Key Points
-
The oxysterols 25-hydroxycholesterol (25-HC) and 7α,25-dihydroxycholesterol (7α,25-HC) are made by a range of cell types in lymphoid and non-lymphoid tissues, and their production is upregulated by inflammatory stimuli.
-
The ability of 25-HC to regulate the synthesis and compartmentalization of lipids enables it to antagonize the replication of a wide diversity of viruses.
-
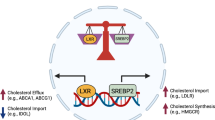
25-HC functions downstream of interferon (IFN) signalling to suppress interleukin-1β (IL-1β) expression and inflammasome activity. This mechanism of IFN-mediated feedback regulation of inflammation occurs at least in part through 25-HC-mediated suppression of sterol response element-binding protein (SREBP) processing.
-
25-HC augments the expression of some inflammatory cytokines, including IL-6. The mechanisms by which the anti-inflammatory and pro-inflammatory actions of 25-HC might be coordinated are discussed.
-
7α,25-HC, the most potent ligand of the G protein-coupled receptor EBI2 (also known as GPR183), guides the migration of B cells and dendritic cells to interfollicular regions of lymphoid tissues. EBI2 and 7α,25-HC deficiency both cause defective antibody responses.
-
A model is discussed in which 25-HC and 7α,25-HC produced in overlapping zones might function in a coordinated manner to recruit EBI2+ cells, promote their resistance to viral infection and control their cytokine production.
Abstract
Cholesterol and components of the cholesterol biosynthetic pathway have fundamental roles in all mammalian cells. Hydroxylated forms of cholesterol are now emerging as important regulators of immune function. This involves effects on the cholesterol biosynthetic pathway and cell membrane properties, which can have antiviral and anti-inflammatory influences. In addition, a dihydroxylated form of cholesterol functions as an immune cell guidance cue by engaging the G protein-coupled receptor EBI2, and it is required for mounting adaptive immune responses. In this Review, we summarize the current understanding of the closely related oxysterols 25-hydroxycholesterol and 7α,25-dihydroxycholesterol, and the growing evidence that they have wide-ranging influences on innate and adaptive immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nature Rev. Mol. Cell. Biol. 9, 125–138 (2008).
Simons, K. & Gerl, M. J. Revitalizing membrane rafts: new tools and insights. Nature Rev. Mol. Cell. Biol. 11, 688–699 (2010).
Heaton, N. S. & Randall, G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 19, 368–375 (2011).
Chukkapalli, V., Heaton, N. S. & Randall, G. Lipids at the interface of virus-host interactions. Curr. Opin. Microbiol. 15, 512–518 (2012).
Goldstein, J. L., DeBose-Boyd, R. A. & Brown, M. S. Protein sensors for membrane sterols. Cell 124, 35–46 (2006). This is a concise review on the pioneering work that defined the molecular mechanism of cholesterol-mediated and 25-HC-mediated feedback regulation of the sterol biosynthetic pathway.
Russell, D. W. Oxysterol biosynthetic enzymes. Biochim. Biophys. Acta 1529, 126–135 (2000).
Russell, D. W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72, 137–174 (2003). This authoritative review describes the enzyme requirements for the synthesis of bile acids and oxysterols in the liver and other tissues.
Brown, M. S. & Goldstein, J. L. Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J. Biol. Chem. 249, 7306–7314 (1974).
Kandutsch, A. A. & Chen, H. W. Inhibition of sterol synthesis in cultured mouse cells by cholesterol derivatives oxygenated in the side chain. J. Biol. Chem. 249, 6057–6061 (1974).
Bjorkhem, I. Are side-chain oxidized oxysterols regulators also in vivo? J. Lipid Res. 50, S213–S218 (2009).
Diczfalusy, U. On the formation and possible biological role of 25-hydroxycholesterol. Biochimie 95, 455–460 (2013).
Diczfalusy, U. et al. Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J. Lipid Res. 50, 2258–2264 (2009).
