Key Points
-
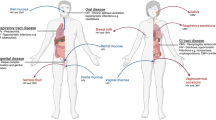
In the current HIV/AIDS pandemic, most new infections are acquired by vaginal transmission.
-
Studies of this route of transmission in an animal model — monkeys infected intravaginally with simian immunodeficiency virus (SIV), a close relative of HIV-1 — have led to a new appreciation of the importance of events in the first weeks after exposure in determining the subsequent course of the slow infections that are caused by HIV-1 and SIV.
-
In their initial encounter at mucosal front lines, the virus and the host both face perils.
-
The host is vulnerable because it continuously renews cells at mucosal surfaces, and these cells — activated and recently activated but ostensibly resting CD4+ memory T cells — support viral replication.
-
The large population of 'resting' host CD4+ T cells at mucosal surfaces enables virus to establish a beachhead at the point of entry, and the huge number of these cells in gut-associated lymphoid tissue provides fuel for virus production, allowing the spread of infection throughout the lymphoid tissues.
-
The host is also vulnerable to massive depletion of CD4+ T cells in the gut and to blunting of immune responses by regulatory T cells.
-
Both vulnerabilities are associated with mechanisms that operate in the mucosal immune system to maintain a state of vigilance without causing immunopathological damage: CD95–CD95-ligand-mediated apoptosis of CD4+ effector memory T cells in the lamina propria, and an immunosuppressive response to immune activation.
-
Virus is most vulnerable at the point of entry for several days after exposure. At this stage, microbicides or a robust vaccine-induced mucosal immune response could prevent establishment of a self-propagating infection, both locally and systemically.
Abstract
HIV-1 and simian immunodeficiency virus (SIV), as well as their hosts, face perils at mucosal front lines in early infection. At these sites, 'resting' CD4+ memory T cells fuel infection (because they are hosts for virus), depleting CD4+ memory T cells throughout the lymphoid tissues, particularly in the gut, and eliciting an immunosuppressive regulatory T-cell response that impairs host defence. But HIV-1 and SIV also risk elimination at the earliest stage of infection, at the mucosal point of entry, if founder populations of infected cells do not expand sufficiently to establish a self-propagating infection. Microbicides and vaccines could increase these viral vulnerabilities at mucosal front lines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kaposi's sarcoma and pneumocystis pneumonia among homosexual men — New York City and California. MMWR Morb. Mortal. Wkly Rep. 30, 305–308 (1981).
Pneumocystis pneumonia — Los Angeles. MMWR Morb. Mortal. Wkly Rep. 30, 250–252 (1981).
Joint United Nations Programme on HIV/AIDS & World Health Organization. AIDS Epidemic Update: 2004 (Joint United Nations Programme on HIV/AIDS, Geneva, 2004).
Annan, K. In Africa, AIDS has a woman's face. New York Times (New York) 9 (29 Dec 2002).
Haase, A. T. Pathogenesis of lentivirus infections. Nature 322, 130–136 (1986).
Anderson, R. M. & May, R. M. Infectious diseases of humans: dynamics and control (Oxford Univ. Press, New York, 1991).
Mowat, A. M. & Viney, J. L. The anatomical basis of intestinal immunity. Immunol. Rev. 156, 145–166 (1997).
Pope, M. & Haase, A. T. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nature Med. 9, 847–852 (2003).
Richman, D. D., Wrin, T., Little, S. J. & Petropoulos, C. J. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl Acad. Sci. USA 100, 4144–4149 (2003).
Allen, T. M. et al. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407, 386–390 (2000).
Goulder, P. J. & Watkins, D. I. HIV and SIV CTL escape: implications for vaccine design. Nature Rev. Immunol. 4, 630–640 (2004).
Borrow, P. et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nature Med. 3, 205–211 (1997).
Price, D. A. et al. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl Acad. Sci. USA 94, 1890–1895 (1997).
Chun, T. W. et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387, 183–188 (1997).
Persaud, D., Zhou, Y., Siliciano, J. M. & Siliciano, R. F. Latency in human immunodeficiency virus type 1 infection: no easy answers. J. Virol. 77, 1659–1665 (2003).
Finzi, D. et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997).
Wong, J. K. et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278, 1291–1295 (1997).
Haase, A. T. et al. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science 274, 985–989 (1996).
