury. Am. J. Pathol. 173, 1795–1805 (2008).
Culmsee, C. et al. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J. Neurosci. 25, 10262–10272 (2005).
Galluzzi, L., Blomgren, K. & Kroemer, G. Mitochondrial membrane permeabilization in neuronal injury. Nature Rev. Neurosci. 10, 481–494 (2009).
Boujrad, H., Gubkina, O., Robert, N., Krantic, S. & Susin, S. A. AIF-mediated programmed necrosis: a highly regulated way to die. Cell Cycle 6, 2612–2619 (2007).
Xu, Y., Huang, S., Liu, Z. G. & Han, J. Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. J. Biol. Chem. 281, 8788–8795 (2006).
Alano, C. C., Ying, W. & Swanson, R. A. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J. Biol. Chem. 279, 18895–18902 (2004).
Brenner, C. et al. Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene 19, 329–336 (2000).
Baines, C. P., Kaiser, R. A., Sheiko, T., Craigen, W. J. & Molkentin, J. D. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nature Cell Biol. 9, 550–555 (2007).
Kokoszka, J. E. et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427, 461–465 (2004).
Schulze-Osthoff, K. et al. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J. Biol. Chem. 267, 5317–5323 (1992). This paper provided the first evidence for a role of complex I-mediated ROS production in TNF-induced necrotic cell death.
Festjens, N. et al. Butylated hydroxyanisole is more than a reactive oxygen species scavenger. Cell Death Differ. 13, 166–169 (2006).
Bouche, C., Serdy, S., Kahn, C. R. & Goldfine, A. B. The cellular fate of glucose and its relevance in type 2 diabetes. Endocr. Rev. 25, 807–830 (2004).
Van Herreweghe, F. et al. Tumor necrosis factor-induced modulation of glyoxalase I activities through phosphorylation by PKA results in cell death and is accompanied by the formation of a specific methylglyoxal-derived AGE. Proc. Natl Acad. Sci. USA 99, 949–954 (2002).
Rabbani, N. & Thornalley, P. J. Dicarbonyls linked to damage in the powerhouse: glycation of mitochondrial proteins and oxidative stress. Biochem. Soc. Trans. 36, 1045–1050 (2008).
Mates, J. M. et al. Glutamine homeostasis and mitochondrial dynamics. Int. J. Biochem. Cell Biol. 41, 2051–2061 (2009).
Albrecht, J. & Norenberg, M. D. Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology 44, 788–794 (2006).
Morgan, M. J., Kim, Y. S. & Liu, Z. G. TNFα and reactive oxygen species in necrotic cell death. Cell Res. 18, 343–349 (2008).
Brookes, P. S. Mitochondrial H+ leak and ROS generation: an odd couple. Free Radic. Biol. Med. 38, 12–23 (2005).
Poyton, R. O., Ball, K. A. & Castello, P. R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 20, 332–340 (2009).
Chen, T. Y., Chi, K. H., Wang, J. S., Chien, C. L. & Lin, W. W. Reactive oxygen species are involved in FasL-induced caspase-independent cell death and inflammatory responses. Free Radic. Biol. Med. 46, 643–655 (2009).
Thon, L. et al. Ceramide mediates caspase-independent programmed cell death. FASEB J. 19, 1945–1956 (2005).
Antosiewicz, J., Ziolkowski, W., Kaczor, J. J. & Herman-Antosiewicz, A. Tumor necrosis factor-α-induced reactive oxygen species formation is mediated by JNK1-dependent ferritin degradation and elevation of labile iron pool. Free Radic. Biol. Med. 43, 265–270 (2007).
Kurz, T., Terman, A. & Brunk, U. T. Autophagy, ageing and apoptosis: the role of oxidative stress and lysosomal iron. Arch. Biochem. Biophys. 462, 220–230 (2007).
Xie, C. et al. Distinct roles of basal steady-state and induced H-ferritin in tumor necrosis factor-induced death in L929 cells. Mol. Cell. Biol. 25, 6673–6681 (2005).
Brune, B. The intimate relation between nitric oxide and superoxide in apoptosis and cell survival. Antioxid. Redox Signal. 7, 497–507 (2005).
Goss, S. P., Singh, R. J., Hogg, N. & Kalyanaraman, B. Reactions of *NO, *NO2 and peroxynitrite in membranes: physiological implications. Free Radic. Res. 31, 597–606 (1999).
