Key Points
-
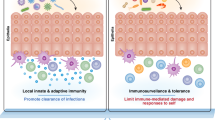
Together with the passive protection of an impermeable barrier, an active adaptive immune system has evolved in the intestine of higher vertebrates.
-
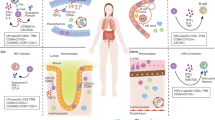
The intestine consists of two immunological compartments: the initiation compartment, containing the organized lymphoid structures of the gut-associated lymphoid tissue (GALT), and the effector compartment, consisting of the diffuse immune cells of the lamina propria and intestinal epithelium.
-
The adaptive immune system, which allows for the formation of immune memory, is prominent at the mucosal site. In mammals, the intestine is an important T-cell organ and two distinct subsets of memory T cells have evolved at mucosal surfaces.
-
The natural and acquired memory T-cell subsets differ from each other in the nature of the antigens they recognize and the differentiation pathway they take to achieve a memory phenotype.
-
Natural memory T cells are self-specific and gained gut tropism and a memory phenotype during agonist selection in the thymus. These natural memory mucosal T cells respond to self-antigens of stressed epithelial cells and regulate immune responses of the conventional mucosal T cells. The γδ-TCR+ subset also has tissue-repair ability.
-
Acquired memory T cells are non-self-specific and gained gut tropism and mucosal memory differentiation through specific recognition of antigen presented by mucosal dendritic cells. The acquired memory mucosal T cells respond to foreign antigens derived from invading pathogens. They clear the pathogen in a highly regulated manner.
-
The joint efforts of these two subsets of memory T cells allow for the most effective protection, while preserving integrity of the barrier at the mucosal front line in higher vertebrates.
Abstract
The intestine is fundamental for the uptake of fluids and nutrients, but it also provides a main entrance for pathogens. This unique challenge has imposed an important evolutionary drive for distinctive specialization of the mucosal defence mechanisms. A hallmark of the modern immune system is its ability to generate antigen-specific memory T cells, which can provide immediate, potent and long-lasting protection. The high demand for effective protection, which is also compatable with vital functions of the intestine, has ensured that unique subsets of memory T cells have evolved at the mucosal front line. This review discusses the various mucosal T-cell subsets, and the new insights we have gained in understanding their specific differentiation and unique functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fasano, A. Cellular microbiology: can we learn cell physiology from microorganisms? Am. J. Physiol. 276, C765–C776 (1999).
Maury, J., Nicoletti, C., Guzzo-Chambraud, L. & Maroux, S. The filamentous brush border glycocalyx, a mucin-like marker of enterocyte hyper-polarization. Eur. J. Biochem. 228, 323–331 (1995).
Husband, A. J. & Gowans, J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J. Exp. Med. 148, 1146–1160 (1978).
Sansonetti, P. Host-pathogen interactions: the seduction of molecular cross talk. Gut 50 (Suppl 3), III2–III8 (2002).
Matsunaga, T. Did the first adaptive immunity evolve in the gut of ancient jawed fish? Cytogenet. Cell Genet. 80, 138–141 (1998).
Sprent, J. & Tough, D. F. T cell death and memory. Science 293, 245–248 (2001).
Schluns, K. S. & Lefrancois, L. Cytokine control of memory T-cell development and survival. Nature Rev. Immunol. 3, 269–279 (2003).
Rocha, B., Vassalli, P. & Guy-Grand, D. The Vβ repertoire of mouse gut homodimeric α CD8+ intraepithelial T cell receptor α/β+ lymphocytes reveals a major extrathymic pathway of T cell differentiation. J. Exp. Med. 173, 483–486 (1991). This paper describes for the first time the presence of autoreactive T-cell receptors (TCRs) among the TCR repertoire of CD8αα+ intraepithelial lymphocytes (IELs).