Schacker, T. et al. Rapid accumulation of human immunodeficiency virus (HIV) in lymphatic tissue reservoirs during acute and early HIV infection: implications for timing of antiretroviral therapy. J. Infect. Dis. 181, 354–357 (2000).
Heath, S. L., Tew, J. G., Tew, J. G., Szakal, A. K. & Burton, G. F. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature 377, 740–744 (1995).
Douek, D. C. et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417, 95–98 (2002).
Li, Q. et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434, 1148–1152 (2005). Documents the role of infected resting CD4+ T cells in peak viral replication and the roles of direct killing and apoptosis in the massive depletion of CD4+ T cells in the gut lamina propria.
Mattapallil, J. J. et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434, 1093–1097 (2005). Shows that a high proportion of CD4+ memory T cells in all lymphoid tissues are infected (that is, viral-DNA positive) and then depleted, which is consistent with direct killing as the mechanism of depletion. Both activated, proliferating cells and 'resting' cells are infected, and the authors show that infection correlates with the amount of CCR5 mRNA in 'resting' cells.
Veazey, R. S. et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280, 427–431 (1998). Definitively shows that the gut is an important site of both SIV replication and massive loss of CD4+ T cells.
Kewenig, S. et al. Rapid mucosal CD4+ T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology 116, 1115–1123 (1999).
Schneider, T. et al. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut 37, 524–529 (1995).
Mehandru, S. et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200, 761–770 (2004).
Brenchley, J. M. et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200, 749–759 (2004).
Guadalupe, M. et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77, 11708–11717 (2003).
Lim, S. G. et al. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin. Exp. Immunol. 92, 448–454 (1993).
Kahn, J. O. & Walker, B. D. Acute human immunodeficiency virus type 1 infection. N. Engl. J. Med. 339, 33–39 (1998).
Lavreys, L. et al. Primary human immunodeficiency virus type 1 infection: clinical manifestations among women in Mombasa, Kenya. Clin. Infect. Dis. 30, 486–490 (2000).
Schacker, T. W., Hughes, J. P., Shea, T., Coombs, R. W. & Corey, L. Biological and virologic characteristics of primary HIV infection. Ann. Intern. Med. 128, 613–620 (1998).
Ho, D. D. et al. Primary human T-lymphotropic virus type III infection. Ann. Intern. Med. 103, 880–883 (1985).
Kinloch-de Loes, S. et al. Symptomatic primary infection due to human immunodeficiency virus type 1: review of 31 cases. Clin. Infect. Dis. 17, 59–65 (1993).
Cooper, D. A. et al. Acute AIDS retrovirus infection. Definition of a clinical illness associated with seroconversion. Lancet 1, 537–540 (1985).
Miller, C. J. et al. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J. Virol. 63, 4277–4284 (1989). Describes the non-human primate model of vaginal transmission of HIV, which is generally considered to be the most relevant model.
Miller, C. J. et al. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J. Virol. 68, 6391–6400 (1994).
Hendrickx, A. G. & Cukierski, M. A. Reproductive and developmental toxicology in nonhuman primates. Prog. Clin. Biol. Res. 235, 73–88 (1987).
Ma, Z., Lu, F. X., Torten, M. & Miller, C. J. The number and distribution of immune cells in the cervicovaginal mucosa remain constant throughout the menstrual cycle of rhesus macaques. Clin. Immunol. 100, 240–249 (2001).
Miller, C. J. & Lu, F. X. Anti-HIV and -SIV immunity in the vagina. Int. Rev. Immunol. 22, 65–76 (2003).
Embretson, J. et al. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 362, 359–362 (1993).
Pantaleo, G. et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 362, 355–358 (1993).
Tenner-Racz, K. et al. HTLV-III/LAV viral antigens in lymph nodes of homosexual men with persistent generalized lymphadenopathy and AIDS. Am. J. Pathol. 123, 9–15 (1986).
Reimann, K. A. et al. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J. Virol. 68, 2362–2370 (1994).
Fox, C. H. Lymphoid germinal centers are reservoirs of HIV infection and account for the apparent latency of infection. AIDS Res. Hum. Retroviruses 8, 756–758 (1992).
Reinhart, T. A. et al. Simian immunodeficiency virus burden in tissues and cellular compartments during clinical latency and AIDS. J. Infect. Dis. 176, 1198–1208 (1997).