Tien, M., Berlett, B. S., Levine, R. L., Chock, P. B. & Stadtman, E. R. Peroxynitrite-mediated modification of proteins at physiological carbon dioxide concentration: pH dependence of carbonyl formation, tyrosine nitration, and methionine oxidation. Proc. Natl Acad. Sci. USA 96, 7809–7814 (1999).
Davis, C. W. et al. Nitration of the mitochondrial complex I subunit NDUFB8 elicits RIP1- and RIP3-mediated necrosis. Free Radic. Biol. Med. 48, 306–317 (2010).
Cauwels, A. et al. Nitrite protects against morbidity and mortality associated with TNF- or LPS-induced shock in a soluble guanylate cyclase-dependent manner. J. Exp. Med. 206, 2915–2924 (2009).
Shiva, S. et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 204, 2089–2102 (2007).
Benedetti, A., Comporti, M. & Esterbauer, H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta 620, 281–296 (1980).
Orrenius, S., Gogvadze, V. & Zhivotovsky, B. Mitochondrial oxidative stress: implications for cell death. Annu. Rev. Pharmacol. Toxicol. 47, 143–183 (2007).
Kurz, T., Terman, A., Gustafsson, B. & Brunk, U. T. Lysosomes in iron metabolism, ageing and apoptosis. Histochem. Cell Biol. 129, 389–406 (2008).
Kurz, T., Gustafsson, B. & Brunk, U. T. Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. FEBS J. 273, 3106–3117 (2006).
Burke, J. E. & Dennis, E. A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 50, S237–S242 (2009).
Suffys, P. et al. Tumour-necrosis-factor-mediated cytotoxicity is correlated with phospholipase-A2 activity, but not with arachidonic acid release per se. Eur. J. Biochem. 195, 465–475 (1991).
Won, J. S. & Singh, I. Sphingolipid signaling and redox regulation. Free Radic. Biol. Med. 40, 1875–1888 (2006).
Kagedal, K., Zhao, M., Svensson, I. & Brunk, U. T. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem. J. 359, 335–343 (2001).
Kim, W. H., Choi, C. H., Kang, S. K., Kwon, C. H. & Kim, Y. K. Ceramide induces non-apoptotic cell death in human glioma cells. Neurochem. Res. 30, 969–979 (2005).
Luberto, C. et al. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J. Biol. Chem. 277, 41128–41139 (2002).
Ono, K., Kim, S. O. & Han, J. Susceptibility of lysosomes to rupture is a determinant for plasma membrane disruption in tumor necrosis factor α- induced cell death. Mol. Cell. Biol. 23, 665–676 (2003).
Yamashima, T. et al. Sustained calpain activation associated with lysosomal rupture executes necrosis of the postischemic CA1 neurons in primates. Hippocampus 13, 791–800 (2003).
Yamashima, T. & Oikawa, S. The role of lysosomal rupture in neuronal death. Prog. Neurobiol. 89, 343–358 (2009).
Yamashima, T. et al. Inhibition of ischaemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: a novel strategy for neuroprotection based on 'calpain-cathepsin hypothesis'. Eur. J. Neurosci. 10, 1723–1733 (1998). Monkeys undergoing temporary whole brain ischaemia were shown to be remarkably protected against hippocampal necrosis by the intravenous administration of a cathepsin B inhibitor, strongly supporting the pathophysiological relevance of the 'calpain–cathepsin' hypothesis in vivo and pointing to cathepsin inhibitors as potential neuroprotective agents.
Bano, D. et al. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell 120, 275–285 (2005).
Kirkegaard, T. et al. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 463, 549–553 (2010).
Nylandsted, J. et al. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med. 200, 425–435 (2004).
Tang, D. et al. Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)-induced HMGB1 cytoplasmic translocation and release. J. Immunol. 178, 7376–7384 (2007).
Doulias, P. T. et al. Involvement of heat shock protein-70 in the mechanism of hydrogen peroxide-induced DNA damage: the role of lysosomes and iron. Free Radic. Biol. Med. 42, 567–577 (2007).
Williamson, C. L., Dabkowski, E. R., Dillmann, W. H. & Hollander, J. M. Mitochondria protection from hypoxia/reoxygenation injury with mitochondria heat shock protein 70 overexpression. Am. J. Physiol. Heart Circ. Physiol. 294, H249–H256 (2008).
Zitvogel, L., Kepp, O. & Kroemer, G. Decoding cell death signals in inflammation and immunity. Cell 140, 798–804 (2010).
Poon, I. K., Hulett, M. D. & Parish, C. R. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ. 17, 381–397 (2010).
