Key Points
-
Host defence peptides (HDPs) display substantial immunomodulatory properties in vitro and in vivo, and these features are becoming increasingly appreciated in the literature.
-
The immune response is a highly complex process, involving multiple interconnected signalling pathways.
-
HDPs influence the entire signalling network of the immune response and, as a result, their effects on biological processes are also complex.
-
The ability of HDPs to influence many different cell types and pathways has implications in various immune-associated diseases.
-
In addition to direct antimicrobial activity and immunomodulatory activities, HDPs may have a role in biological functions such as anticancer activity, wound healing and angiogenesis.
Abstract
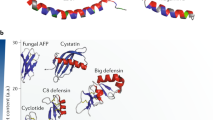
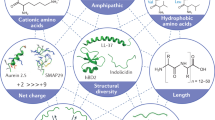
Host defence peptides (HDPs) are short, cationic amphipathic peptides with diverse sequences that are produced by various cells and tissues in all complex life forms. HDPs have important roles in the body's response to infection and inflammation. This Review focuses on human HDPs and explores the diverse immunomodulatory effects of HDPs from a systems biology perspective, which highlights the interconnected nature of the effect (or effects) of HDPs on the host. Studies have demonstrated that HDPs are expressed throughout the body and mediate a broad range of activities, which explains their association with various inflammatory diseases and autoimmune disorders. The diverse actions of HDPs, such as their roles in wound healing and in the maintenance of the microbiota, are also explored, in addition to potential therapeutic applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
28 April 2016
In the original version of this article, the first subheading of Table 1 was incorrect. It should read Cathelicidin LL-37. This has now been corrected online and for the print version. We apologize for this error.
References
Hancock, R. E. W. & Sahl, H. G. Antimicrobial and host-defence peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24, 1551–1557 (2006).
Fjell, C. D., Hiss, J. A., Hancock, R. E. W. & Schneider, G. Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov. 11, 37–51 (2012).
Wang, G., Li, X. & Wang, Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 44, D1087–D1093 (2016).
Hilchie, A. L., Wuerth, K. & Hancock, R. E. W. Immune modulation by multifaceted cationic host defence (antimicrobial) peptides. Nat. Chem. Biol. 9, 761–768 (2013). This review summarizes many of the immunomodulatory roles of HDPs, with a specific emphasis on animal studies.
Scott, M. G. et al. An anti-infective peptide that selectively modulates the innate immune response. Nat. Biotechnol. 25, 465–472 (2007).
Mansour, S. C., Pena, O. M. & Hancock, R. E. W. Host defence peptides: front-line immunomodulators. Trends Immunol. 35, 443–450 (2014).
Breuer, K. et al. InnateDB: systems biology of innate immunity and beyond—recent updates and continuing curation. Nucleic Acids Res. 41, D1228–D1233 (2013).
Xia, J., Gill, E. E. & Hancock, R. E. W. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat. Protoc. 10, 823–844 (2015).
Hultmark, D., Steiner, H., Rasmuson, T. & Boman, H. G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 106, 7–16 (1980).
Gallo, R. L. & Hooper, L. V. Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 12, 503–516 (2012). This review describes numerous HDPs and human defence proteins, and summarizes their biological role at epithelial surfaces.
Ganz, T. Extracellular release of antimicrobial defensins by human polymorphonuclear leukocytes. Infect. Immun. 55, 568–571 (1987).
Ayabe, T. et al. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1, 113–118 (2000).
Bowdish, D. M. et al. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 77, 451–459 (2005).
Vandamme, D., Landuyt, B., Luyten, W. & Schoofs, L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell. Immunol. 280, 22–35 (2012). This review provides a thorough summary of the structure, expression and diverse activities of LL-37.
Nijnik, A. & Hancock, R. E. W. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr. Opin. Hematol. 16, 41–47 (2009).
Wang, T.-T. et al. Cutting edge: 1,25-Dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 173, 2909–2912 (2004).
Mily, A. et al. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: a randomized controlled trial. PLoS ONE 10, e0138340 (2015).
Mookherjee, N. et al. Systems biology evaluation of immune responses induced by human host defence peptide LL-37 in mononuclear cells. Mol. Biosyst. 5, 483–496 (2009). This article describes a systems analysis of CD14+ monocytes that are exposed to LL-37 and outlines the various genes and pathways that respond to this stimulus; in particular, MAPK signalling proteins and their targets are investigated.
