Key Points
-
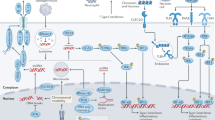
Actin, microtubules, intermediate filaments and septins are four major components of the cytoskeleton. They all have crucial roles in cell-autonomous immunity.
-
There is currently intense investigation of how cytoskeletal dynamics are regulated, and how cytoskeletal components interact and work in conjunction with cell-autonomous immunity.
-
Sensing of pathogenic bacteria is a first step in orchestrating their elimination by the immune system. During infection, bacterial effector proteins and toxins have effects on the host cytoskeleton, and new studies have identified innate immune sensors that guard against pathogen-induced manipulation of cytoskeleton proteins.
-
Crucial functions of the cytoskeleton in cell-autonomous immunity include their scaffolding role for protein recruitment at the plasma membrane and in the cytosol, and their role in subcellular compartmentalization.
-
Innate immune proteins also regulate cytoskeletal dynamics to effectively restrict bacterial pathogens, highlighting the interdependence between the cytoskeleton and cell-autonomous immunity.
-
Further investigations into the multiple roles of the cytoskeleton in host defence will help to decipher the molecular mechanisms underlying human diseases, such as cancer, neurological disorders and autoinflammatory conditions, as well as susceptibility to infections.
Abstract
Host cells use antimicrobial proteins, pathogen-restrictive compartmentalization and cell death in their defence against intracellular pathogens. Recent work has revealed that four components of the cytoskeleton — actin, microtubules, intermediate filaments and septins, which are well known for their roles in cell division, shape and movement — have important functions in innate immunity and cellular self-defence. Investigations using cellular and animal models have shown that these cytoskeletal proteins are crucial for sensing bacteria and for mobilizing effector mechanisms to eliminate them. In this Review, we highlight the emerging roles of the cytoskeleton as a structural determinant of cell-autonomous host defence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Randow, F., MacMicking, J. D. & James, L. C. Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science 340, 701–706 (2013). This paper provides a summary of mechanisms of cell-autonomous immunity in host defence.
Pasparakis, M. & Vandenabeele, P. Necroptosis and its role in inflammation. Nature 517, 311–320 (2015).
Sridharan, H. & Upton, J. W. Programmed necrosis in microbial pathogenesis. Trends Microbiol. 22, 199–207 (2014).
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011).
Yoneyama, M., Onomoto, K., Jogi, M., Akaboshi, T. & Fujita, T. Viral RNA detection by RIG-I-like receptors. Curr. Opin. Immunol. 32C, 48–53 (2015).
Philpott, D. J., Sorbara, M. T., Robertson, S. J., Croitoru, K. & Girardin, S. E. NOD proteins: regulators of inflammation in health and disease. Nat. Rev. Immunol. 14, 9–23 (2014).
von Moltke, J., Ayres, J. S., Kofoed, E. M., Chavarría-Smith, J. & Vance, R. E. Recognition of bacteria by inflammasomes. Ann. Rev. Immunol. 31, 73–106 (2013).
Schattgen, S. A. & Fitzgerald, K. A. The PYHIN protein family as mediators of host defenses. Immunol. Rev. 243, 109–118 (2011).
Dambuza, I. M. & Brown, G. D. C-type lectins in immunity: recent developments. Curr. Opin. Immunol. 32C, 21–27 (2014).
Deretic, V. Autophagy as an innate immunity paradigm: expanding the scope and repertoire of pattern recognition receptors. Curr. Opin. Immunol. 24, 21–31 (2012).
Stolz, A., Ernst, A. & Dikic, I. Cargo recognition and trafficking in selective autophagy. Nat. Cell Biol. 16, 495–501 (2014).
Bonizzi, G. & Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25, 280–288 (2004).
Arthur, J. S. C. & Ley, S. C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 13, 679–692 (2013).
Tamura, T., Yanai, H., Savitsky, D. & Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Ann. Rev. Immunol. 26, 535–584 (2008).
MacMicking, J. D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol. 12, 367–382 (2012).
Schneider, W. M., Chevillotte, M. D. & Rice, C. M. Interferon-stimulated genes: a complex web of host defenses. Ann. Rev. Immunol. 32, 513–545 (2014).