Lauber, K. et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113, 717–730 (2003).
Elliott, M. R. et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 (2009).
Martin, S. J. et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182, 1545–1556 (1995).
Krysko, D. V. et al. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ. 13, 2011–2022 (2006).
Krysko, O., De Ridder, L. & Cornelissen, M. Phosphatidylserine exposure during early primary necrosis (oncosis) in JB6 cells as evidenced by immunogold labeling technique. Apoptosis 9, 495–500 (2004).
Brouckaert, G. et al. Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production. Mol. Biol. Cell 15, 1089–1100 (2004).
Hirt, U. A. & Leist, M. Rapid, noninflammatory and PS-dependent phagocytic clearance of necrotic cells. Cell Death Differ. 10, 1156–1164 (2003).
Krysko, D. V., Brouckaert, G., Kalai, M., Vandenabeele, P. & D'Herde, K. Mechanisms of internalization of apoptotic and necrotic L929 cells by a macrophage cell line studied by electron microscopy. J. Morphol. 258, 336–345 (2003).
Hodge, S., Hodge, G., Scicchitano, R., Reynolds, P. N. & Holmes, M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol. 81, 289–296 (2003).
O'Brien, B. A. et al. A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J. Autoimmun. 26, 104–115 (2006).
Schrijvers, D. M., De Meyer, G. R., Herman, A. G. & Martinet, W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc. Res. 73, 470–480 (2007).
Munoz, L. E. et al. SLE — a disease of clearance deficiency? Rheumatology (Oxford) 44, 1101–1107 (2005).
Zitvogel, L. et al. Immune response against dying tumor cells. Adv. Immunol. 84, 131–179 (2004).
Casares, N. et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 202, 1691–1701 (2005).
Xu, J. et al. Extracellular histones are major mediators of death in sepsis. Nature Med. 15, 1318–1321 (2009).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Menard, C. et al. Role of murine cytomegalovirus US22 gene family members in replication in macrophages. J. Virol. 77, 5557–5570 (2003).
Mack, C., Sickmann, A., Lembo, D. & Brune, W. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc. Natl Acad. Sci. USA 105, 3094–3099 (2008).
Massoumi, R., Chmielarska, K., Hennecke, K., Pfeifer, A. & Fassler, R. CYLD inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-κB signaling. Cell 125, 665–677 (2006).
Zhang, J. et al. Impaired regulation of NF-κB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J. Clin. Invest. 116, 3042–3049 (2006).
Roach, H. I. & Clarke, N. M. Physiological cell death of chondrocytes in vivo is not confined to apoptosis. New observations on the mammalian growth plate. J. Bone Joint Surg. Br. 82, 601–613 (2000).
Barkla, D. H. & Gibson, P. R. The fate of epithelial cells in the human large intestine. Pathology 31, 230–238 (1999).
Chautan, M., Chazal, G., Cecconi, F., Gruss, P. & Golstein, P. Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr. Biol. 9, 967–970 (1999).
Lin, M. T. & Beal, M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 (2006).
Doraiswamy, P. M. & Finefrock, A. E. Metals in our minds: therapeutic implications for neurodegenerative disorders. Lancet Neurol. 3, 431–434 (2004).
Lewis, J. et al. Disruption of Hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-κB activation. J. Biol. Chem. 275, 10519–10526 (2000).
Vanden Berghe, T., Kalai, M., van Loo, G., Declercq, W. & Vandenabeele, P. Disruption of HSP90 function reverts tumor necrosis factor-induced necrosis to apoptosis. J. Biol. Chem. 278, 5622–5629 (2003).
Yuan, J., Lipinski, M. & Degterev, A. Diversity in the mechanisms of neuronal cell death. Neuron 40, 401–413 (2003).
Lim, S. Y., Davidson, S. M., Mocanu, M. M., Yellon, D. M. & Smith, C. C. The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc. Drugs Ther. 21, 467–469 (2007).
You, Z. et al. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 28, 1564–1573 (2008).
Liaudet, L. et al. Protection against hemorrhagic shock in mice genetically deficient in poly(ADP-ribose)polymerase. Proc. Natl Acad. Sci. USA 97, 10203–10208 (2000).
Mota, R. A. et al. Inhibition of poly(ADP-ribose) polymerase attenuates the severity of acute pancreatitis and associated lung injury. Lab. Invest. 85, 1250–1262 (2005).
Bonventre, J. V. et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature 390, 622–625 (1997).