Peyssonnaux, C. et al. Critical role of HIF-1α in keratinocyte defence against bacterial infection. J. Invest. Dermatol. 128, 1964–1968 (2008).
Yu, J. et al. Host defence peptide LL-37, in synergy with inflammatory mediator IL-1β, augments immune responses by multiple pathways. J. Immunol. 179, 7684–7691 (2007).
Lai, Y. et al. LL37 and cationic peptides enhance TLR3 signaling by viral double-stranded RNAs. PLoS ONE 6, e26632 (2011).
Nijnik, A., Pistolic, J., Filewod, N. C. J. & Hancock, R. E. W. Signaling pathways mediating chemokine induction in keratinocytes by cathelicidin LL-37 and flagellin. J. Innate Immun. 4, 377–386 (2012).
Hurtado, P. & Peh, C. A. LL-37 promotes rapid sensing of CpG oligodeoxynucleotides by B lymphocytes and plasmacytoid dendritic cells. J. Immunol. 184, 1425–1435 (2010).
Chen, X. et al. Human antimicrobial peptide LL-37 modulates proinflammatory responses induced by cytokine milieus and double-stranded RNA in human keratinocytes. Biochem. Biophys. Res. Commun. 433, 532–537 (2013).
van der Does, A. M. et al. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J. Immunol. 185, 1442–1449 (2010).
Davidson, D. J. et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 172, 1146–1156 (2004).
Lande, R. et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 (2007).
Chamilos, G. et al. Cytosolic sensing of extracellular self-DNA transported into monocytes by the antimicrobial peptide LL37. Blood 120, 3699–3707 (2012).
Ries, M. et al. Identification of novel oligonucleotides from mitochondrial DNA that spontaneously induce plasmacytoid dendritic cell activation. J. Leukoc. Biol. 94, 123–135 (2013).
Elssner, A., Duncan, M., Gavrilin, M. & Wewers, M. D. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1β processing and release. J. Immunol. 172, 4987–4994 (2004).
Mookherjee, N. et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defence peptide LL-37. J. Immunol. 176, 2455–2464 (2006).
Jin, G. et al. An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis. PLoS ONE 5, e10993 (2010).
Kandler, K. et al. The anti-microbial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int. Immunol. 18, 1729–1736 (2006).
Di Nardo, A. et al. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J. Immunol. 178, 1829–1834 (2007).
Hazlett, L. & Wu, M. Defensins in innate immunity. Cell Tissue Res. 343, 175–188 (2011). This review outlines the structures, expression patterns and biological activities of defensins.
Semple, F. & Dorin, J. R. β-Defensins: multifunctional modulators of infection, inflammation and more? J. Innate Immun. 4, 337–348 (2012).
Rodríguez- García, M. et al. Human immature monocyte-derived dendritic cells produce and secrete α-defensins 1–3. J. Leukoc. Biol. 82, 1143–1146 (2007).
Yamamoto-Furusho, J. K., Barnich, N., Hisamatsu, T. & Podolsky, D. K. MDP-NOD2 stimulation induces HNP-1 secretion, which contributes to NOD2 antibacterial function. Inflamm. Bowel Dis. 16, 736–742 (2010).
Negroni, A. et al. Activation of NOD2-mediated intestinal pathway in a pediatric population with Crohn's disease. Inflamm. Bowel Dis. 15, 1145–1154 (2009).
Harder, J., Bartels, J., Christophers, E. & Schroder, J. M. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276, 5707–5713 (2001).
García, J. R. et al. Human β-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 15, 1819–1821 (2001).
Miles, K. et al. Dying and necrotic neutrophils are anti-inflammatory secondary to the release of α-defensins. J. Immunol. 183, 2122–2132 (2009).
Soehnlein, O. et al. Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages. J. Clin. Invest. 118, 3491–3502 (2008).
Niyonsaba, F. et al. Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Invest. Dermatol. 127, 594–604 (2007).
Boniotto, M. et al. Human β-defensin 2 induces a vigorous cytokine response in peripheral blood mononuclear cells. Antimicrob. Agents Chemother. 50, 1433–1441 (2006).
Funderburg, N. et al. Human β-defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc. Natl Acad. Sci. USA 104, 18631–18635 (2007).