Kim, S. K., Reed, D. S., Heath, W. R., Carbone, F. & Lefrancois, L. Activation and migration of CD8+ T cells in the intestinal mucosa. J. Immunol. 159, 4295–4306 (1997). In this paper, it was shown that after activation of adoptively transferred OT-I TCR-transgenic T cells, the CD8+ effector T cells migrated to the lamina propria and intestinal epithelium, indicating that CD8+ T cells activated in the periphery can readily enter the mucosa during an ongoing immune response.
Schieferdecker, H. L., Ullrich, R., Hirseland, H. & Zeitz, M. T cell differentiation antigens on lymphocytes in the human intestinal lamina propria. J. Immunol. 149, 2816–2822 (1992).
Zeitz, M. et al. Phenotype and function of lamina propria T lymphocytes. Immunol. Res. 10, 199–206 (1991).
Zeitz, M., Ullrich, R., Schneider, T., Schieferdecker, H. L. & Riecken, E. O. Cell differentiation and proliferation in the gastrointestinal tract with respect to the local immune system. Ann. NY Acad. Sci. 733, 75–86 (1994).
Kim, S. K., Schluns, K. S. & Lefrancois, L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J. Immunol. 163, 4125–4132 (1999). This paper showed that the intestinal mucosa generates antigen-specific memory T cells, which express a distinct array of adhesion molecules as compared with secondary lymphoid memory cells and have ex vivo lytic activity that is upregulated in a CD28-independent manner after antigen re-encounter in vivo.
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Blumberg, R. S., Yockey, C. E., Gross, G. G., Ebert, E. C. & Balk, S. P. Human intestinal intraepithelial lymphocytes are derived from a limited number of T cell clones that utilize multiple Vβ T cell receptor genes. J. Immunol. 150, 5144–5153 (1993).
Arstila, T. et al. Identical T cell clones are located within the mouse gut epithelium and lamina propia and circulate in the thoracic duct lymph. J. Exp. Med. 191, 823–834 (2000).
Regnault, A., Cumano, A., Vassalli, P., Guy-Grand, D. & Kourilsky, P. Oligoclonal repertoire of the CD8αα and the CD8αβ TCR-α/β murine intestinal intraepithelial T lymphocytes: evidence for the random emergence of T cells. J. Exp. Med. 180, 1345–1358 (1994).
Beagley, K. W. et al. Differences in intraepithelial lymphocyte T cell subsets isolated from murine small versus large intestine. J. Immunol. 154, 5611–5619 (1995).
Camerini, V., Panwala, C. & Kronenberg, M. Regional specialization of the mucosal immune system. Intraepithelial lymphocytes of the large intestine have a different phenotype and function than those of the small intestine. J. Immunol. 151, 1765–1776 (1993).
Latthe, M., Terry, L. & MacDonald, T. T. High frequency of CD8αα homodimer-bearing T cells in human fetal intestine. Eur. J. Immunol. 24, 1703–1705 (1994).
Lin, T. et al. Thymus ontogeny and the development of TCRαβ intestinal intraepithelial lymphocytes. Cell Immunol. 171, 132–139 (1996). Using neonatal thymectomy, it was shown that most αβ-TCR+ IELs are of thymic origin. Accordingly, the transfer of thymus grafts from normal mice to nude mice gave rise to CD4−CD8αα+ and CD4+CD8αα+ IELs, depending on the age of the thymus.
Kuo, S., El Guindy, A., Panwala, C. M., Hagan, P. M. & Camerini, V. Differential appearance of T cell subsets in the large and small intestine of neonatal mice. Pediatr. Res. 49, 543–551 (2001).
Guy-Grand, D. et al. Different use of T cell receptor transducing modules in two populations of gut intraepithelial lymphocytes are related to distinct pathways of T cell differentiation. J. Exp. Med. 180, 673–679 (1994).
Park, S. Y. et al. Differential contribution of the FcR γ-chain to the surface expression of the T cell receptor among T cells localized in epithelia: analysis of FcRγ-deficient mice. Eur. J. Immunol. 25, 2107–2110 (1995).