Miller, C. J. et al. Propagation and dissemination of infection after vaginal transmission of SIV. J. Virol. 79, 9217–9227 (2005). Documents small founder populations of infected cells at the point of entry following intravaginal inoculation with SIV, and describes the requirement for local proliferation and for continued seeding of distal lymphoid tissues to establish systemic infection. These findings point to opportunities to prevent systemic infection using microbicides or vaccines that target viral vulnerabilities at the earliest stages of infection at the point of entry.
Shattock, R. J. & Moore, J. P. Inhibiting sexual transmission of HIV-1 infection. Nature Rev. Microbiol. 1, 25–34 (2003).
Zhang, Z. et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286, 1353–1357 (1999). First paper to show the infection of resting cells with SIV and HIV in vivo.
Hu, J., Gardner, M. B. & Miller, C. J. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74, 6087–6095 (2000).
Gray, R. H. et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 357, 1149–1153 (2001).
Cohn, M. A. et al. Chronic inflammation with increased human immunodeficiency virus (HIV) RNA expression in the vaginal epithelium of HIV-infected Thai women. J. Infect. Dis. 184, 410–417 (2001).
Serwadda, D. et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case–control study in Rakai, Uganda. J. Infect. Dis. 188, 1492–1497 (2003).
Gray, R. H., Wawer, M. J., Sewankambo, N. & Serwadda, D. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 350, 1780 (1997).
Martin, H. L. et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J. Infect. Dis. 180, 1863–1868 (1999).
Sewankambo, N. et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 350, 546–550 (1997).
Van Damme, L. et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360, 971–977 (2002).
Schnittman, S. M. et al. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc. Natl Acad. Sci. USA 87, 6058–6062 (1990).
Zhang, Z. Q. et al. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc. Natl Acad. Sci. USA 101, 5640–5645 (2004).
Stevenson, M., Stanwick, T. L., Dempsey, M. P. & Lamonica, C. A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9, 1551–1560 (1990).
Chou, C. S., Ramilo, O. & Vitetta, E. S. Highly purified CD25− resting T cells cannot be infected de novo with HIV-1. Proc. Natl Acad. Sci. USA 94, 1361–1365 (1997). Clearly shows that HIV cannot infect resting CD4+ T cells in vitro.
Tang, S., Patterson, B. & Levy, J. A. Highly purified quiescent human peripheral blood CD4+ T cells are infectible by human immunodeficiency virus but do not release virus after activation. J. Virol. 69, 5659–5665 (1995).
Spina, C. A., Guatelli, J. C. & Richman, D. D. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J. Virol. 69, 2977–2988 (1995).
Barre-Sinoussi, F. et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220, 868–871 (1983).
Popovic, M., Sarngadharan, M. G., Read, E. & Gallo, R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224, 497–500 (1984).
Chun, T. W. et al. Gene expression and viral production in latently infected, resting CD4+ T cells in viremic versus aviremic HIV-infected individuals. Proc. Natl Acad. Sci. USA 100, 1908–1913 (2003).
Berger, E. A., Murphy, P. M. & Farber, J. M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17, 657–700 (1999).
Pierson, T. et al. Characterization of chemokine receptor utilization of viruses in the latent reservoir for human immunodeficiency virus type 1. J. Virol. 74, 7824–7833 (2000).
Korin, Y. D. & Zack, J. A. Nonproductive human immunodeficiency virus type 1 infection in nucleoside-treated G0 lymphocytes. J. Virol. 73, 6526–6532 (1999).
Zack, J. A. et al. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61, 213–222 (1990).
Greene, W. C. & Peterlin, B. M. Charting HIV's remarkable voyage through the cell: basic science as a passport to future therapy. Nature Med. 8, 673–680 (2002).
Anton, P. A. et al. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS 14, 1761–1765 (2000).
Zeitz, M., Greene, W. C., Peffer, N. J. & James, S. P. Lymphocytes isolated from the intestinal lamina propria of normal nonhuman primates have increased expression of genes associated with T-cell activation. Gastroenterology 94, 647–655 (1988).
Reilly, C., Schacker, T., Haase, A. T., Wietgrefe, S. & Krason, D. The clustering of infected SIV cells in lymphatic tissue. J. Am. Stat. Assoc. 97, 943–954 (2002).
Boirivant, M. et al. HIV-1 gp120 accelerates Fas-mediated activation-induced human lamina propria T cell apoptosis. J. Clin. Immunol. 18, 39–47 (1998). Shows the susceptibility of lamina propria CD4+ T cells to apoptosis induced by HIV gp120.