Chaly, Y. V. et al. Neutrophil α-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur. Cytokine Netw. 11, 257–266 (2000).
Tewary, P. et al. β-Defensin 2 and 3 promote the uptake of self or CpG DNA, enhance IFN-α production by human plasmacytoid dendritic cells, and promote inflammation. J. Immunol. 191, 865–874 (2013).
Economopoulou, M. et al. Inhibition of pathologic retinal neovascularization by α-defensins. Blood 106, 3831–3838 (2005).
Chavakis, T. et al. Regulation of neovascularization by human neutrophil peptides (α-defensins): a link between inflammation and angiogenesis. FASEB J. 18, 1306–1308 (2004).
Lu, W. & de Leeuw, E. Pro-inflammatory and pro-apoptotic properties of Human Defensin 5. Biochem. Biophys. Res. Commun. 436, 557–562 (2013).
Nagaoka, I., Niyonsaba, F., Tsutsumi-Ishii, Y., Tamura, H. & Hirata, M. Evaluation of the effect of human β-defensins on neutrophil apoptosis. Int. Immunol. 20, 543–553 (2008).
Semple, F. et al. Human β-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur. J. Immunol. 40, 1073–1078 (2010).
Semple, F. et al. Human β-defensin 3 affects the activity of pro-inflammatory pathways associated with MyD88 and TRIF. Eur. J. Immunol. 41, 3291–3300 (2011). This article describes the response of mouse macrophages to HBD3 in combination with TLR4 stimuli. The specific pathways that are modulated by this HDP to achieve its anti-inflammatory effects are outlined.
Suarez-Carmona, M. et al. ΔNp63 isoform-mediated β-defensin family up-regulation is associated with (lymph)angiogenesis and poor prognosis in patients with squamous cell carcinoma. Oncotarget 5, 1856–1868 (2014).
Baroni, A. et al. Antimicrobial human β-defensin-2 stimulates migration, proliferation and tube formation of human umbilical vein endothelial cells. Peptides 30, 267–272 (2009).
Pena, O. M. et al. Synthetic cationic peptide IDR- 1018 modulates human macrophage differentiation. PLoS ONE 8, e52449 (2013). This paper describes the effects of a synthetic HDP, IDR-1018, on macrophage differentiation; macrophages stimulated with IDR-1018 display a phenotype in-between the classical M1 and M2 states.
Mansour, S. C., de la Fuente-Núñez, C. & Hancock, R. E. Peptide IDR-1018: modulating the immune system and targeting bacterial biofilms to treat antibiotic-resistant bacterial infections. J. Pept. Sci. 21, 323–329 (2015).
Zhao, A., Lu, W. & de Leeuw, E. Functional synergism of Human Defensin 5 and Human Defensin 6. Biochem. Biophys. Res. Commun. 467, 967–972 (2015). This paper describes one of the first examples of immunomodulatory synergy between two natural HDPs.
Büchau, A. S. et al. The host defence peptide cathelicidin is required for NK cell-mediated suppression of tumor growth. J. Immunol. 184, 369–378 (2010).
Taudien, S. et al. Association studies of the copy-number variable ß-defensin cluster on 8p23.1 in adenocarcinoma and chronic pancreatitis. BMC Res. Notes 5, 629 (2012).
Han, Q. et al. Human β-defensin-1 suppresses tumor migration and invasion and is an independent predictor for survival of oral squamous cell carcinoma patients. PLoS ONE 9, e91867 (2014).
Winter, J. et al. Human β-defensin-1, -2, and -3 exhibit opposite effects on oral squamous cell carcinoma cell proliferation. Cancer Invest. 29, 196–201 (2011).
Gunes, M. et al. Plasma human neutrophil proteins-1, -2, and -3 levels in patients with bladder cancer. J. Cancer Res. Clin. Oncol. 139, 195–199 (2012).
Chuang, C.-M., Monie, A., Wu, A., Mao, C.-P. & Hung, C.-F. Treatment with LL-37 peptide enhances antitumor effects induced by CpG oligodeoxynucleotides against ovarian cancer. Hum. Gene Ther. 20, 303–313 (2009).
Kuroda, K., Okumura, K., Isogai, H. & Isogai, E. The human cathelicidin antimicrobial peptide LL-37 and mimics are potential anticancer drugs. Front. Oncol. 5, 344 (2015).
