Key Points
-
Vaccines against cancer aim to induce both tumour-specific effector T cells that can reduce the tumour mass and tumour-specific memory T cells that can control tumour relapse.
-
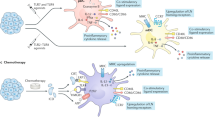
Dendritic cells (DCs) induce and regulate immune responses; therefore, they are a crucial target and tool for vaccination. The immunogenicity of antigens delivered by DCs has now been shown in patients with cancer.
-
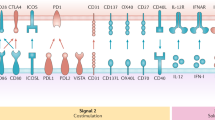
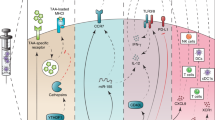
Basic principles of DC biology in the context of vaccination are discussed. By examining these principles, it is evident that certain parameters of vaccination with DCs need to be refined to improve efficacy. These include strategies for loading DCs with tumour antigens; manipulation of the different DC-maturation signals that can lead to different types of induced immune response; and promotion of the migration of DCs from the site of injection to draining lymph nodes.
-
The importance of different DC subsets leading to the induction of distinct immune responses is reviewed. This complexity of the DC system requires that each DC subset be tested for efficacy at inducing antitumour responses in vivo. The ultimate ex vivo-generated DC vaccine will be heterogeneous and composed of several subsets, each of which will target a specific immune effector.
-
The challenges of effective vaccination against chronic diseases, including cancer, are highlighted, particularly the exhaustion of antigen-specific T cells (owing to chronic activation) and the existence of other immune mechanisms that might hinder vaccine efficacy (for example, the development of tumour-antigen-specific regulatory T cells).
-
Immune correlates of efficacy of DC vaccines are defined, including breadth of induced tumour-specific immunity, induction of tumour-specific effector and memory T cells, induction of T cells that kill tumour cells, and decreased numbers of T cells with regulatory function.
Abstract
Mouse studies have shown that the immune system can reject tumours, and the identification of tumour antigens that can be recognized by human T cells has facilitated the development of immunotherapy protocols. Vaccines against cancer aim to induce tumour-specific effector T cells that can reduce the tumour mass, as well as tumour-specific memory T cells that can control tumour relapse. Owing to their capacity to regulate T-cell immunity, dendritic cells are increasingly used as adjuvants for vaccination, and the immunogenicity of antigens delivered by dendritic cells has now been shown in patients with cancer. A better understanding of how dendritic cells regulate immune responses will allow us to better exploit these cells to induce effective antitumour immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Steinman, R. M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9, 271–296 (1991). A comprehensive and excellent review of DC biology that laid the groundwork for current approaches to investigating DCs and their use as a therapy.
Banchereau, J. et al. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811 (2000).
Mellman, I. & Steinman, R. M. Dendritic cells: specialized and regulated antigen processing machines. Cell 106, 255–258 (2001).
Caux, C. et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte–macrophage colony-stimulating factor plus tumor necrosis factor α: II. Functional analysis. Blood 90, 1458–1470 (1997).
Jego, G. et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19, 225–234 (2003).
Fernandez, N. C. et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nature Med. 5, 405–411 (1999).
Kadowaki, N. et al. Distinct cytokine profiles of neonatal natural killer T cells after expansion with subsets of dendritic cells. J. Exp. Med. 193, 1221–1226 (2001).
Nossal, G. J. Host immunobiology and vaccine development. Lancet 350, 1316–1319 (1997).
Davis, I. D., Jefford, M., Parente, P. & Cebon, J. Rational approaches to human cancer immunotherapy. J. Leukoc. Biol. 73, 3–29 (2003). A comprehensive and excellent review of the development of immunotherapy for cancer, including DC-based vaccination trials.
Gilboa, E., Nair, S. K. & Lyerly, H. K. Immunotherapy of cancer with dendritic-cell-based vaccines. Cancer Immunol. Immunother. 46, 82–87 (1998).
Lu, W., Arraes, L. C., Ferreira, W. T. & Andrieu, J. M. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nature Med. 10, 1359–1365 (2004).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nature Med. 10, 909–915 (2004).
Foley, E. J. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res. 13, 835–837 (1953).
Dighe, A. S., Richards, E., Old, L. J. & Schreiber, R. D. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN γ receptors. Immunity 1, 447–456 (1994).
Euvrard, S., Kanitakis, J. & Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 348, 1681–1691 (2003).