Eldridge, M. J. G. & Shenoy, A. R. Antimicrobial inflammasomes: unified signalling against diverse bacterial pathogens. Curr. Opin. Microbiol. 23, 32–41 (2015).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Aachoui, Y. et al. Caspase-11 protects against bacteria that escape the vacuole. Science 339, 975–978 (2013). References 18 and 19 report the identification of cell-autonomous roles for caspase 4 (previously known as caspase 11) and caspase 5 in detecting cytosolic LPS and protecting against bacterial infections.
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Rathinam, V. A. K. et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150, 606–619 (2012).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Aachoui, Y., Sagulenko, V., Miao, E. A. & Stacey, K. J. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr. Opin. Microbiol. 16, 319–326 (2013).
Levine, B., Mizushima, N. & Virgin, H. W. Autophagy in immunity and inflammation. Nature 469, 323–335 (2011).
Pollard, T. D. & Cooper, J. A. Actin, a central player in cell shape and movement. Science 326, 1208–1212 (2009).
Desai, A. & Mitchison, T. J. Microtubule polymerization dynamics. Ann. Rev. Cell Dev. Biol. 13, 83–117 (1997).
Herrmann, H., Strelkov, S. V., Burkhard, P. & Aebi, U. Intermediate filaments: primary determinants of cell architecture and plasticity. J. Clin. Invest. 119, 1772–1783 (2009).
Mostowy, S. & Cossart, P. Septins: the fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 13, 183–194 (2012).
Haglund, C. M. & Welch, M. D. Pathogens and polymers: microbe–host interactions illuminate the cytoskeleton. J. Cell Biol. 195, 7–17 (2011).
Keestra, A. M. et al. Manipulation of small Rho GTPases is a pathogen-induced process detected by NOD1. Nature 496, 233–237 (2013). This is the first report on an NLR that acts as a guardian that senses pathogen-induced changes to host proteins.
Hardt, W.-D., Chen, L.-M., Schuebel, K. E., Bustelo, X. R. & Galán, J. E. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93, 815–826 (1998).
Kufer, T. A., Kremmer, E., Adam, A. C., Philpott, D. J. & Sansonetti, P. J. The pattern-recognition molecule Nod1 is localized at the plasma membrane at sites of bacterial interaction. Cell. Microbiol. 10, 477–486 (2008).
Fukazawa, A. et al. GEF-H1 mediated control of NOD1 dependent NF-κB activation by Shigella effectors. PLoS Pathog. 4, e1000228 (2008).
Bielig, H. et al. The cofilin phosphatase slingshot homolog 1 (SSH1) links NOD1 signaling to actin remodeling. PLoS Pathog. 10, e1004351 (2014).
Bravo-Cordero, J. J., Magalhaes, M. A. O., Eddy, R. J., Hodgson, L. & Condeelis, J. Functions of cofilin in cell locomotion and invasion. Nat. Rev. Mol. Cell Biol. 14, 405–415 (2013).
Le Bourhis, L. et al. Role of Nod1 in mucosal dendritic cells during Salmonella pathogenicity island 1-independent Salmonella enterica serovar Typhimurium infection. Infect. Immun. 77, 5203–5203 (2009).
Eitel, J. et al. β-PIX and Rac1 GTPase mediate trafficking and negative regulation of NOD2. J. Immunol. 181, 2664–2671 (2008).
Legrand-Poels, S. et al. Modulation of Nod2-dependent NF-κB signaling by the actin cytoskeleton. J. Cell Sci. 120, 1299–1310 (2007).
Zhao, Y. et al. Control of NOD2 and Rip2-dependent innate immune activation by GEF-H1. Inflamm. Bowel Dis. 18, 603–612 (2012).
Stevens, C. et al. The intermediate filament protein, vimentin, is a regulator of NOD2 activity. Gut 62, 695–707 (2013).
Dangl, J. L., Horvath, D. M. & Staskawicz, B. J. Pivoting the plant immune system from dissection to deployment. Science 341, 746–751 (2013).
Savic, S., Dickie, L. J., Battellino, M. & McDermott, M. F. Familial mediterranean fever and related periodic fever syndromes/autoinflammatory diseases. Curr. Opin. Rheum. 24, 103–112 (2012).
Xu, H. et al. Innate immune sensing of bacterial modifications of Rho GTPases by the pyrin inflammasome. Nature 513, 237–241 (2014). This study identified the first non-NLR protein, pyrin, as an inflammasome sensor of covalent modifications of RHO GTPases.