Gaspar, D., Veiga, A. S. & Castanho, M. A. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 4, 294 (2013). This review summarizes the relationship between HDPs and anticancer peptides.
von Haussen, J. et al. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer 59, 12–23 (2008).
Sainz, B. et al. Microenvironmental hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by activating its cancer stem cell compartment. Gut 64, 1921–1935 (2015).
Lecaille, F., Lalmanach, G. & Andrault, P.-M. Antimicrobial proteins and peptides in human lung diseases: a friend and foe partnership with host proteases. Biochimie 122, 151–168 (2016).
Ratjen, F. & Döring, G. Cystic fibrosis. Lancet 361, 681–689 (2003).
Chen, C. I.-U., Schaller-Bals, S., Paul, K. P., Wahn, U. & Bals, R. β-defensins and LL-37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. J. Cyst. Fibros. 3, 45–50 (2004).
Bergsson, G. et al. LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline. J. Immunol. 183, 543–551 (2009).
Mayer, M. L. et al. Rescue of dysfunctional autophagy attenuates hyperinflammatory responses from cystic fibrosis cells. J. Immunol. 190, 1227–1238 (2013).
de la Fuente-Núñez, C., Reffuveille, F., Haney, E. F., Straus, S. K. & Hancock, R. E. W. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 10, e1004152 (2014). This paper details the mechanism of antibiofilm activity for a synthetic HDP.
Xiao, W., Hsu, Y.-P., Ishizaka, A., Kirikae, T. & Moss, R. B. Sputum cathelicidin, urokinase plasminogen activation system components, and cytokines discriminate cystic fibrosis, COPD, and asthma inflammation. Chest 128, 2316–2326 (2005).
Paone, G. et al. Human neutrophil peptides sputum levels in symptomatic smokers and COPD patients. Eur. Rev. Med. Pharmacol. Sci. 15, 556–562 (2011).
Olin, J. T. & Wechsler, M. E. Asthma: pathogenesis and novel drugs for treatment. BMJ 349, g5517 (2014).
Sun, J., Dahlén, B., Agerberth, B. & Haeggström, J. Z. The antimicrobial peptide LL-37 induces synthesis and release of cysteinyl leukotrienes from human eosinophils – implications for asthma. Allergy 68, 304–311 (2013).
Rohde, G. et al. CXC chemokines and antimicrobial peptides in rhinovirus-induced experimental asthma exacerbations. Clin. Exp. Allergy 44, 930–939 (2014).
Brauner, H. et al. Markers of innate immune activity in patients with type 1 and type 2 diabetes mellitus and the effect of the anti-oxidant coenzyme Q10 on inflammatory activity. Clin. Exp. Immunol. 177, 478–482 (2014).
Diana, J. et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat. Med. 19, 65–73 (2013).
Allen, J. S. et al. Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes 58, 138–145 (2009).
Sun, J. et al. Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 43, 304–317 (2015).
Gonzalez-Curiel, I. et al. Differential expression of antimicrobial peptides in active and latent tuberculosis and its relationship with diabetes mellitus. Hum. Immunol. 72, 656–662 (2011).
Rivas-Santiago, B. et al. Expression of antimicrobial peptides in diabetic foot ulcer. J. Dermatol. Sci. 65, 19–26 (2012).
Kahlenberg, J. M. & Kaplan, M. J. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J. Immunol. 191, 4895–4901 (2013).
Hall, J. C. & Rosen, A. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat. Rev. Rheumatol. 6, 40–49 (2010).
Murakami, M. et al. Cathelicidin anti-microbial peptide expression in sweat, an innate defence system for the skin. J. Invest. Dermatol. 119, 1090–1095 (2002).
Nardo, A. D., Vitiello, A. & Gallo, R. L. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J. Immunol. 170, 2274–2278 (2003).
Frohm, M. et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272, 15258–15263 (1997).
Clausen, M.-L., Slotved, H.-C., Krogfelt, K. A., Andersen, P. S. & Agner, T. In vivo expression of antimicrobial peptides in atopic dermatitis. Exp. Dermatol. 25, 3–9 (2016).
Nomura, I. et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J. Immunol. 171, 3262–3269 (2003).
Harder, J. et al. Enhanced expression and secretion of antimicrobial peptides in atopic dermatitis and after superficial skin injury. J. Invest. Dermatol. 130, 1355–1364 (2010).