Guy-Grand, D., Cuenod-Jabri, B., Malassis-Seris, M., Selz, F. & Vassalli, P. Complexity of the mouse gut T cell immune system: identification of two distinct natural killer T cell intraepithelial lineages. Eur. J. Immunol. 26, 2248–2256 (1996).
Klein, J. R. Ontogeny of the Thy-1−, Lyt-2+ murine intestinal intraepithelial lymphocyte. Characterization of a unique population of thymus-independent cytotoxic effector cells in the intestinal mucosa. J. Exp. Med. 164, 309–314 (1986).
Ohteki, T. & MacDonald, H. R. Expression of the CD28 co-stimulatory molecule on subsets of murine intestinal intraepithelial lymphocytes correlates with lineage and responsiveness. Eur. J. Immunol. 23, 1251–1255 (1993).
Van Houten, N., Mixter, P. F., Wolfe, J. & Budd, R. C. CD2 expression on murine intestinal intraepithelial lymphocytes is bimodal and defines proliferative capacity. Int. Immunol. 5, 665–672 (1993).
Rocha, B., Vassalli, P. & Guy-Grand, D. Thymic and extrathymic origins of gut intraepithelial lymphocyte populations in mice. J. Exp. Med. 180, 681–686 (1994).
Rocha, B., von Boehmer, H. & Guy-Grand, D. Selection of intraepithelial lymphocytes with CD8αα co-receptors by self-antigen in the murine gut. Proc. Natl Acad. Sci. USA 89, 5336–5340 (1992).
Lefrancois, L. & Puddington, L. Extrathymic intestinal T-cell development: virtual reality? Immunol. Today 16, 16–21 (1995).
Lefrancois L & Olson, S. Reconstitution of the extrathymic intestinal T cell compartment in the absence of irradiation. J. Immunol. 159, 538–541 (1997).
Guy-Grand, D. et al. Extrathymic T cell lymphopoiesis: ontogeny and contribution to gut intraepithelial lymphocytes in athymic and euthymic mice. J. Exp. Med. 197, 333–341 (2003).
Guy-Grand, D. et al. Contribution of double-negative thymic precursors to CD8αα+ intraepithelial lymphocytes of the gut in mice bearing TCR transgenes. Eur. J. Immunol. 31, 2593–2602 (2001).
Leishman, A. J. et al. Precursors of functional MHC class I- or class II-restricted CD8αα+ T cells are positively selected in the thymus by agonist self-peptides. Immunity 16, 355–364 (2002). This paper showed for the first time that precursors of functional CD8αα+ T cells are positively selected in the thymus in the presence of agonist self-peptides. It was also shown that the agonist-selection process requires a functional TCR α-chain connecting-peptide domain motif (α-CPM) and is CD8β independent. CD8αα on mature, agonist-selected T cells does not imply MHC class I restriction, and CD8αα+ T cells can be either MHC class I or class II restricted.
Poussier, P., Ning, T., Banerjee, D. & Julius, M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J. Exp. Med. 195, 1491–1497 (2002). This study demonstrates that prior reconstitution of severe combined immunodeficient (SCID) recipients with CD8αα+ αβ-TCR+, but not γδ-TCR+ or CD8αβ+ αβ-TCR+, IELs prevents induced colitis in an interleukin-10 (IL-10)-dependent manner. Moreover, self-specific CD8αα+ αβ-TCR+ H-Y TCR-transgenic IELs also prevent the onset of colitis.
Sydora, B. C., Brossay, L., Hagenbaugh, A., Kronenberg, M. & Cheroutre, H. TAP-independent selection of CD8+ intestinal intraepithelial lymphocytes. J. Immunol. 156, 4209–4216 (1996).