Bauman, D. R. et al. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc. Natl Acad. Sci. USA 106, 16764–16769 (2009). This paper is an early demonstration that 25-HC can affect the adaptive immune system with the discovery of increased IgA levels in CH25H-deficient mice and decreased IgA levels in CYP7B1-deficient mice.
Park, K. & Scott, A. L. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J. Leukoc. Biol. 88, 1081–1087 (2010).
Zou, T., Garifulin, O., Berland, R. & Boyartchuk, V. L. Listeria monocytogenes infection induces prosurvival metabolic signaling in macrophages. Infect. Immun. 79, 1526–1535 (2011). This study shows that L. monocytogenes infection and type I IFN treatment of macrophages upregulate Ch25h and this leads to the repression of caspase 1 activation.
Blanc, M. et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 38, 106–118 (2013). This study shows that 25-HC is strongly induced following viral infection and by IFN, and has a broad ability to antagonize the replication of enveloped viruses in vitro.
Liu, S. Y. et al. Interferon-inducible cholesterol-25- hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 38, 92–105 (2013). This study is complementary to reference 16 and shows that CH25H and 25-HC inhibit the replication of a wide range of enveloped viruses in vitro , as well as demonstrating the in vivo effects of 25-HC and CH25H deficiency on viral replication.
Gatto, D., Paus, D., Basten, A., Mackay, C. R. & Brink, R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity 31, 259–269 (2009).
Pereira, J. P., Kelly, L. M., Xu, Y. & Cyster, J. G. EBI2 mediates B cell segregation between the outer and centre follicle. Nature 460, 1122–1126 (2009). References 18 and 19 show, using independently generated EBI2-deficient mice, an in vivo function for EBI2 in guiding immune cell migration.
Hannedouche, S. et al. Oxysterols direct immune cell migration via EBI2. Nature 475, 524–527 (2011).
Liu, C. et al. Oxysterols direct B-cell migration through EBI2. Nature 475, 519–523 (2011). References 20 and 21 both use classical biochemical methods to de-orphanize EBI2 and identify 7α,25-HC as the most potent ligand.
Gold, E. S. et al. ATF3 protects against atherosclerosis by suppressing 25-hydroxycholesterol-induced lipid body formation. J. Exp. Med. 209, 807–817 (2012). This study shows that Ch25h is a major target gene repressed by ATF3 and that increased levels of 25-HC in the absence of ATF3 are associated with macrophage foam cell formation.
Gold, E. S. et al. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc. Natl Acad. Sci. USA 111, 10666–10671 (2014). This study shows that 25-HC can augment the expression of some inflammatory genes in macrophages and correlates these findings with reduced inflammation-mediated pathology following influenza virus infection in CH25H-deficient mice.
Koarai, A. et al. 25-Hydroxycholesterol enhances cytokine release and Toll-like receptor 3 response in airway epithelial cells. Respir. Res. 13, 63 (2012).
Reboldi, A. et al. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science 345, 679–684 (2014). This study shows that CH25H deficiency leads to the increased production of IL-1 family cytokines following inflammatory insults in vivo , and demonstrates that 25-HC represses Il1b expression and inflammasome activation in activated macrophages.
Gatto, D. et al. The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nature Immunol. 14, 446–453 (2013).
Yi, T. & Cyster, J. G. EBI2-mediated bridging channel positioning supports splenic dendritic cell homeostasis and particulate antigen capture. Elife 2, e00757 (2013). References 26 and 27 establish that CD4+ DCs in the spleen require EBI2 and 7α,25-HC for positioning in marginal zone bridging channels, and show that this positioning is important for maintaining their homeostasis and for mounting T cell-dependent antibody responses against certain blood-borne antigens.
Lund, E. G., Kerr, T. A., Sakai, J., Li, W. P. & Russell, D. W. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. J. Biol. Chem. 273, 34316–34327 (1998).