Ballardini, N. et al. Enhanced expression of the antimicrobial peptide LL-37 in lesional skin of adults with atopic eczema. Br. J. Dermatol. 161, 40–47 (2009).
Kopfnagel, V., Harder, J. & Werfel, T. Expression of antimicrobial peptides in atopic dermatitis and possible immunoregulatory functions. Curr. Opin. Allergy Clin. Immunol. 13, 531–536 (2013).
Ong, P. Y. et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347, 1151–1160 (2002).
Lande, R. et al. Cationic antimicrobial peptides in psoriatic skin cooperate to break innate tolerance to self-DNA. Eur. J. Immunol. 45, 203–213 (2015).
Nestle, F. O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 202, 135–143 (2005).
Morizane, S. et al. Cathelicidin antimicrobial peptide LL-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J. Invest. Dermatol. 132, 135–143 (2012).
Lande, R. et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 5, 5621 (2014).
Nijnik, A. & Hancock, R. Host defence peptides: antimicrobial and immunomodulatory activity and potential applications for tackling antibiotic-resistant infections. Emerg. Health Threats J. 2, e1 (2009).
Gonzalez-Curiel, I. et al. 1,25-dihydroxyvitamin D3 induces LL-37 and HBD-2 production in keratinocytes from diabetic foot ulcers promoting wound healing: an in vitro model. PLoS ONE 9, e111355 (2014).
Raftery, T. et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn's disease: results from a randomised double-blind placebo-controlled study. United European Gastroenterol. J. 3, 294–302 (2015).
Mily, A. et al. Oral intake of phenylbutyrate with or without vitamin D3 upregulates the cathelicidin LL-37 in human macrophages: a dose finding study for treatment of tuberculosis. BMC Pulm. Med. 13, 23 (2013).
Gambichler, T. et al. Expression of antimicrobial peptides and proteins in etanercept-treated psoriasis patients. Regul. Pept. 167, 163–166 (2011).
Heilborn, J. D. et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Invest. Dermatol. 120, 379–389 (2003).
Shaykhiev, R. et al. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L842–L848 (2005).
Tokumaru, S. et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J. Immunol. 175, 4662–4668 (2005).
Niyonsaba, F. et al. Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Invest. Dermatol. 127, 594–604 (2006).
Grönberg, A., Mahlapuu, M., Ståhle, M., Whately-Smith, C. & Rollman, O. Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: a randomized, placebo-controlled clinical trial. Wound Repair Regen. 22, 613–621 (2014).
Hirsch, T. et al. Human β-defensin-3 promotes wound healing in infected diabetic wounds. J. Gene Med. 11, 220–228 (2009).
Steinstraesser, L. et al. Innate defence regulator peptide 1018 in wound healing and wound infection. PLoS ONE. 7, e39373 (2012).
Caterino, J. M. et al. A Prospective, observational pilot study of the use of urinary antimicrobial peptides in diagnosing emergency department patients with positive urine cultures. Acad. Emerg. Med. 22, 1226–1230 (2015).
Albrethsen, J., Møller, C. H., Olsen, J., Raskov, H. & Gammeltoft, S. Human neutrophil peptides 1, 2 and 3 are biochemical markers for metastatic colorectal cancer. Eur. J. Cancer 42, 3057–3064 (2006).
Kim, D. H. et al. Antimicrobial peptide, lumbricusin, ameliorates motor dysfunction and dopaminergic neurodegeneration in a mouse model of parkinson's disease. J. Microbiol. Biotechnol. 25, 1640–1647 (2015).
Zhang, Z. & Shively, J. E. Generation of novel bone forming cells (monoosteophils) from the cathelicidin-derived peptide LL-37 treated monocytes. PLoS ONE 5, e13985 (2010).
Bevins, C. L. & Salzman, N. H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 9, 356–368 (2011).
Cullen, T. W. et al. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science 347, 170–175 (2015).
Gellatly, S. L., Needham, B., Madera, L., Trent, M. S. & Hancock, R. E. W. The Pseudomonas aeruginosa PhoP-PhoQ two-component regulatory system is induced upon interaction with epithelial cells and controls cytotoxicity and inflammation. Infect. Immun. 80, 3122–3131 (2012).