Levine, A. D. & Fiocchi, C. Regulation of life and death in lamina propria T cells. Semin. Immunol. 13, 195–199 (2001).
Bu, P. et al. Apoptosis: one of the mechanisms that maintains unresponsiveness of the intestinal mucosal immune system. J. Immunol. 166, 6399–6403 (2001).
Boirivant, M. et al. Stimulated human lamina propria T cells manifest enhanced Fas-mediated apoptosis. J. Clin. Invest. 98, 2616–2622 (1996).
De Maria, R. et al. Functional expression of Fas and Fas ligand on human gut lamina propria T lymphocytes. A potential role for the acidic sphingomyelinase pathway in normal immunoregulation. J. Clin. Invest. 97, 316–322 (1996).
Backhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A. & Gordon, J. I. Host–bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005).
Davenport, M. P., Ribeiro, R. M. & Perelson, A. S. Kinetics of virus-specific CD8+ T cells and the control of human immunodeficiency virus infection. J. Virol. 78, 10096–10103 (2004).
Reynolds, M. R. et al. The CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to SIV: too late and too little. J. Virol. 79, 9228–9235 (2005). Analyses CD8+ T-cell responses to immunodominant epitopes of SIV in lymphoid-tissue compartments after vaginal exposure to SIV, and shows that the immune response occurs after peak viral replication and is of insufficient magnitude to prevent infection or the massive CD4+ T-cell loss in the gut that is described in references 25 and 51. The robust response in female reproductive tissues might be an encouraging sign that a vaccine-induced response at the earliest stages of infection at the point of entry might be able to contain infection there and prevent systemic infection.
Mothe, B. R. et al. Dominance of CD8 responses specific for epitopes bound by a single major histocompatibility complex class I molecule during the acute phase of viral infection. J. Virol. 76, 875–884 (2002).
Koup, R. A. et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68, 4650–4655 (1994).
Borrow, P., Lewicki, H., Hahn, B. H., Shaw, G. M. & Oldstone, M. B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68, 6103–6110 (1994).
Kuroda, M. J. et al. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 162, 5127–5133 (1999).
Phillips, A. N. Reduction of HIV concentration during acute infection: independence from a specific immune response. Science 271, 497–499 (1996). Original proposal that the decrease in HIV concentration in peripheral blood in the acute stage of infection is a result of exhaustion of the substrate — that is, activated CD4+ T cells — rather than a result of the virus-specific immune response.
De Boer, R. J. & Perelson, A. S. Target cell limited and immune control models of HIV infection: a comparison. J. Theor. Biol. 190, 201–214 (1998).
Schwartz, E. J. et al. Effect of target cell availability on HIV-1 production in vitro. AIDS 16, 341–345 (2002).
Heise, C. et al. Human immunodeficiency virus infection of enterocytes and mononuclear cells in human jejunal mucosa. Gastroenterology 100, 1521–1527 (1991).
Heise, C., Vogel, P., Miller, C. J., Halsted, C. H. & Dandekar, S. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological, and morphological changes. Am. J. Pathol. 142, 1759–1771 (1993).
Kotler, D. P., Gaetz, H. P., Lange, M., Klein, E. B. & Holt, P. R. Enteropathy associated with the acquired immunodeficiency syndrome. Ann. Intern. Med. 101, 421–428 (1984).
Kotler, D. P., Reka, S. & Clayton, F. Intestinal mucosal inflammation associated with human immunodeficiency virus infection. Dig. Dis. Sci. 38, 1119–1127 (1993).
Ullrich, R. et al. Mucosal HIV infection. Pathobiology 66, 145–150 (1998).
Ullirch, R. et al. Small intestinal structure and function in patients infected with human immunodeficiency virus (HIV): evidence for HIV-induced enteropathy. Ann. Intern. Med. 111, 15–21 (1989).
Abel, K. et al. γ-Interferon-mediated inflammation is associated with lack of protection from intravaginal simian immunodeficiency virus SIVmac239 challenge in simian–human immunodeficiency virus 89.6-immunized rhesus macaques. J. Virol. 78, 841–854 (2004).
Guy-Grand, D., DiSanto, J. P., Henchoz, P., Malassis-Seris, M. & Vassalli, P. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-γ, TNF) in the induction of epithelial cell death and renewal. Eur. J. Immunol. 28, 730–744 (1998).