Neuhaus, O., Emoto, M., Blum, C., Yamamoto, S. & Kaufmann, S. H. Control of thymus-independent intestinal intraepithelial lymphocytes by β2-microglobulin. Eur. J. Immunol. 25, 2332–2339 (1995).
Fujiura, Y. et al. Development of CD8αα+ intestinal intraepithelial T cells in β2-microglobulin- and/or TAP1-deficient mice. J. Immunol. 156, 2710–2715 (1996).
Gapin, L., Cheroutre, H. & Kronenberg, M. Cutting edge: TCRαβ+ CD8αα+ T cells are found in intestinal intraepithelial lymphocytes of mice that lack classical MHC class I molecules. J. Immunol. 163, 4100–4104 (1999).
Park, S. H. et al. Selection and expansion of CD8α/α(1) T cell receptor α/β(1) intestinal intraepithelial lymphocytes in the absence of both classical major histocompatibility complex class I and nonclassical CD1 molecules. J. Exp. Med. 190, 885–890 (1999).
Das, G. & Janeway, C. A., Jr. Development of CD8α/α and CD8α/β T cells in major histocompatibility complex class I-deficient mice. J. Exp. Med. 190, 881–884 (1999).
Leishman, A. J. et al. T cell responses modulated through interaction between CD8αα and the nonclassical MHC class I molecule, TL. Science 294, 1936–1939 (2001). This paper indicates that, in contrast to other MHC class I molecules that bind CD8αβ, TL preferentially binds CD8αα. This high-affinity binding of CD8αα to TL modifies TCR-activation responses and defines a new mechanism of lymphocyte regulation through CD8αα and MHC class I.
Lambolez, F. & Rocha, B. Immunology. A molecular gut reaction. Science 294, 1848–1849 (2001).
Mosley, R. L., Styre, D. & Klein, J. R. CD4+CD8+ murine intestinal intraepithelial lymphocytes. Int. Immunol. 2, 361–365 (1990).
Morrissey, P. J., Charrier, K., Horovitz, D. A., Fletcher, F. A. & Watson, J. D. Analysis of the intra-epithelial lymphocyte compartment in SCID mice that received co-isogenic CD4+ T cells. Evidence that mature post-thymic CD4+ T cells can be induced to express CD8α in vivo. J. Immunol. 154, 2678–2686 (1995).
Aranda, R. et al. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J. Immunol. 158, 3464–3473 (1997).
Madakamutil, L. et al. CD8αα mediates survival and differentiation of CD8 memory T cell precursors. Science (in the press). This paper shows that CD8αα expression can be induced on activated conventional T cells, and interaction of CD8αα with its ligand TL on conventional antigen-presenting cells (APCs) leads to increased survival and differentiation of the effector T cells to memory T cells.
Kaech, S. M. et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature Immunol. 4, 1191–1198 (2003).
Nicoletti, C. Unsolved mysteries of intestinal M cells. Gut 47, 735–739 (2000).
Kelsall, B. L. & Strober, W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer's patch. J. Exp. Med. 183, 237–247 (1996).
Hershberg, R. M. et al. Intestinal epithelial cells use two distinct pathways for HLA class II antigen processing. J. Clin. Invest. 100, 204–215 (1997).
Hershberg, R. M. & Mayer, L. F. Antigen processing and presentation by intestinal epithelial cells- polarity and complexity. Immunol. Today 21, 123–128 (2000).
Rescigno, M. et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature Immunol. 2, 361–367 (2001). This paper demonstrates an alternative dendritic cell (DC)-mediated mechanism by which antigens are taken up in the intestine. DCs can open the tight junctions between epithelial cells and send dendrites out to sample the gut lumen directly. In addition, it shows that these DCs express tightjunction proteins, which preserve the integrity of the epithelial-cell barrier.
Sprent, J. Fate of H2-activated T lymphocytes in syngeneic hosts. I. Fate in lymphoid tissues and intestines traced with 3H-thymidine, 125I-deoxyuridine and 51chromium. Cell Immunol. 21, 278–302 (1976).