Hakim, F. T., Flomerfelt, F. A., Boyiadzis, M. & Gress, R. E. Aging, immunity and cancer. Curr. Opin. Immunol. 16, 151–156 (2004).
Steinman, R. M. & Mellman, I. Immunotherapy: bewitched, bothered, and bewildered no more. Science 305, 197–200 (2004).
Townsend, A. R., Gotch, F. M. & Davey, J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell 42, 457–467 (1985).
Boon, T., Cerottini, J. C., Van den Eynde, B., van der Bruggen, P. & Van Pel, A. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 12, 337–365 (1994).
Rosenberg, S. A. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol. Today 18, 175–182 (1997).
Pardoll, D. M. Cancer vaccines. Nature Med. 4, 525–531 (1998).
Gilboa, E. The makings of a tumor rejection antigen. Immunity 11, 263–270 (1999).
Finn, O. J. Cancer vaccines: between the idea and the reality. Nature Rev. Immunol. 3, 630–641 (2003).
Antonia, S., Mule, J. J. & Weber, J. S. Current developments of immunotherapy in the clinic. Curr. Opin. Immunol. 16, 130–136 (2004).
Hsueh, E. C. & Morton, D. L. Antigen-based immunotherapy of melanoma: Canvaxin therapeutic polyvalent cancer vaccine. Semin. Cancer Biol. 13, 401–407 (2003).
Sondak, V. K. & Sosman, J. A. Results of clinical trials with an allogenic melanoma tumor cell lysate vaccine: Melacine. Semin. Cancer Biol. 13, 409–415 (2003).
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003). An outstanding review that summarizes studies on tolerogenic DCs, written by the researchers who were the first to propose and show that DCs can induce immune tolerance.
Caux, C., Dezutter-Dambuyant, C., Schmitt, D. & Banchereau, J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature 360, 258–261 (1992).
Romani, N. et al. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180, 83–93 (1994).
Sallusto, F. & Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179, 1109–1118 (1994).
Shortman, K. & Liu, Y. J. Mouse and human dendritic cell subtypes. Nature Rev. Immunol. 2, 151–161 (2002). A comprehensive and excellent review that summarizes our current knowledge of subsets of DCs that have different biological functions.
Caux, C. et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF + TNF α. J. Exp. Med. 184, 695–706 (1996).
Siegal, F. P. et al. The nature of the principal type 1 interferon-producing cells in human blood. Science 284, 1835–1837 (1999).
Cella, M. et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce high levels of type I IFN. Nature Med. 5, 919–923 (1999).
Kadowaki, N., Antonenko, S., Lau, J. Y. & Liu, Y. J. Natural interferon α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 192, 219–226 (2000).
Fonteneau, J. F. et al. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood 101, 3520–3526 (2003).
Randolph, G. J., Inaba, K., Robbiani, D. F., Steinman, R. M. & Muller, W. A. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11, 753–761 (1999).
Chomarat, P., Banchereau, J., Davoust, J. & Palucka, A. K. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nature Immunol. 1, 510–514 (2000).
Peters, J. H. et al. Signals required for differentiating dendritic cells from human monocytes in vitro. Adv. Exp. Med. Biol. 329, 275–280 (1993).
Paquette, R. L. et al. Interferon-α and granulocyte– macrophage colony-stimulating factor differentiate peripheral blood monocytes into potent antigen-presenting cells. J. Leukoc. Biol. 64, 358–367 (1998).
Luft, T. et al. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 161, 1947–1953 (1998).
Santini, S. M. et al. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 191, 1777–1788 (2000).
Blanco, P., Palucka, A. K., Gill, M., Pascual, V. & Banchereau, J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 294, 1540–1543 (2001).
Chomarat, P., Dantin, C., Bennett, L., Banchereau, J. & Palucka, A. K. TNF skews monocyte differentiation from macrophages to dendritic cells. J. Immunol. 171, 2262–2269 (2003).
Mohamadzadeh, M. et al. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J. Exp. Med. 194, 1013–1020 (2001).
Seifert, U. et al. An essential role for tripeptidyl peptidase in the generation of an MHC class I epitope. Nature Immunol. 4, 375–379 (2003).
Maldonado-Lopez, R. et al. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189, 587–592 (1999).
Pulendran, B. et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl Acad. Sci. USA 96, 1036–1041 (1999).
Pulendran, B. et al. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 167, 5067–5076 (2001).
Rissoan, M. C. et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science 283, 1183–1186 (1999).
Brocker, T. The role of dendritic cells in T cell selection and survival. J. Leukoc. Biol. 66, 331–335 (1999).