Galluzzi, L. et al. No death without life: vital functions of apoptotic effectors. Cell Death Differ. 15, 1113–1123 (2008).
Galluzzi, L. et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 16, 1093–1107 (2009).
Krysko, D. V., Vanden Berghe, T., D'Herde, K. & Vandenabeele, P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods 44, 205–221 (2008).
Hayden, M. S. & Ghosh, S. Signaling to NF-κB. Genes Dev. 18, 2195–2224 (2004).
Tokunaga, F. et al. Involvement of linear polyubiquitylation of NEMO in NF-κB activation. Nature Cell Biol. 11, 123–132 (2009).
Baud, V. & Karin, M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nature Rev. Drug Discov. 8, 33–40 (2009).
Brummelkamp, T. R., Nijman, S. M., Dirac, A. M. & Bernards, R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature 424, 797–801 (2003).
Trompouki, E. et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-κB activation by TNFR family members. Nature 424, 793–796 (2003). References 167 and 168 elucidate the molecular determinants linking CYLD mutations and familial cylindromatosis. CYLD turned out to be a deubiquitylating enzyme that exerts oncosuppressive functions by negatively regulating NF-κB activation.
Nakanishi, C. & Toi, M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nature Rev. Cancer 5, 297–309 (2005).
Weinstein, I. B. Addiction to oncogenes — the Achilles heal of cancer. Science 297, 63–64 (2002).
Sun, X. et al. RIP3, a novel apoptosis-inducing kinase. J. Biol. Chem. 274, 16871–16875 (1999).
Acknowledgements
We apologize to our colleagues for not citing all primary research papers owing to space restrictions, and we thank W. Declercq for fruitful discussions. Electron microscopy pictures in Box 1 were kindly provided by D. Krysko, Ghent University, VIB, Belgium. P.V. holds a Methusalem grant from the Flemish Government (BOF09/01M00709) and is supported by the Flanders Institute for Biotechnology (VIB), the Interuniversity Poles of Attraction-Belgian Science Policy (IAP6/18), Fonds voor Wetenschappelijk Onderzoek – Vlaanderen (FWO, G.0133.05 and 3G.0218.06), The Special Research Fund of Ghent University (Geconcerteerde Onderzoekstacties 12.0505.02) and the European Commission (EU Marie Curie Training and Mobility Program, ApopTrain, MRTN-CT-035,624; EU FP7 Integrated Project, APO-SYS, HEALTH-F4-2007-200,767; EU FP6 Integrated Project, Epistem, LSHB-CT-2005-019,067; Marie Curie Training and Mobility Program). L.G. and T.V.B. are financed by APO-SYS and FWO, respectively. G.K. is supported by Ligue Nationale contre le Cancer (Equipe labellisée), Agence Nationale pour la Recherche (ANR), the European Commission (APO-SYS, ChemoRes, ApopTrain, Active p53), Fondation pour la Recherche Médicale (FRM), Institut National du Cancer (INCa) and Cancéropôle Ile-de-France.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (Table)
Components of the molecular machinery for programmed necrosis. (PDF 137 kb)
Supplementary information S2 (Table)
Examples of RIP-targeting strategies underscoring the pathophysiological relevance of necroptosis in vitro (PDF 249 kb)
Supplementary Information S3 (Table)
Examples of pharmacological interventions underscoring the pathophysiological relevance of necroptosis-related factors in vivo (PDF 177 kb)
Supplementary Information S4 (Table)
Examples of genetic interventions underscoring the pathophysiological relevance of necroptosis-related factors in vivo (PDF 226 kb)
Related links
Glossary
- Heterophagy
-
A term of Greek origin indicating the cellular digestion of an exogenous substance, cell or subcellular particle that has been taken up from the extracellular microenvironment.
- Autophagy
-
A pathway for the recycling of cellular contents, in which materials inside the cell are packaged into vesicles and are then targeted to the vacuole or lysosome for bulk turnover. Autophagy is thought to be prominently cytoprotective.
- Caspase
-
A Cys protease that cleaves its substrate after an Asp residue. Caspases play a crucial part in both the initiation (caspase 2, caspase 8, caspase 9 and caspase 10) and execution (caspase 3, caspase 6 and caspase 7) of apoptosis, and they are also required for many processes that are unrelated to cell death, such as the differentiation of several cell types161.
- Glutaminolysis
-
The bioenergetic pathway by which Glu or Gln is converted to α-ketoglutarate, an intermediate of the Krebs cycle. Thus, glutaminolysis can provide substrates for ATP generation by oxidative phosphorylation or stimulate the generation of pyruvate through malate decarboxylation.