Martin, C. A. & Panja, A. Cytokine regulation of human intestinal primary epithelial cell susceptibility to Fas-mediated apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 282, G92–G104 (2002).
Zhang, L. et al. Effects of antibody on viral kinetics in simian/human immunodeficiency virus infection: implications for vaccination. J. Virol. 78, 5520–5522 (2004).
Belkaid, Y. & Rouse, B. T. Natural regulatory T cells in infectious disease. Nature Immunol. 6, 353–360 (2005).
Maloy, K. J. & Powrie, F. Regulatory T cells in the control of immune pathology. Nature Immunol. 2, 816–822 (2001).
Mills, K. H. Regulatory T cells: friend or foe in immunity to infection? Nature Rev. Immunol. 4, 841–855 (2004).
Sakaguchi, S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 (2004).
Kornfeld, C. et al. Anti-inflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Invest. 115, 1082–1091 (2005).
Andersson, J. et al. The prevalence of regulatory cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 174, 3143–3147 (2005).
Veazey, R. S. et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nature Med. 9, 343–346 (2003).
Lederman, M. M. et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science 306, 485–487 (2004).
Kaul, R. et al. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164, 1602–1611 (2000).
Miller, C. J. et al. Rhesus macaques previously-infected with SHIV are protected from vaginal challenge with pathogenic SIVmac239 . J. Virol. 71, 1911–1921 (1997).
Abel, K. et al. Simian–human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and α interferon responses. J. Virol. 77, 3099–3118 (2003).
Crotty, S. et al. Protection against simian immunodeficiency virus vaginal challenge by using Sabin poliovirus vectors. J. Virol. 75, 7435–7452 (2001).
Johnson, R. P. et al. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J. Virol. 73, 4952–4961 (1999).
Joag, S. V. et al. Oral immunization of macaques with attenuated vaccine virus induces protection against vaginally transmitted AIDS. J. Virol. 72, 9069–9078 (1998).
Enose, Y. et al. Protection by intranasal immunization of a nef-deleted, nonpathogenic SHIV against intravaginal challenge with a heterologous pathogenic SHIV. Virology 298, 306–316 (2002).
Johnson, R. P. & Desrosiers, R. C. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr. Opin. Immunol. 10, 436–443 (1998).
Lifson, J. D. et al. Transient early post-inoculation anti-retroviral treatment facilitates controlled infection with sparing of CD4+ T cells in gut-associated lymphoid tissues in SIVmac239-infected rhesus macaques, but not resistance to rechallenge. J. Med. Primatol. 32, 201–210 (2003).
Acknowledgements
The author thanks J. Carlis, Q. Li and J. Estes for helpful discussions, C. O'Neill and T. Leonard for help with the manuscript and figures, and the National Institutes of Health (United States) for research support.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
FURTHER INFORMATION
Glossary
- LAMINA PROPRIA
-
The layer of mucosal tissue directly under the mucosal epithelial-cell surface, in which effector cells for mucosal immunity reside.
- EFFECTOR MEMORY T CELL
-
A terminally differentiated T cell that lacks lymph-node-homing receptor but expresses receptors that enable it to home to inflamed tissues. Effector memory cells can exert immediate effector functions without the need for further differentiation.
- IMMUNODOMINANT EPITOPE
-
An antigen within a complex mixture (such as a whole virus), or a region of an antigen, that is recognized preferentially during an immune response.
Rights and permissions
About this article
Cite this article
Haase, A. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol 5, 783–792 (2005). https://doi.org/10.1038/nri1706
Issue Date:
DOI: https://doi.org/10.1038/nri1706
This article is cited by
-
Molecular mechanisms by which the HIV-1 latent reservoir is established and therapeutic strategies for its elimination
Archives of Virology (2023)
-
Clinical Features of Ocular Pathology in Patients with Acquired Immunodeficiency Syndrome and Syphilis
Advances in Therapy (2021)
-
Durable protection against repeated penile exposures to simian-human immunodeficiency virus by broadly neutralizing antibodies
Nature Communications (2020)
-
Impact of Endemic Infections on HIV Susceptibility in Sub-Saharan Africa
Tropical Diseases, Travel Medicine and Vaccines (2019)
-
MAdCAM costimulation through Integrin-α4β7 promotes HIV replication
Mucosal Immunology (2018)