Stiles, A. R., McDonald, J. G., Bauman, D. R. & Russell, D. W. CYP7B1: one cytochrome P450, two human genetic diseases, and multiple physiological functions. J. Biol. Chem. 284, 28485–28489 (2009).
Schwarz, M. et al. The bile acid synthetic gene 3β-hydroxy-δ5-C27-steroid oxidoreductase is mutated in progressive intrahepatic cholestasis. J. Clin. Invest. 106, 1175–1184 (2000).
Yi, T. et al. Oxysterol gradient generation by lymphoid stromal cells guides activated B cell movement during humoral responses. Immunity 37, 535–548 (2012). This study demonstrates that CH25H and CYP7B1 are abundant in interfollicular stromal cells and low in the central region of follicles. It also shows that HSD3B7 is needed to inactivate 7α,25-HC as an EBI2 ligand and thereby establish 7α,25-HC 'gradients' that guide cell movement.
Brown, M. S. & Goldstein, J. L. Cholesterol feedback: from Schoenheimer's bottle to Scap's MELADL. J. Lipid Res. 50, S15–S27 (2009).
Spann, N. J. & Glass, C. K. Sterols and oxysterols in immune cell function. Nature Immunol. 14, 893–900 (2013).
Sever, N., Yang, T., Brown, M. S., Goldstein, J. L. & DeBose-Boyd, R. A. Accelerated degradation of HMG CoA reductase mediated by binding of insig-1 to its sterol-sensing domain. Mol. Cell 11, 25–33 (2003).
Mackenzie, J. M., Khromykh, A. A. & Parton, R. G. Cholesterol manipulation by West Nile virus perturbs the cellular immune response. Cell Host Microbe 2, 229–239 (2007).
Rothwell, C. et al. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology 389, 8–19 (2009).
Robinzon, S. et al. Impaired cholesterol biosynthesis in a neuronal cell line persistently infected with measles virus. J. Virol. 83, 5495–5504 (2009).
Petersen, J. et al. The major cellular sterol regulatory pathway is required for Andes virus infection. PLoS Pathog. 10, e1003911 (2014).
Lu, Y. E., Cassese, T. & Kielian, M. The cholesterol requirement for sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J. Virol. 73, 4272–4278 (1999).
Danthi, P. & Chow, M. Cholesterol removal by methyl-β-cyclodextrin inhibits poliovirus entry. J. Virol. 78, 33–41 (2004).
Daya, M., Cervin, M. & Anderson, R. Cholesterol enhances mouse hepatitis virus-mediated cell fusion. Virology 163, 276–283 (1988).
Phalen, T. & Kielian, M. Cholesterol is required for infection by Semliki Forest virus. J. Cell Biol. 112, 615–623 (1991).
Blanc, M. et al. Host defense against viral infection involves interferon mediated down-regulation of sterol biosynthesis. PLoS Biol. 9, e1000598 (2011). This study demonstrates that several viral infections and type I IFNs repress SREBP2 cleavage and sterol biosynthesis in macrophages and fibroblasts.
Shibata, N. et al. 25-Hydroxycholesterol activates the integrated stress response to reprogram transcription and translation in macrophages. J. Biol. Chem. 288, 35812–35823 (2013).
Liu, S. Y., Sanchez, D. J., Aliyari, R., Lu, S. & Cheng, G. Systematic identification of type I and type II interferon-induced antiviral factors. Proc. Natl Acad. Sci. USA 109, 4239–4244 (2012). This study identifies CH25H as a major ISG that has antiviral activity.
Moog, C., Aubertin, A. M., Kirn, A. & Luu, B. Oxysterols, but not cholesterol, inhibit human immunodeficiency virus replication in vitro. Antivir. Chem. Chemother. 9, 491–496 (1998).
Su, A. I. et al. Genomic analysis of the host response to hepatitis C virus infection. Proc. Natl Acad. Sci. USA 99, 15669–15674 (2002).
Arita, M. et al. Oxysterol-binding protein family I is the target of minor enviroxime-like compounds. J. Virol. 87, 4252–4260 (2013).