- Mitochondrial permeability transition
-
Long-lasting openings of the PTPC lead to an abrupt increase in the inner mitochondrial membrane's permeability to ions and low-molecular-mass solutes, thus provoking osmotic swelling of the mitochondrial matrix and rupture of the mitochondrial outer membrane.
- Apoptotic body
-
A membrane-surrounded vesicle that is shed from dying cells during the late stages of apoptosis and that may include portions of the nucleus and/or apparently normal organelles.
- Inflammasome
-
A supramolecular complex comprising a pattern recognition receptor (such as NLRP3) and an adaptor protein (such as ASC) that is required for the autocatalytic activation of pro-caspase 1. Active caspase 1 catalyses the proteolytic maturation of interleukin-1β, a potent pro-inflammatory cytokine.
- Activation-induced cell death
-
After an adaptive immune response, superfluous lymphocytes are eliminated on T cell receptor re-stimulation by a mechanism that may involve the CD95–CD95L system.
- Polyubiquitylation
-
The attachment of chains of the small protein ubiquitin to Lys residues of proteins, often as a tag for rapid cellular degradation.
- Necrostatin 1
-
A Trp-based molecule (5-(1H-indol-3-ylmethyl)-3-methyl-2-thioxo-4- imidazolidinone) that was first identified as a specific and potent inhibitor of necroptosis7.
- Oncogene addiction
-
An expression coined by Weinberg in 2002 (Ref. 170) to describe the observation that tumour maintenance often depends on the continued activity of some oncogenes.
- Mitochondrial transmembrane potential (Δψm)
-
The electrochemical gradient built across the inner mitochondrial membrane by the proton pumps associated with the respiratory chain. The Δψm creates a proton-moving force that is required for mitochondrial ATP generation by the F1–FO ATP synthase, and its permanent dissipation is considered an early sign of apoptosis.
- Advanced glycation end product (AGE)
-
The product of a chain of chemical reactions that most often is initiated by non-enzymatic protein glycosylation. Increased extracellular glucose favours the accumulation of AGEs, which interact with specific receptors on the plasma membrane to stimulate the generation of intracellular ROS.
- Haber–Weiss reaction
-
The generation of hydroxyl radicals from hydrogen peroxide and superoxide (H2O2 + O.2− ← OH.+HO−+O2). The reaction is very slow, but is catalysed by ferric ions (Fe3+).
- Fenton reaction
-
The ferrous ion (Fe2+)-dependent decomposition of dihydrogen peroxide, generating the highly reactive hydroxyl radical (Fe2+ + H2O2 ← Fe3+ + OH. + OH−).
- Lipid peroxidation
-
The biochemical reaction whereby free radicals 'steal' electrons from lipids in cell membranes, resulting in ultrastructural damage to organelles.
- Labile iron pool
-
A cytosolic fraction of iron ions loosely bound to macromolecules (for example, ferritin) — also known as a chelatable iron pool — that harbours the metabolically active (and hence potentially toxic) forms of ferrous (Fe2+) and ferric (Fe3+) ions.
- Oxidative phosphorylation
-
The process whereby respiratory chain complexes embedded in the inner mitochondrial membrane catalyse a series of redox reactions that provide the free energy to generate the Δψm.
- Macropinosome
-
A large intracellular vesicle filled with extracellular fluids and macromolecules that is formed by macropinocytosis.
Rights and permissions
About this article
Cite this article
Vandenabeele, P., Galluzzi, L., Vanden Berghe, T. et al. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11, 700–714 (2010). https://doi.org/10.1038/nrm2970
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2970
This article is cited by
-
Ancestral allele of DNA polymerase gamma modifies antiviral tolerance
Nature (2024)
-
Venetoclax efficacy on acute myeloid leukemia is enhanced by the combination with butyrate
Scientific Reports (2024)
-
Antioxidant and anti-inflammatory properties of hydroalcoholic extracts from Mammillaria candida and Turbinicarpus laui (Cactaceae) in vitro cultures
Plant Cell, Tissue and Organ Culture (PCTOC) (2024)
-
Novel PIKfyve/Tubulin Dual-target Inhibitor as a Promising Therapeutic Strategy for B-cell Acute Lymphoblastic Leukemia
Current Medical Science (2024)
-
Necroptosis inhibitors: mechanisms of action and therapeutic potential
Apoptosis (2024)