Jonuleit, H., Schmitt, E., Schuler, G., Knop, J. & Enk, A. H. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192, 1213–1222 (2000).
Dhodapkar, M. V., Steinman, R. M., Krasovsky, J., Munz, C. & Bhardwaj, N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193, 233–238 (2001).
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196, 1627–1638 (2002).
Bonifaz, L. C. et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 199, 815–824 (2004).
Yamazaki, S. et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 198, 235–247 (2003).
Nestle, F. O. et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nature Med. 4, 328–332 (1998).
Thurner, B. et al. Vaccination with Mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J. Exp. Med. 190, 1669–1678 (1999).
Hsu, F. J. et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nature Med. 2, 52–58 (1996).
Small, E. J. et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J. Clin. Oncol. 18, 3894–3903 (2000).
Fong, L. et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc. Natl Acad. Sci. USA 98, 8809–8814 (2001).
Banchereau, J. et al. Immune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 61, 6451–6458 (2001).
Figdor, C. G., de Vries, I. J., Lesterhuis, W. J. & Melief, C. J. Dendritic cell immunotherapy: mapping the way. Nature Med. 10, 475–480 (2004).
Jonuleit, H. et al. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int. J. Cancer 93, 243–251 (2001).
Schuler-Thurner, B. et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J. Exp. Med. 195, 1279–1288 (2002).
Jonuleit, H. et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum- free conditions. Eur. J. Immunol. 27, 3135–3142 (1997).
Mailliard, R. B. et al. α-Type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 64, 5934–5937 (2004).
Reis e Sousa, C. Dendritic cells as sensors of infection. Immunity 14, 495–498 (2001). This paper summarizes the interactions of DCs with pathogens, and it provides background and rationale for the use of microbial products for DC maturation.
Pasare, C. & Medzhitov, R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity 21, 733–741 (2004).
Eriksson, U. et al. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nature Med. 9, 1484–1490 (2003).
Kadowaki, N. et al. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194, 863–869 (2001).
Morse, M. A. et al. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 59, 56–58 (1999).
Martin-Fontecha, A. et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198, 615–621 (2003).
Scandella, E., Men, Y., Gillessen, S., Forster, R. & Groettrup, M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood 100, 1354–1361 (2002).
Luft, T. et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E2 regulates the migratory capacity of specific DC subsets. Blood 100, 1362–1372 (2002).
Sallusto, F. et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 28, 2760–2769 (1998).
Fong, L., Brockstedt, D., Benike, C., Wu, L. & Engleman, E. G. Dendritic cells injected via different routes induce immunity in cancer patients. J. Immunol. 166, 4254–4259 (2001).
Mora, J. R. et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424, 88–93 (2003).
Mullins, D. W. et al. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J. Exp. Med. 198, 1–13 (2003).
Palucka, A. K. et al. Single injection of CD34+ progenitor-derived dendritic cell vaccine can lead to induction of T-cell immunity in patients with stage IV melanoma. J. Immunother. 26, 432–439 (2003).
Paczesny, S. et al. Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells. J. Exp. Med. 199, 1503–1511 (2004).
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Kaech, S. M., Wherry, E. J. & Ahmed, R. Effector and memory T-cell differentiation: implications for vaccine development. Nature Rev. Immunol. 2, 251–262 (2002).
Zinkernagel, R. M. On natural and artificial vaccinations. Annu. Rev. Immunol. 21, 515–546 (2003).
Zajac, A. J. et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998).
Wherry, E. J., Blattman, J. N., Murali-Krishna, K., van der Most, R. & Ahmed, R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003).
Wang, R. F., Wang, X., Atwood, A. C., Topalian, S. L. & Rosenberg, S. A. Cloning genes encoding MHC class II-restricted antigens: mutated CDC27 as a tumor antigen. Science 284, 1351–1354 (1999).
Salgaller, M. L., Marincola, F. M., Cormier, J. N. & Rosenberg, S. A. Immunization against epitopes in the human melanoma antigen gp100 following patient immunization with synthetic peptides. Cancer Res. 56, 4749–4757 (1996).
Solinger, A. M., Ultee, M. E., Margoliash, E. & Schwartz, R. H. T-lymphocyte response to cytochrome c. I. Demonstration of a T-cell heteroclitic proliferative response and identification of a topographic antigenic determinant on pigeon cytochrome c whose immune recognition requires two complementing major histocompatibility complex-linked immune response genes. J. Exp. Med. 150, 830–848 (1979).