Gale, S. E. et al. Side chain oxygenated cholesterol regulates cellular cholesterol homeostasis through direct sterol-membrane interactions. J. Biol. Chem. 284, 1755–1764 (2009).
Olsen, B. N., Schlesinger, P. H., Ory, D. S. & Baker, N. A. 25-Hydroxycholesterol increases the availability of cholesterol in phospholipid membranes. Biophys. J. 100, 948–956 (2011).
Chang, T. Y., Chang, C. C., Ohgami, N. & Yamauchi, Y. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 22, 129–157 (2006).
Arita, M. Phosphatidylinositol-4 kinase III β and oxysterol-binding protein accumulate unesterified cholesterol on poliovirus-induced membrane structure. Microbiol. Immunol. 58, 239–256 (2014).
Mesmin, B. et al. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell 155, 830–843 (2013).
Amini- Bavil-Olyaee, S. et al. The antiviral effector IFITM3 disrupts intracellular cholesterol homeostasis to block viral entry. Cell Host Microbe 13, 452–464 (2013).
Trinchieri, G. Type I interferon: friend or foe? J. Exp. Med. 207, 2053–2063 (2010).
Gonzalez-Navajas, J. M., Lee, J., David, M. & Raz, E. Immunomodulatory functions of type I interferons. Nature Rev. Immunol. 12, 125–135 (2012).
Ludigs, K., Parfenov, V., Du Pasquier, R. A. & Guarda, G. Type I IFN-mediated regulation of IL-1 production in inflammatory disorders. Cell. Mol. Life Sci. 69, 3395–3418 (2012).
Inoue, M. & Shinohara, M. L. The role of interferon-β in the treatment of multiple sclerosis and experimental autoimmune encephalomyelitis — in the perspective of inflammasomes. Immunology 139, 11–18 (2013).
Guarda, G. et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011). This is an elegant study demonstrating that type I IFN suppresses inflammasome activation by an undefined mechanism.
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Franchi, L., Munoz-Planillo, R. & Nunez, G. Sensing and reacting to microbes through the inflammasomes. Nature Immunol. 13, 325–332 (2012).
Kuijk, L. M. et al. HMG-CoA reductase inhibition induces IL-1β release through Rac1/PI3K/PKB-dependent caspase-1 activation. Blood 112, 3563–3573 (2008).
Liao, Y. H. et al. HMG-CoA reductase inhibitors activate caspase-1 in human monocytes depending on ATP release and P2X7 activation. J. Leukoc. Biol. 93, 289–299 (2013).
Mayer-Barber, K. D. et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 511, 99–103 (2014).
Berry, M. P. et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977 (2010).
Masters, S. L., Simon, A., Aksentijevich, I. & Kastner, D. L. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*). Annu. Rev. Immunol. 27, 621–668 (2009).
Mandey, S. H., Kuijk, L. M., Frenkel, J. & Waterham, H. R. A role for geranylgeranylation in interleukin-1β secretion. Arthritis Rheum. 54, 3690–3695 (2006).
Pontillo, A., Paoluzzi, E. & Crovella, S. The inhibition of mevalonate pathway induces upregulation of NALP3 expression: new insight in the pathogenesis of mevalonate kinase deficiency. Eur. J. Hum. Genet. 18, 844–847 (2010).
Kim, J. et al. Sufficient production of geranylgeraniol is required to maintain endotoxin tolerance in macrophages. J. Lipid Res. 54, 3430–3437 (2013).
Gibson, K. M. et al. 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured fibroblasts from patients with mevalonate kinase deficiency: differential response to lipid supplied by fetal bovine serum in tissue culture medium. J. Lipid Res. 31, 515–521 (1990).
Umetani, M. et al. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell. Metab. 20, 172–182 (2014).
Umetani, M. et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nature Med. 13, 1185–1192 (2007).