Gavrilin, M. A. et al. Activation of the pyrin inflammasome by intracellular Burkholderia cenocepacia. J. Immunol. 188, 3469–3477 (2012).
Clemens, D. L., Lee, B.-Y. & Horwitz, M. A. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect. Immun. 73, 5892–5902 (2005).
Gavrilin, M. A. et al. Pyrin critical to macrophage IL-1β response to Francisella challenge. J. Immunol. 182, 7982–7989 (2009).
Waite, A. L. et al. Pyrin and ASC co-localize to cellular sites that are rich in polymerizing actin. Exp. Biol. Med. 234, 40–52 (2009).
Gavrilin, M. A. & Wewers, M. D. Francisella recognition by inflammasomes: differences between mice and men. Front. Microbiol. 2, 11 (2011).
Fernandes-Alnemri, T. et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393 (2010).
Jones, J. W. et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl Acad. Sci. USA 107, 9771–9776 (2010).
Rathinam, V. A. K. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Kim, M. L. et al. Aberrant actin depolymerization triggers the pyrin inflammasome and autoinflammatory disease that is dependent on IL-18, not IL-1β. J. Exp. Med. 212, 927–938 (2015).
Kile, B. T. et al. Mutations in the cofilin partner Aip1/Wdr1 cause autoinflammatory disease and macrothrombocytopenia. Blood 110, 2371–2380 (2007).
Muñoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Lamkanfi, M. & Dixit, V. M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Misawa, T. et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 14, 454–460 (2013). This study proposed a role for microtubules in mitochondrial trafficking and activation of NLRP3 inflammasomes.
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
dos Santos, G. et al. Vimentin regulates activation of the NLRP3 inflammasome. Nat. Commun. 6, 6574 (2015). This is the first report on NLRP3 activation requiring vimentin, highlighting a link between the NLRP3 inflammasome and the cytoskeleton.
Shenoy, A. R. et al. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 336, 481–485 (2012).
Mishra, B. B. et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nat. Immunol. 14, 52–60 (2013).
Park, S. et al. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J. Immunol. 191, 4358–4366 (2013).
Subramanian, N., Natarajan, K., Clatworthy, M. R., Wang, Z. & Germain, R. N. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell 153, 348–361 (2013).
Rees, F., Hui, M. & Doherty, M. Optimizing current treatment of gout. Nat. Rev. Rheumatol. 10, 271–283 (2014).
Goldbach-Mansky, R. Current status of understanding the pathogenesis and management of patients with NOMID/CINCA. Curr. Rheumatol. Rep. 13, 123–131 (2011).
Strowig, T., Henao-Mejia, J., Elinav, E. & Flavell, R. Inflammasomes in health and disease. Nature 481, 278–286 (2012).
Zhang, J.-G. et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity 36, 646–657 (2012).
Ahrens, S. et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity 36, 635–645 (2012). References 67 and 68 report the identification of actin as a ligand for CLEC9A and its role in acting as danger signal that signifies cell death.
Sancho, D. et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 458, 899–903 (2009).
Hanc, P. et al. Structure of the complex of F-actin and DNGR-1, a C-type lectin receptor involved in dendritic cell cross-presentation of dead cell-associated antigens. Immunity 42, 839–849 (2015).
Zelenay, S. et al. The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice. J. Clin. Invest. 122, 1615–1627 (2012).
Caudron, F. & Barral, Y. Septins and the lateral compartmentalization of eukaryotic membranes. Dev. Cell 16, 493–506 (2009).
Tran Van Nhieu, G. et al. Actin-based confinement of calcium responses during Shigella invasion. Nat. Commun. 4, 1567 (2013).
Iborra, S. et al. The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice. J. Clin. Invest. 122, 1628–1643 (2012).
Sandiford, S. L. et al. Cytoplasmic actin is an extracellular insect immune factor which is secreted upon immune challenge and mediates phagocytosis and direct killing of bacteria, and is a Plasmodium antagonist. PLoS Pathog. 11, e1004631 (2015).
Aktories, K., Lang, A. E., Schwan, C. & Mannherz, H. G. Actin as target for modification by bacterial protein toxins. FEBS J. 278, 4526–4543 (2011).
Goodridge, H. S., Underhill, D. M. & Touret, N. Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic 13, 1062–1071 (2012).