Parkhurst, M. R. et al. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J. Immunol. 157, 2539–2548 (1996).
Stuge, T. B. et al. Diversity and recognition efficiency of T cell responses to cancer. PLoS Med. 1, e28 (2004).
Zitvogel, L. et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nature Med. 4, 594–600 (1998).
Ribas, A., Butterfield, L. H., Glaspy, J. A. & Economou, J. S. Cancer immunotherapy using gene-modified dendritic cells. Curr. Gene Ther. 2, 57–78 (2002).
Boczkowski, D., Nair, S. K., Snyder, D. & Gilboa, E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 184, 465–472 (1996).
Regnault, A. et al. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189, 371–380 (1999).
Fong, L. & Engleman, E. G. Dendritic cells in cancer immunotherapy. Annu. Rev. Immunol. 18, 245–273 (2000).
Albert, M. L., Sauter, B. & Bhardwaj, N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392, 86–89 (1998).
Berard, F. et al. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J. Exp. Med. 192, 1535–1544 (2000).
Neidhardt-Berard, E. M., Berard, F., Banchereau, J. & Palucka, A. K. Dendritic cells loaded with killed breast cancer cells induce differentiation of tumor-specific cytotoxic T lymphocytes. Breast Cancer Res. 6, R322–R328 (2004).
Jordan, M. S. et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nature Immunol. 2, 301–306 (2001).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunol. 4, 330–336 (2003).
Chen, Y., Kuchroo, V. K., Inobe, J., Hafler, D. A. & Weiner, H. L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 265, 1237–1240 (1994).
Groux, H. et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389, 737–742 (1997).
Roncarolo, M. G., Bacchetta, R., Bordignon, C., Narula, S. & Levings, M. K. Type 1 T regulatory cells. Immunol. Rev. 182, 68–79 (2001).
Steitz, J., Bruck, J., Lenz, J., Knop, J. & Tuting, T. Depletion of CD25+ CD4+ T cells and treatment with tyrosinase-related protein 2-transduced dendritic cells enhance the interferon α-induced, CD8+ T-cell-dependent immune defense of B16 melanoma. Cancer Res. 61, 8643–8646 (2001).
Sutmuller, R. P. et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J. Exp. Med. 194, 823–832 (2001).
Woo, E. Y. et al. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 61, 4766–4772 (2001).
Viguier, M. et al. Foxp3 expressing CD4+CD25high regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J. Immunol. 173, 1444–1453 (2004).
Wang, H. Y. et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity 20, 107–118 (2004). This study was the first to show tumour-specific inducible regulatory T cells in humans.
Terabe, M. et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R–STAT6 pathway. Nature Immunol. 1, 515–520 (2000).
North, R. J. The murine antitumor immune response and its therapeutic manipulation. Adv. Immunol. 35, 89–155 (1984). This paper shows the existence of T Reg cells and describes early approaches to improve immunity to tumours by removal of these regulatory cells with cyclophosphamide. This strategy has been rediscovered recently and is the focus of several studies.
Ma, J. et al. Anti-tumor T cell response and protective immunity in mice that received sublethal irradiation and immune reconstitution. Eur. J. Immunol. 33, 2123–2132 (2003).
Asavaroengchai, W., Kotera, Y. & Mule, J. J. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc. Natl Acad. Sci. USA 99, 931–936 (2002).
Berd, D. & Mastrangelo, M. J. Effect of low dose cyclophosphamide on the immune system of cancer patients: depletion of CD4+, 2H4+ suppressor-inducer T-cells. Cancer Res. 48, 1671–1675 (1988).
Hoon, D. S., Foshag, L. J., Nizze, A. S., Bohman, R. & Morton, D. L. Suppressor cell activity in a randomized trial of patients receiving active specific immunotherapy with melanoma cell vaccine and low dosages of cyclophosphamide. Cancer Res. 50, 5358–5364 (1990).
Pardoll, D. & Allison, J. Cancer immunotherapy: breaking the barriers to harvest the crop. Nature Med. 10, 887–892 (2004).
Gabrilovich, D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nature Rev. Immunol. 4, 941–952 (2004).
Marrack, P., Kappler, J. & Mitchell, T. Type I interferons keep activated T cells alive. J. Exp. Med. 189, 521–530 (1999).
Tough, D. F., Sun, S., Zhang, X. & Sprent, J. Stimulation of naive and memory T cells by cytokines. Immunol. Rev. 170, 39–47 (1999).