Weninger, W., Manjunath, N. & von Andrian, U. H. Migration and differentiation of CD8+ T cells. Immunol. Rev. 186, 221–233 (2002).
Stagg, A. J., Kamm, M. A. & Knight, S. C. Intestinal dendritic cells increase T cell expression of α4β7 integrin. Eur. J. Immunol. 32, 1445–1454 (2002). The authors show for the first time that the α 4 β 7hi subset of circulating memory cells is primed in mucosal tissues. DCs of the mesenteric lymph nodes (MLNs) induced more α 4 β 7hi cells than peripheral lymph-node DCs, irrespective of the source of T cells, indicating that DCs can shape immune responses by influencing the homing of the lymphocytes they activate.
Johansson-Lindbom, B. et al. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 198, 963–969 (2003).
Mora, J. R. et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424, 88–93 (2003).
Berlin, C. et al. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 80, 413–422 (1995).
Berlin, C. et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–185 (1993).
Hamann, A., Andrew, D. P., Jablonski-Westrich, D., Holzmann, B. & Butcher, E. C. Role of α4-integrins in lymphocyte homing to mucosal tissues in vivo. J. Immunol. 152, 3282–3293 (1994).
Bargatze, R. F., Jutila, M. A. & Butcher, E. C. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity 3, 99–108 (1995).
Wagner, N. et al. Critical role for β7 integrins in formation of the gut-associated lymphoid tissue. Nature 382, 366–370 (1996).
Rott, L. S., Briskin, M. J., Andrew, D. P., Berg, E. L. & Butcher, E. C. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. Comparison with vascular cell adhesion molecule-1 and correlation with β7 integrins and memory differentiation. J. Immunol. 156, 3727–3736 (1996).
Rose, J. R., Williams, M. B., Rott, L. S., Butcher, E. C. & Greenberg, H. B. Expression of the mucosal homing receptor α4β7 correlates with the ability of CD8+ memory T cells to clear rotavirus infection. J. Virol. 72, 726–730 (1998).
Lefrancois, L. et al. The role of β7 integrins in CD8 T cell trafficking during an antiviral immune response. J. Exp. Med. 189, 1631–1638 (1999).
Sydora, B. C. et al. β7 Integrin expression is not required for the localization of T cells to the intestine and colitis pathogenesis. Clin. Exp. Immunol. 129, 35–42 (2002).
Kuklin, N. A. et al. α4β7 independent pathway for CD8+ T cell-mediated intestinal immunity to rotavirus. J. Clin. Invest. 106, 1541–1552 (2000).
Austrup, F., Rebstock, S., Kilshaw, P. J. & Hamann, A. Transforming growth factor-β1-induced expression of the mucosa-related integrin αE on lymphocytes is not associated with mucosa-specific homing. Eur. J. Immunol. 25, 1487–1491 (1995).
Cepek, K. L. et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature 372, 190–193 (1994). This paper reported that heterotypic adhesive interactions between epithelial cells and IELs in vitro are mediated by E-cadherin and α E β 7 integrin.
Kilshaw, P. J. & Murant, S. J. Expression and regulation of β7 (βp) integrins on mouse lymphocytes: relevance to the mucosal immune system. Eur. J. Immunol. 21, 2591–2597 (1991). This study shows that after stimulation in the presence of transforming growth factor-β (TGF-β), activated T cells express increased levels of β 7 integrins and exchange α 4 for α E , which, in most cells, was expressed de novo . The authors suggest that in vivo , this change in α-chain usage occurs on mucosal T cells in response to TGF-β and provides particular mucosa-adhesion properties.
Kunkel, E. J. et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192, 761–768 (2000).
Svensson, M. et al. CCL25 mediates the localization of recently activated CD8αβ+ lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 110, 1113–1121 (2002).