Rosas, M. et al. The induction of inflammation by dectin-1 in vivo is dependent on myeloid cell programming and the progression of phagocytosis. J. Immunol. 181, 3549–3557 (2008).
Watts, C., West, M. A. & Zaru, R. TLR signalling regulated antigen presentation in dendritic cells. Curr. Opin. Immunol. 22, 124–130 (2010).
Underhill, D. M. & Goodridge, H. S. Information processing during phagocytosis. Nat. Rev. Immunol. 12, 492–502 (2012).
Kagan, J. C. et al. TRAM couples endocytosis of toll-like receptor 4 to the induction of interferon-β. Nat. Immunol. 9, 361–368 (2008).
Sasai, M., Linehan, M. M. & Iwasaki, A. Bifurcation of toll-like receptor 9 signaling by adaptor protein 3. Science 329, 1530–1534 (2010).
Gotoh, K. et al. Selective control of type I IFN induction by the Rac activator DOCK2 during TLR-mediated plasmacytoid dendritic cell activation. J. Exp. Med. 207, 721–730 (2010). References 82 and 83 show that actin-dependent maturation of TLR9 endosomes controls expression of type I IFNs.
Parker, D. & Prince, A. Staphylococcus aureus induces type I IFN signaling in dendritic cells via TLR9. J. Immunol. 189, 4040–4046 (2012).
Torchinsky, M. B., Garaude, J., Martin, A. P. & Blander, J. M. Innate immune recognition of infected apoptotic cells directs TH17 cell differentiation. Nature 458, 78–82 (2009).
Kong, L. et al. An essential role for RIG-I in toll-like receptor-stimulated phagocytosis. Cell Host Microbe 6, 150–161 (2009). This study identified a key role for the cytosolic RNA sensor RIG-I in actin dynamics downstream of TLR4.
Mostowy, S. et al. Septins regulate bacterial entry into host cells. PLoS ONE 4, e4196 (2009).
Mostowy, S. et al. Septin 11 restricts InlB-mediated invasion by Listeria. J. Biol. Chem. 284, 11613–11621 (2009).
Huang, Y.-W. et al. Mammalian septins are required for phagosome formation. Mol. Biol. Cell 19, 1717–1726 (2008).
Golebiewska, U. et al. Evidence for a fence that impedes the diffusion of phosphatidylinositol 4,5-bisphosphate out of the forming phagosomes of macrophages. Mol. Biol. Cell 22, 3498–3507 (2011).
Mizushima, N., Yoshimori, T. & Ohsumi, Y. The role of Atg proteins in autophagosome formation. Ann. Rev. Cell Dev. Biol. 27, 107–132 (2011).
Mostowy, S. & Cossart, P. Bacterial autophagy: restriction or promotion of bacterial replication? Trends Cell Biol. 22, 283–291 (2012).
Huang, J. & Brumell, J. H. Bacteria–autophagy interplay: a battle for survival. Nat. Rev. Micro. 12, 101–114 (2014).
Mostowy, S. Multiple roles of the cytoskeleton in bacterial autophagy. PLoS Pathog. 10, e1004409 (2014).
Monastyrska, I., Rieter, E., Klionsky, D. J. & Reggiori, F. Multiple roles of the cytoskeleton in autophagy. Biol. Rev. 84, 431–448 (2009).
Mostowy, S. et al. Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe 8, 433–444 (2010). This study is the first report on the septin cage and provides the first link between septins and cell-autonomous immunity.
Mostowy, S. et al. p62 and NDP52 proteins target intracytosolic Shigella and Listeria to different autophagy pathways. J. Biol. Chem. 286, 26987–26995 (2011).
Ogawa, M. et al. Escape of intracellular Shigella from autophagy. Science 307, 727–731 (2005).
Ogawa, M. et al. A Tecpr1-dependent selective autophagy pathway targets bacterial pathogens. Cell Host Microbe 9, 376–389 (2011).
Chen, D. et al. A mammalian autophagosome maturation mechanism mediated by TECPR1 and the Atg12–Atg5 conjugate. Mol. Cell 45, 629–641 (2012).
Yoshikawa, Y. et al. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat. Cell Biol. 11, 1233–1240 (2009).
Schroeder, N., Mota, L. J. & Méresse, S. Salmonella-induced tubular networks. Trends Microbiol. 19, 268–277 (2011).