Phan, G. Q., Wang, E. & Marincola, F. M. T-cell-directed cancer vaccines: mechanisms of immune escape and immune tolerance. Expert Opin. Biol. Ther. 1, 511–523 (2001).
Lanzavecchia, A. & Sallusto, F. Regulation of T cell immunity by dendritic cells. Cell 106, 263–266 (2001).
Rossi, D. & Zlotnik, A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18, 217–242 (2000).
Sun, J. C. & Bevan, M. J. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300, 339–342 (2003).
Shedlock, D. J. & Shen, H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300, 337–339 (2003).
Janssen, E. M. et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421, 852–856 (2003). References 123–125 show the crucial role of CD4+ T cells for priming functional CD8+ T-cell immunity, including CD8+ T-cell memory.
Northrop, J. K. & Shen, H. CD8+ T-cell memory: only the good ones last. Curr. Opin. Immunol. 16, 451–455 (2004).
Kronenberg, M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 19 Jan 2005 (10.1146/annurev.immunol.23.021704.115742).
Cerwenka, A. & Lanier, L. L. Natural killer cells, viruses and cancer. Nature Rev. Immunol. 1, 41–49 (2001).
Qin, Z. et al. B cells inhibit induction of T cell-dependent tumor immunity. Nature Med. 4, 627–630 (1998).
Harris, D. P. et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nature Immunol. 1, 475–482 (2000).
Figdor, C. G., van Kooyk, Y. & Adema, G. J. C-type lectin receptors on dendritic cells and Langerhans cells. Nature Rev. Immunol. 2, 77–84 (2002).
Valladeau, J. et al. The monoclonal antibody DCGM4 recognizes Langerin, a protein specific of Langerhans cells, and is rapidly internalized from the cell surface. Eur. J. Immunol. 29, 2695–2704 (1999).
Valladeau, J. et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity 12, 71–81 (2000).
Dzionek, A. et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 165, 6037–6046 (2000).
Dzionek, A. et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J. Exp. Med. 194, 1823–1834 (2001).
Albert, M. L. et al. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nature Med. 4, 1321–1324 (1998).
Inaba, K., Metlay, J. P., Crowley, M. T. & Steinman, R. M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J. Exp. Med. 172, 631–640 (1990).
Sornasse, T. et al. Antigen-pulsed dendritic cells can efficiently induce an antibody response in vivo. J. Exp. Med. 175, 15–21 (1992).
Zitvogel, L. et al. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J. Exp. Med. 183, 87–97 (1996).
Celluzzi, C. M., Mayordomo, J. I., Storkus, W. J., Lotze, M. T. & Falo, L. D. Jr. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J. Exp. Med. 183, 283–287 (1996).
Nair, S. K., Snyder, D., Rouse, B. T. & Gilboa, E. Regression of tumors in mice vaccinated with professional antigen-presenting cells pulsed with tumor extracts. Int. J. Cancer 70, 706–715 (1997).
Fu, F. et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86−) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation 62, 659–665 (1996).
Soumelis, V. et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nature Immunol. 3, 673–680 (2002).
Scheibenbogen, C. et al. Phase 2 trial of vaccination with tyrosinase peptides and granulocyte–macrophage colony-stimulating factor in patients with metastatic melanoma. J. Immunother. 23, 275–281 (2000).
Cebon, J. et al. Two Phase I studies of low dose recombinant human IL-12 with Melan-A and influenza peptides in subjects with advanced malignant melanoma. Cancer Immun. 3, 7–20 (2003).
Soiffer, R. et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte–macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA 95, 13141–13146 (1998).
Mitchell, M. S. et al. Phase I trial of large multivalent immunogen derived from melanoma lysates in patients with disseminated melanoma. Clin. Cancer Res. 10, 76–83 (2004).
Hersey, P. et al. Phase I/II study of treatment with dendritic cell vaccines in patients with disseminated melanoma. Cancer Immunol. Immunother. 53, 125–134 (2004).