Wurbel, M. A. et al. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor γδ+ gut intraepithelial lymphocytes. Blood 98, 2626–2632 (2001).
Uehara, S., Grinberg, A., Farber, J. M. & Love, P. E. A role for CCR9 in T lymphocyte development and migration. J. Immunol. 168, 2811–2819 (2002).
Gosling, J. et al. Cutting edge: identification of a novel chemokine receptor that binds dendritic cell- and T cell-active chemokines including ELC, SLC, and TECK. J. Immunol. 164, 2851–2856 (2000).
Agace, W. W. et al. Constitutive expression of stromal derived factor-1 by mucosal epithelia and its role in HIV transmission and propagation. Curr. Biol. 10, 325–328 (2000).
Pan, J. et al. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J. Immunol. 165, 2943–2949 (2000).
Muehlhoefer, A. et al. Fractalkine is an epithelial and endothelial cell-derived chemoattractant for intraepithelial lymphocytes in the small intestinal mucosa. J. Immunol. 164, 3368–3376 (2000).
Iqbal, N. et al. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J. Exp. Med. 195, 71–84 (2002).
Kweon, M. N. et al. Development of antigen induced colitis in SCID mice reconstituted with spleen derived memory type CD4+ CD45RB+ T cells. Gut 50, 299–306 (2002).
Masopust, D., Jiang, J., Shen, H. & Lefrancois, L. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol. 166, 2348–2356 (2001).
Muller, S., Buhler-Jungo, M. & Mueller, C. Intestinal intraepithelial lymphocytes exert potent protective cytotoxic activity during an acute virus infection. J. Immunol. 164, 1986–1994 (2000). In this study, it was shown that although αβ TCR+ CD8αα+ IELs have only minimal virus-specific cytotoxicity, maximum specific killing was mediated by αβ-TCR+CD8αβ+ IELs, exceeding that mediated by CD8+ splenocytes. These data indicate a potent priming of lymphocytic choriomeningitis virus (LCMV)-specific IELs in situ and indicate that cytotoxic CD8αβ+ IELs markedly contribute to the elimination of virus-infected cells in vivo.
Pope, C. et al. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166, 3402–3409 (2001).
Lepage, A. C., Buzoni-Gatel, D., Bout, D. T. & Kasper, L. H. Gut-derived intraepithelial lymphocytes induce long term immunity against Toxoplasma gondii. J. Immunol. 161, 4902–4908 (1998).
Hershberg, R. et al. Expression of the thymus leukemia antigen in mouse intestinal epithelium. Proc. Natl Acad. Sci. USA 87, 9727–9731 (1990).
Boismenu, R. & Havran, W. L. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science 266, 1253–1255 (1994). This paper shows that γδ-TCR+ IELs uniquely express the epithelial-cell mitogen keratinocyte growth factor (KGF) and promote the growth of cultured epithelial cells, indicating that γδ-TCR+ IELs function in the surveillance and repair of damaged gut epithelial tissues.
Imaoka, A., Matsumoto, S., Setoyama, H., Okada, Y. & Umesaki, Y. Proliferative recruitment of intestinal intraepithelial lymphocytes after microbial colonization of germ-free mice. Eur. J. Immunol. 26, 945–948 (1996).
Chidgey, A. & Boyd, R. Agonist peptide modulates T cell selection thresholds through qualitative and quantitative shifts in CD8 co-receptor expression. Int. Immunol. 9, 1527–1536 (1997).
Chidgey, A. P. & Boyd, R. L. Positive selection of low responsive, potentially autoreactive T cells induced by high avidity, non-deleting interactions. Int. Immunol. 10, 999–1008 (1998).
Mintern, J. D., Maurice, M. M., Ploegh, H. L. & Schott, E. Thymic selection and peripheral activation of CD8 T cells by the same class I MHC/peptide complex. J. Immunol. 172, 699–708 (2004).