Jones, E. A., Kananurak, A., Bevins, C. L., Hollox, E. J. & Bakaletz, L. O. Copy number variation of the β defensin gene cluster on chromosome 8p influences the bacterial microbiota within the nasopharynx of otitis-prone children. PLoS ONE 9, e98269 (2014).
Guo, L. et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl Acad. Sci. USA 112, 7569–7574 (2015).
Gardy, J. L., Lynn, D. J., Brinkman, F. S. L. & Hancock, R. E. W. Enabling a systems biology approach to immunology: focus on innate immunity. Trends Immunol. 30, 249–262 (2009).
Haney, E. F., Mansour, S. C., Hilchie, A. L., de la Fuente-Núñez, C. & Hancock, R. E. W. High throughput screening methods for assessing antibiofilm and immunomodulatory activities of synthetic peptides. Peptides 71, 276–285 (2015).
Cherkasov, A. et al. Use of artificial intelligence in the design of small peptide antibiotics effective against a broad spectrum of highly antibiotic-resistant superbugs. ACS Chem. Biol. 4, 65–74 (2009).
Barrett, T. et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res. 41, D991–D995 (2013).
Suarez-Carmona, M., Hubert, P., Delvenne, P. & Herfs, M. Defensins: 'simple' antimicrobial peptides or broad-spectrum molecules? Cytokine Growth Factor Rev. 26, 361–370 (2015).
Croft, D. et al. The Reactome pathway knowledgebase. Nucleic Acids Res. 42, D472–D477 (2014).
Acknowledgements
The authors' peptide research has been generously supported by the Canadian Institutes of Health Research (MOP-74493). R.E.W.H. holds a Canada Research Chair.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
R.E.W.H. is developing innate defence regulator (IDR) peptides as therapeutics and vaccine adjuvants, and has filed several patents in this area, all of which are assigned to his employer, the University of British Columbia. Two of his IDR peptides have been licensed to Elanco Animal Health Inc., for use in treatment of animals and as vaccine adjuvants, and one has been licensed to the Pan-provincial Vaccine Enterprise, for development as a component of vaccine adjuvant formulations. E.F.H. is a co-inventor on one of these patents. Recently, R.E.W.H. formed a new virtual company, ABT Innovations Inc., to promote commercialization of the University of British Columbia peptide patents. E.E.G. declares no competing interests.
Related links
DATABASES
Supplementary information
Supplementary information S1 (table)
HDP expression patterns and affected cell types and functions. (PDF 195 kb)
Glossary
- Host defence peptides
-
(HDPs). Short (<50 amino acids) cationic amphipathic peptides with immunomodulatory and/or antimicrobial activities.
- Antimicrobial peptides
-
Cationic peptides with an emphasis on their antimicrobial activities; traditional terminology for host defence peptides.
- Immunomodulatory
-
The ability to modulate the immune response, including by influencing the production of chemokines and cytokines.
- Innate defence regulator
-
(IDR). Synthetic immunomodulatory peptide derived from natural host defence peptides.
- Pleiotropic
-
Having more than one effect on the biological system.
- Diapedesis
-
The migration of leukocytes across the endothelium, which occurs by leukocytes squeezing through the junctions between adjacent endothelial cells.
- Global transcriptional profiling
-
Measurement of the entire gene expression profile to obtain an overall picture of the activity of genes in a cell or biological system.
- Neurotropic
-
Localization to nerve tissue.
Rights and permissions
About this article
Cite this article
Hancock, R., Haney, E. & Gill, E. The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol 16, 321–334 (2016). https://doi.org/10.1038/nri.2016.29
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2016.29
This article is cited by
-
The protective role of chicken cathelicidin-1 against Streptococcus suis serotype 2 in vitro and in vivo
Veterinary Research (2023)
-
Effects of diets with different amino acid release characteristics on the gut microbiota and barrier function of weaned pigs
BMC Microbiology (2023)
-
Characterization of a novel peptide mined from the Red Sea brine pools and modified to enhance its anticancer activity
BMC Cancer (2023)
-
Bactericidal synergism between phage endolysin Ply2660 and cathelicidin LL-37 against vancomycin-resistant Enterococcus faecalis biofilms
npj Biofilms and Microbiomes (2023)
-
Regulation of dermal fibroblasts by human neutrophil peptides
Scientific Reports (2023)