Thurston, T. L. M., Ryzhakov, G., Bloor, S., von Muhlinen, N. & Randow, F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat. Immunol. 10, 1215–1221 (2009). This study describes the discovery of NDP52 as the first autophagy receptor for antibacterial autophagy.
Zheng, Y. T. et al. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J. Immunol. 183, 5909–5916 (2009).
Wild, P. et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333, 228–233 (2011).
Yu, H. B. et al. Autophagy facilitates Salmonella replication in HeLa cells. mBio 5, e00865-14 (2014).
Wang, R. C. et al. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 338, 956–959 (2012). This study identified a fundamental link between autophagy and the cytoskeleton, showing a role for vimentin in autophagy stabilization via interaction with beclin 1.
Kumar, Y. & Valdivia, R. H. Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds. Cell Host Microbe 4, 159–169 (2008).
Al-Zeer, M. A., Al-Younes, H. M., Lauster, D., Abu Lubad, M. & Meyer, T. F. Autophagy restricts Chlamydia trachomatis growth in human macrophages via IFNG-inducible guanylate binding proteins. Autophagy 9, 50–62 (2012).
Kim, B.-H. et al. A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science 332, 717–721 (2011).
Ostler, N. et al. Gamma interferon-induced guanylate binding protein 1 is a novel actin cytoskeleton remodeling factor. Mol. Cell. Biol. 34, 196–209 (2014).
Tanaka-Takiguchi, Y., Kinoshita, M. & Takiguchi, K. Septin-mediated uniform bracing of phospholipid membranes. Curr. Biol. 19, 140–145 (2009).
Mostowy, S. et al. The zebrafish as a new model for the in vivo study of Shigella flexneri interaction with phagocytes and bacterial autophagy. PLoS Pathog. 9, e1003588 (2013).
Sanjuan, M. A. et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450, 1253–1257 (2007). This is the first study to show close links between TLR signalling, phagocytosis and non-canonical autophagy.
West, A. P. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 (2011).
Randow, F. & Youle, R. J. Self and nonself: how autophagy targets mitochondria and bacteria. Cell Host Microbe 15, 403–411 (2014).
Manzanillo, P. S. et al. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature 501, 512–516 (2013). This is an important study in understanding the parallel mechanisms underlying bacterial autophagy and mitophagy.
Li, J. et al. Caspase-11 regulates cell migration by promoting Aip1–Cofilin-mediated actin depolymerization. Nat. Cell Biol. 9, 276–286 (2007).
Li, J., Yin, H. L. & Yuan, J. Flightless-I regulates proinflammatory caspases by selectively modulating intracellular localization and caspase activity. J. Cell Biol. 181, 321–333 (2008). References 118 and 119 report important roles for caspase 4 in actin dynamics in vitro and in vivo.
Man, S. M. et al. Actin polymerization as a key innate immune effector mechanism to control Salmonella infection. Proc. Natl Acad. Sci. USA 111, 17588–17593 (2014). This study identified a role for NLRC4 in actin dynamics during S . Typhimurium infection.
Jin, J. et al. LRRFIP2 negatively regulates NLRP3 inflammasome activation in macrophages by promoting Flightless-I-mediated caspase-1 inhibition. Nat. Commun. 4, 2075 (2013).
Akhter, A. et al. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 37, 35–47 (2012). This paper reports the identification of a cell-autonomous role for caspase 4 in restricting L. pneumophila infection in mice.
Vance, R. E. The NAIP/NLRC4 inflammasomes. Curr. Opin. Immunol. 32, 84–89 (2015).
Ablasser, A. et al. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 503, 530–534 (2013).
Cai, X., Chiu, Y.-H. & Chen, Zhijian, J. The cGAS–cGAMP–STING pathway of cytosolic DNA sensing and signaling. Mol. Cell 54, 289–296 (2014).
McEwan, W. A. et al. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat. Immunol. 14, 327–336 (2013).
Tam, J. C. H., Bidgood, S. R., McEwan, W. A. & James, L. C. Intracellular sensing of complement C3 activates cell autonomous immunity. Science 345, 1256070 (2014).
Xia, P. et al. Sox2 functions as a sequence-specific DNA sensor in neutrophils to initiate innate immunity against microbial infection. Nat. Immunol. 16, 366–375 (2015). This paper reports the discovery of SOX2 as a cytosolic DNA sensor in neutrophils for host defence against bacterial infection.