Acknowledgements
We thank our patients for volunteering to participate in our studies. We thank our colleagues and collaborators, including J. Connolly, M. Dhodapkar, J. Fay, S. Paczesny, R. Steinman and H. Ueno, for their contributions to our progress. We are grateful to all former and current members of the Baylor Institute for Immunology Research (Dallas, United States). We thank M. Ramsay and W. Duncan for support. We thank M. Dhodapkar, R. Steinman and J. Weber for comments on the manuscript. We dedicate this review to P. Pascual. This research was supported by grants from the Baylor Health Care System Foundation and the National Institutes of Health (United States). J.B. is the recipient of the W. W. Caruth Jr Chair in Organ Transplantation Immunology. A.K.P. is the recipient of the Michael A. E. Ramsay Chair for Cancer Immunology Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Jacques Banchereau has been a consultant for Argos Therapeutics and is a scientific founder of ODC Therapy, Inc., which are both private companies. He has stock options in both. A. Karolina Palucka is a scientific founder of ODC Therapy, Inc., and she also has stock options. These private companies undertake research associated with dendritic-cell vaccines. Neither one of them has in any way supported the clinical trials, the results of which are described in this paper.
Glossary
- IMMUNOSENESCENCE
-
The decreased function of the immune system with age. In particular, the number of naive T cells decreases as thymic function decreases.
- CENTRAL TOLERANCE
-
The lack of self-responsiveness that occurs as lymphocytes develop. It is associated with the deletion of autoreactive clones. For T cells, this occurs in the thymus.
- PERIPHERAL TOLERANCE
-
The lack of self-responsiveness of mature lymphocytes to specific antigens. It is associated with suppression of production of self-reactive antibodies by B cells and inhibition of self-reactive effector cells, such as cytotoxic T lymphocytes and natural killer cells.
- ANERGY
-
A state of T cells that have been stimulated through their T-cell receptor in the absence of ligation of the co-stimulatory molecule CD28. On restimulation, these T cells cannot produce interleukin-2 or proliferate, even in the presence of co-stimulatory signals.
- INDUCIBLE REGULATORY T CELLS
-
A subset of CD4+ T cells that mediate their effects through the secretion of cytokines such as interleukin-10 and transforming growth factor-β, which inhibit other T cells. Their role is to maintain self-tolerance.
- CD4+CD25+ REGULATORY T CELLS
-
(TReg cells). A subset of CD4+ T cells that express high levels of CD25 (also known as the interleukin-2 receptor α-chain), are naturally anergic and require stimulation through the T-cell receptor for induction of their cell-mediated suppressive function. Their role is to maintain self-tolerance.
- ELISPOT
-
(Enzyme-linked immunosorbent spot). An antibody-capture-based method for enumerating specific T cells (CD4+ and CD8+) that secrete a particular cytokine (often interferon-γ).
- TUMOUR-ASSOCIATED ANTIGENS
-
Antigens that are expressed by tumour cells. These belong to three main categories: tissue-differentiation antigens, which are also expressed by non-malignant cells; mutated or aberrantly expressed molecules; and cancer testis antigens, which are normally expressed only by spermatocytes and occasionally in the placenta.
- MODIFIED HETEROCLITIC PEPTIDES
-
Short amino-acid sequences in which certain amino acids are replaced to ensure increased binding to MHC class I molecules.
- EXOSOMES
-
Small lipid-bilayer vesicles that are released from dendritic cells and other cells. They are composed of cell membranes or are derived from the membranes of intracellular vesicles. They might contain antigen–MHC complexes and interact with antigen-specific lymphocytes directly, or they might be taken up by other antigen-presenting cells.
- CROSS-PRIMING
-
The initiation of a CD8+ T-cell response to an antigen that is not present within antigen-presenting cells: in this case, dendritic cells. This occurs through the ability of dendritic cells to present peptides that are derived from exogenous antigens in the context of MHC class I molecules. This property is atypical, as most cells exclusively present peptides derived from endogenous proteins in the context of MHC class I molecules.
- IMMUNOEDITING
-
The generation of tumour variants with reduced immunogenicity, which might therefore escape from immune responses.
Rights and permissions
About this article
Cite this article
Banchereau, J., Palucka, A. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol 5, 296–306 (2005). https://doi.org/10.1038/nri1592
Issue Date:
DOI: https://doi.org/10.1038/nri1592
This article is cited by
-
Emerging strategies for biomaterial-assisted cancer immunotherapy
Korean Journal of Chemical Engineering (2022)
-
Low efficiency of leucocyte plugging-based drug delivery to cancer in mice
Drug Delivery and Translational Research (2022)
-
Infiltration of CD1a-positive dendritic cells in advanced laryngeal cancer correlates with unfavorable outcomes post-laryngectomy
BMC Cancer (2021)
-
Postponing tumor onset and tumor progression can be achieved by alteration of local tumor immunity
Cancer Cell International (2021)
-
A DNA nanodevice-based vaccine for cancer immunotherapy
Nature Materials (2021)