Schweighoffer, E. & Fowlkes, B. J. Positive selection is not required for thymic maturation of transgenic γδ T cells. J. Exp. Med. 183, 2033–2041 (1996).
Alberola-Ila, J., Hogquist, K. A., Swan, K. A., Bevan, M. J. & Perlmutter, R. M. Positive and negative selection invoke distinct signaling pathways. J. Exp. Med. 184, 9–18 (1996).
Cruz, D. et al. An opposite pattern of selection of a single T cell antigen receptor in the thymus and among intraepithelial lymphocytes. J. Exp. Med. 188, 255–265 (1998).
Guehler, S. R., Bluestone, J. A. & Barrett, T. A. Immune deviation of 2C transgenic intraepithelial lymphocytes in antigen-bearing hosts. J. Exp. Med. 184, 493–503 (1996).
Levelt, C. N. et al. High- and low-affinity single-peptide/MHC ligands have distinct effects on the development of mucosal CD8αα and CDαβ T lymphocytes. Proc. Natl Acad. Sci. USA 96, 5628–5633 (1999).
Corazza, N., Muller, S., Brunner, T., Kagi, D. & Mueller, C. Differential contribution of Fas- and perforin-mediated mechanisms to the cell-mediated cytotoxic activity of naive and in vivo-primed intestinal intraepithelial lymphocytes. J. Immunol. 164, 398–403 (2000).
Podd, B. S. et al. MHC class I allele dosage alters CD8 expression by intestinal intraepithelial lymphocytes. J. Immunol. 167, 2561–2568 (2001).
Yoshikai, Y., Reis, M. D. & Mak, T. W. Athymic mice express a high level of functional γ-chain but greatly reduced levels of α- and β-chain T-cell receptor messages. Nature 324, 482–485 (1986).
Lin, T., Takimoto, H., Matsuzaki, G. & Nomoto, K. Effect of neonatal thymectomy on murine small intestinal intraepithelial lymphocytes expressing T cell receptor αβ and 'clonally forbidden Vβs'. Adv. Exp. Med. Biol. 371A, 129–131 (1995).
Legendre, V. et al. Selection of phenotypically distinct NK1. 1+ T cells upon antigen expression in the thymus or in the liver. Eur. J. Immunol. 29, 2330–2343 (1999).
Wurbel, M. A. et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur. J. Immunol. 30, 262–271 (2000).
Marsal, J. et al. Involvement of CCL25 (TECK) in the generation of the murine small-intestinal CD8αα+CD3+ intraepithelial lymphocyte compartment. Eur. J. Immunol. 32, 3488–3497 (2002).
Poussier, P., Ning, T., Chen, J., Banerjee, D. & Julius, M. Intestinal inflammation observed in IL-2R/IL-2 mutant mice is associated with impaired intestinal T lymphopoiesis. Gastroenterology 118, 880–891 (2000).
Fuss, I. J., Boirivant, M., Lacy, B. & Strober, W. The interrelated roles of TGF-β and IL-10 in the regulation of experimental colitis. J. Immunol. 168, 900–908 (2002).
Schild, H. et al. The nature of major histocompatibility complex recognition by γδ T cells. Cell 76, 29–37 (1994).
Steinle, A., Groh, V. & Spies, T. Diversification, expression, and γδ T cell recognition of evolutionarily distant members of the MIC family of major histocompatibility complex class I-related molecules. Proc. Natl Acad. Sci. USA 95, 12510–12515 (1998).
Groh, V., Steinle, A., Bauer, S. & Spies, T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science 279, 1737–1740 (1998).
Wu, J., Groh, V. & Spies, T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial γδ T cells. J. Immunol. 169, 1236–1240 (2002).
Bauer, S. et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 (1999).
Poccia, F. et al. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive Vγ9 Vδ2 T lymphocytes. J. Immunol. 159, 6009–6017 (1997).
Das, G. & Janeway, C. A., Jr. MHC specificity of iIELs. Trends Immunol. 24, 88–93 (2003).