Knodler, L. A. et al. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16, 249–256 (2014).
Ma, Y., Galluzzi, L., Zitvogel, L. & Kroemer, G. Autophagy and cellular immune responses. Immunity 39, 211–227 (2013).
Snider, N. T. & Omary, M. B. Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat. Rev. Mol. Cell Biol. 15, 163–177 (2014).
Terman, J. R. & Kashina, A. Post-translational modification and regulation of actin. Curr. Opin. Cell Biol. 25, 30–38 (2013).
Song, Y. & Brady, S. T. Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol. 25, 125–136 (2014).
Swanson, J. A. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 9, 639–649 (2008).
Cameron, L. A., Robbins, J. R., Footer, M. J. & Theriot, J. A. Biophysical parameters influence actin-based movement, trajectory, and initiation in a cell-free system. Mol. Biol. Cell 15, 2312–2323 (2004).
Cabeen, M. T. & Jacobs-Wagner, C. The bacterial cytoskeleton. Ann. Rev. Genet. 44, 365–392 (2010).
Jones, L. J. F., Carballido- López, R. & Errington, J. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell 104, 913–922 (2001).
Bi, E. & Lutkenhaus, J. FtsZ ring structure associated with division in Escherichia coli. Nature 354, 161–164 (1991).
Ausmees, N., Kuhn, J. R. & Jacobs-Wagner, C. The bacterial cytoskeleton: an intermediate filament-like function in cell shape. Cell 115, 705–713 (2003).
Weirich, C. S., Erzberger, J. P. & Barral, Y. The septin family of GTPases: architecture and dynamics. Nat. Rev. Mol. Cell Biol. 9, 478–489 (2008).
Ghosal, D., Trambaiolo, D., Amos, L. A. & Löwe, J. MinCD cell division proteins form alternating copolymeric cytomotive filaments. Nat. Commun. 5, 5341 (2014).
Angus, K. L. & Griffiths, G. M. Cell polarisation and the immunological synapse. Curr. Opin. Cell Biol. 25, 85–91 (2013).
Harwood, N. E. & Batista, F. D. The cytoskeleton coordinates the early events of B-cell activation. Cold Spring Harb. Perspect. Biol. 3, a002360 (2011).
Thrasher, A. J. & Burns, S. O. WASP: a key immunological multitasker. Nat. Rev. Immunol. 10, 182–192 (2010).
Frade, A. F. et al. Polymorphism in the α cardiac muscle actin 1 gene is associated to susceptibility to chronic inflammatory cardiomyopathy. PLoS ONE 8, e83446 (2013).
Solomon, J. P., Page, L. J., Balch, W. E. & Kelly, J. W. Gelsolin amyloidosis: genetics, biochemistry, pathology and possible strategies for therapeutic intervention. Crit. Rev. Biochem. Mol. Biol. 47, 282–296 (2012).
Kuhlenbaumer, G. et al. Mutations in SEPT9 cause hereditary neuralgic amyotrophy. Nat. Genet. 37, 1044–1046 (2005).
Blanchoin, L., Boujemaa-Paterski, R., Sykes, C. & Plastino, J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 94, 235–263 (2014).
Hyams, J. S. & Lloyd, C. W. Microtubules (eds Hyams, J. S. & Lloyd, C. W.) (Wiley-Liss, 1994).
Saarikangas, J. & Barral, Y. The emerging functions of septins in metazoans. EMBO Rep. 12, 1118–1126 (2011).
Cossart, P. & Sansonetti, P. J. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304, 242–248 (2004).
Pizarro-Cerdá, J., Kühbacher, A. & Cossart, P. Entry of Listeria monocytogenes in mammalian epithelial cells: an updated view. Cold Spring Harb. Perspect. Med. 2, a010009 (2012).
Carayol, N. & Tran Van Nhieu, G. Tips and tricks about Shigella invasion of epithelial cells. Curr. Opin. Microbiol. 16, 32–37 (2013).
Caron, E. et al. Subversion of actin dynamics by EPEC and EHEC. Curr. Opin. Microbiol. 9, 40–45 (2006).
Jaffe, A. B. & Hall, A. RHO GTPases: biochemistry and biology. Ann. Rev. Cell Dev. Biol. 21, 247–269 (2005).
Aktories, K. Bacterial protein toxins that modify host regulatory GTPases. Nat. Rev. Micro. 9, 487–498 (2011).
Welch, M. D. & Way, M. Arp2/3-mediated actin-based motility: a tail of pathogen abuse. Cell Host Microbe 14, 242–255 (2013).
Reed, S. C. O., Lamason, R. L., Risca, V. I., Abernathy, E. & Welch, M. D. Rickettsia actin-based motility occurs in distinct phases mediated by different actin nucleators. Curr. Biol. 24, 98–103 (2014).
Benanti, E. L., Nguyen, C. M. & Welch, M. D. Virulent Burkholderia species mimic host actin polymerases to drive actin-based motility. Cell 161, 348–360 (2015). This study highlights that intracellular bacteria imitate various different host actin-polymerizing pathways.
Asrat, S., de Jesús, D. A., Hempstead, A. D., Ramabhadran, V. & Isberg, R. R. Bacterial pathogen manipulation of host membrane trafficking. Ann. Rev. Cell Dev. Biol. 30, 79–109 (2014).
Kokes, M. et al. Integrating chemical mutagenesis and whole-genome sequencing as a platform for forward and reverse genetic analysis of Chlamydia. Cell Host Microbe 17, 716–725 (2015).
Rubinsztein, D. C., Bento, C. F. & Deretic, V. Therapeutic targeting of autophagy in neurodegenerative and infectious diseases. J. Exp. Med. 212, 979–990 (2015).
Mackeh, R., Perdiz, D., Lorin, S., Codogno, P. & Poüs, C. Autophagy and microtubules – new story, old players. J. Cell Sci. 126, 1071–1080 (2013).
Dobbs, K. et al. Inherited DOCK2 deficiency in patients with early-onset invasive infections. N. Engl. J. Med. 372, 2409–2422 (2015).
Acknowledgements
The authors apologize that, owing to space limitations, many primary research articles of importance to the field could not be acknowledged in this Review. They thank A. Willis for preparation of some of the original figures. Work in the laboratory of S.M. is supported by a Wellcome Trust Research Career Development Fellowship (WT097411MA) and the Lister Institute of Preventive Medicine. A.R.S. would like to acknowledge funds from the Wellcome Trust (WT108246AIA), Imperial College and the Royal Society (RG130811).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Bacterial mechanisms to avoid or exploit autophagy (PDF 293 kb)
Glossary
- Pyrin
-
An inflammasome-activating protein that is not part of the nucleotide-binding and oligomerization domain (NOD)- and leucine-rich repeat (LRR)-containing protein (NLR) family. It has a PAAD (pyrin, AIM, ASC death-domain-like) domain, a DAPIN (domain in apoptosis and interferon response) domain, a PYD (pyrin amino-terminal homology domain), a tripartite motif (TRIM; containing the RING domain, B-box and coiled coil domains), and a carboxy-terminal B30.2 domain.
- Inflammasomes
-
Large oligomeric multiprotein complexes that act as signalling platforms to catalytically activate pro-caspase 1 into its active p20 and p10 subunits, promote the maturation of interleukin-1β (IL-1β) and IL-18, and triggers pyroptosis.
- Non-canonical inflammasome activation
-
NOD-, LRR- and pyrin domain-containing 3 (NLRP3)- and ASC-dependent activation of caspase 1 by caspase 4-mediated detection of lipo-polysaccharide that promotes caspase 1-dependent maturation of interleukin-1β (IL-1β) and IL-18, and triggers caspase 4-dependent pyroptosis.
- Pyroptosis
-
A form of necrotic cell death that is activated by caspase 1, caspase 4 or caspase 5 and results in inflammation; morphological features include cell swelling and the formation of membrane pores, eventually leading to cell lysis.
- Autophagy
-
A catabolic, membrane-trafficking process that involves degradation of cellular components through the actions of lysosomes.
- Autolysosome
-
The compartment resulting from the fusion of an autophagosome with a lysosome.
- Actin
-
A globular protein (G-actin) that forms microfilaments (F-actin) by polymerizing in an ATP-dependent manner.
- Microtubules
-
Highly dynamic cylindrical filaments made from α- and β-tubulin heterodimers. They are formed by polymerization of tubulin in a GTP-dependent manner.