Raulet, D. Roles of the NKG2D immunoreceptor and its ligands. Nature Rev. Immunol. 3, 781–790 (2003).
Cerwenka, A. et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 12, 721–727 (2000).
Diefenbach, A., Jamieson, A. M., Liu, S. D., Shastri, N. & Raulet, D. H. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nature Immunol. 1, 119–126 (2000).
Bye, W. A., Allan, C. H. & Trier, J. S. Structure, distribution, and origin of M cells in Peyer's patches of mouse ileum. Gastroenterology 86, 789–801 (1984).
Neutra, M. R., Phillips, T. L., Mayer, E. L. & Fishkind, D. J. Transport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer's patch. Cell Tissue Res. 247, 537–546 (1987).
Drubin, D. G. & Nelson, W. J. Origins of cell polarity. Cell 84, 335–344 (1996).
Borghesi, C., Taussig, M. J. & Nicoletti, C. Rapid appearance of M cells after microbial challenge is restricted at the periphery of the follicle-associated epithelium of Peyer's patch. Lab. Invest. 79, 1393–1401 (1999).
Acknowledgements
We thank M. Cheroutre for her important contribution and M. Kronenberg for helpful discussions and his continuous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- TIGHT JUNCTIONS
-
Dynamic structures that consist of proteins, including occludin, claudin 1 and junctional adhesion molecule, that seal neighbouring cells together to prevent paracellular traffic of macromolecules and microorganisms.
- ENTEROCYTES
-
Absorbing epithelial cells that form a single-cell layer that lines the luminal side of the intestine.
- PEYER'S PATCHES
-
Organized lymphoid compartments that are integrated into the walls of the small intestine.
- LAMINA PROPRIA
-
A meshwork of connective tissue that underlies the gut epithelium, containing a broad spectrum of myeloid and lymphoid cells.
- OLIGOCLONAL
-
A T-cell receptor (TCR) repertoire with a limited number of TCR clones.
- AGONIST PEPTIDES
-
Cognate antigenic peptides that can stimulate the T-cell receptor in a productive manner.
- M CELLS
-
Specialized epithelial cells that deliver antigens by trans-epithelial vesicular transport from the gut lumen directly to the sub-epithelial lymphoid tissues of the Peyer's patch.
- MHC TETRAMERS
-
Reagents composed of four peptide–MHC complexes linked by biotinylation, which can be fluorescently labelled and used to detect antigen-specific T cells by flow cytometry.
- COLITIS
-
An inflammatory bowel disease characterized by contiguous inflammation of the gut mucosa, mainly of the large intestine.
- ANTAGONIST PEPTIDE
-
Cognate peptide that stimulates the T-cell receptor in a non-productive manner.
- NUDE
-
A mutation in mice that causes both hairlessness and defective thymus formation, which results in the absence of mature T cells.
Rights and permissions
About this article
Cite this article
Cheroutre, H., Madakamutil, L. Acquired and natural memory T cells join forces at the mucosal front line. Nat Rev Immunol 4, 290–300 (2004). https://doi.org/10.1038/nri1333
Issue Date:
DOI: https://doi.org/10.1038/nri1333
This article is cited by
-
Microinflammation in the intestinal mucosa and symptoms of irritable bowel syndrome
Journal of Gastroenterology (2022)
-
Commensal viruses maintain intestinal intraepithelial lymphocytes via noncanonical RIG-I signaling
Nature Immunology (2019)
-
Human immunodeficiency virus and the gastrointestinal immune system: does highly active antiretroviral therapy restore gut immunity?
Mucosal Immunology (2012)
-
Skin-Resident T Cells: The Ups and Downs of On Site Immunity
Journal of Investigative Dermatology (2010)
-
Dynamics of viral replication in blood and lymphoid tissues during SIVmac251 infection of macaques
Retrovirology (2009)