- Intermediate filaments
-
A heterogeneous protein family that contains both nuclear and cytoplasmic proteins, which assemble into filaments intermediate in size between smaller microfilaments and larger microtubules.
- Septins
-
Conserved family of GTP-binding proteins that assemble to form hetero-oligomeric complexes, filaments and rings.
- RHO family GTPases
-
Small (~21 kDa) GTP-hydrolysing enzymes that cycle between their active (GTP-bound) and inactive (GDP-bound) forms and act as molecular switches.
- Type III secretion system
-
(T3SS). A needle-like structure, also called the injectisome, used by Gram-negative bacteria to secrete proteins from the bacterial cell into the eukaryotic cell.
- Guanine nucleotide exchange factor
-
(GEF). Factor that activates monomeric GTPases by stimulating the release of GDP to allow binding of GTP; some GEFs can activate multiple GTPases, whereas others are specific to a single GTPase.
- Druggable human genes
-
A collection of human genes — typically from large gene families such as heterotrimeric G protein-coupled receptors, protein kinases, ion channels and ubiquitin ligases — predicted to bind small molecules that could alter their function in a therapeutically beneficial way.
- Cytochalasin D
-
A fungal metabolite that binds to actin filaments and is used to block the polymerization and elongation of actin.
- Resistance genes
-
(R-genes). Genes in plants that encode proteins with nucleotide-binding oligomerization domain (NOD) and leucine-rich repeat (LRR) domains, and amino-terminal interaction motifs such as Toll–IL-1 receptor (TIR) motif or coiled-coil domains.
- Switch I regions
-
Flexible loops present in RHO GTPases that contain residues that coordinate Mg2+ and allow binding to GTP or GDP.
- Necrosis
-
A form of lytic cell death that frequently results from toxic injury, hypoxia or stress, which is in contrast to genetically controlled programmed cell death pathways such as apoptosis. This form of cell death is usually pro-inflammatory.
- CARD9-, BCL-10- and MALT1-containing complex
-
(CBM complex). A complex containing caspase activation and recruitment domain 9 (CARD9), B cell lymphoma 10 (BCL-10) and MALT lymphoma-associated translocation protein 1 (MALT1) that triggers nuclear factor-κB signalling downstream of immunoreceptor tyrosine-based activation motif (ITAM)-containing receptors in myeloid cells.
- Frustrated phagocytosis
-
The phenomenon of incomplete or stalled phagocytosis: that is, when a phagocyte is unable to completely engulf and internalize a target particle.
- Filopodia
-
Actin-rich, slender projections at the leading edge of motile cells with roles in sensing, migration and cell–cell interactions.
- Lamellipodia
-
Actin-rich projections at the leading edge of motile cells that coexist with filopodia and are essential for cellular movement along a substratum.
- Diffusion barrier
-
A mechanism to compartmentalize cellular membranes into separate domains; diffusion barriers can be found in continuous membranes, such as the plasma and endoplasmic reticulum membranes, and restrict the diffusion of membrane-associated proteins.
- Rapamycin
-
A pharmacological inhibitor of mammalian target of rapamycin (mTOR), which is a central regulator of cell metabolism, growth, proliferation and survival. It is used to induce autophagy or when used at high doses, it can suppress the immune system.
- Leading edge
-
Region of intense F-actin accumulation at the front end of a migrating cell.
Rights and permissions
About this article
Cite this article
Mostowy, S., Shenoy, A. The cytoskeleton in cell-autonomous immunity: structural determinants of host defence. Nat Rev Immunol 15, 559–573 (2015). https://doi.org/10.1038/nri3877
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3877
This article is cited by
-
Immunity to Cryptosporidium: insights into principles of enteric responses to infection
Nature Reviews Immunology (2024)
-
The role of antifreeze genes in the tolerance of cold stress in the Nile tilapia (Oreochromis niloticus)
BMC Genomics (2023)
-
Investigating mechanisms underlying genetic resistance to Salmon Rickettsial Syndrome in Atlantic salmon using RNA sequencing
BMC Genomics (2021)
-
Rescue of maternal immune activation-induced behavioral abnormalities in adult mouse offspring by pathogen-activated maternal Treg cells
Nature Neuroscience (2021)
-
Molecular mechanisms of phenotypic variability in monogenic autoinflammatory diseases
Nature Reviews Rheumatology (2021)