Lundberg, B. Chemical composition and physical state of lipid deposits in atherosclerosis. Atherosclerosis 56, 93–110 (1985).
Brown, A. J. & Jessup, W. Oxysterols and atherosclerosis. Atherosclerosis 142, 1–28 (1999).
Brown, M. S., Dana, S. E. & Goldstein, J. L. Cholesterol ester formation in cultured human fibroblasts. Stimulation by oxygenated sterols. J. Biol. Chem. 250, 4025–4027 (1975).
Miller, S. C. & Melnykovych, G. Regulation of cholesterol biosynthesis and esterification by 25-hydroxycholesterol in a macrophage-like cell line: uncoupling by progesterone. J. Lipid Res. 25, 991–999 (1984).
Cheng, D., Chang, C. C., Qu, X. & Chang, T. Y. Activation of acyl-coenzyme A:cholesterol acyltransferase by cholesterol or by oxysterol in a cell-free system. J. Biol. Chem. 270, 685–695 (1995).
Li-Hawkins, J., Lund, E. G., Turley, S. D. & Russell, D. W. Disruption of the oxysterol 7α-hydroxylase gene in mice. J. Biol. Chem. 275, 16536–16542 (2000).
Gilchrist, M. et al. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature 441, 173–178 (2006).
McDonald, J. G. & Russell, D. W. 25-Hydroxycholesterol: a new life in immunology. J. Leukoc. Biol. 88, 1071–1072 (2010).
Birkenbach, M., Josefsen, K., Yalmanchili, R., Lenoir, G. & Kieff, E. Epstein-Barr virus induced genes: first lymphocyte-specific G-protein coupled peptide receptors. J. Virol. 67, 2209–2220 (1993).
Rosenkilde, M. M. et al. Molecular pharmacological phenotyping of EBI2. An orphan seven-transmembrane receptor with constitutive activity. J. Biol. Chem. 281, 13199–13208 (2006).
Benned-Jensen, T. et al. Ligand modulation of the Epstein-Barr virus-induced seven-transmembrane receptor EBI2: identification of a potent and efficacious inverse agonist. J. Biol. Chem. 286, 29292–29302 (2011).
Benned-Jensen, T. et al. Small molecule antagonism of oxysterol-induced Epstein-Barr virus induced gene 2 (EBI2) activation. FEBS Open Bio. 3, 156–160 (2013).
Benned-Jensen, T. & Rosenkilde, M. M. Structural motifs of importance for the constitutive activity of the orphan 7TM receptor EBI2: analysis of receptor activation in the absence of an agonist. Mol. Pharmacol. 74, 1008–1021 (2008).
Benned-Jensen, T. et al. Molecular characterization of oxysterol binding to the Epstein-Barr virus-induced gene 2 (GPR183). J. Biol. Chem. 287, 35470–35483 (2012).
Zhang, L. et al. Identification of structural motifs critical for Epstein-Barr virus-induced molecule 2 function and homology modeling of the ligand docking site. Mol. Pharmacol. 82, 1094–1103 (2012).
Hanson, M. A. et al. Crystal structure of a lipid G protein-coupled receptor. Science 335, 851–855 (2012).
Gessier, F. et al. Identification and characterization of small molecule modulators of the Epstein-Barr virus-induced gene 2 (EBI2) receptor. J. Med. Chem. 57, 3358–3368 (2014).
Gatto, D. & Brink, R. B cell localization: regulation by EBI2 and its oxysterol ligand. Trends Immunol. 34, 336–341 (2013). This is an excellent review of EBI2 biology in B cells.
Kelly, L. M., Pereira, J. P., Yi, T., Xu, Y. & Cyster, J. G. EBI2 guides serial movements of activated B cells and ligand activity is detectable in lymphoid and nonlymphoid tissues. J. Immunol. 187, 3026–3032 (2011). This study establishes the time course of EBI2 influence on B cell behaviour in vivo and uses a sensitive bioassay to show that an EBI2 ligand is widely distributed in tissues.
Gatto, D., Wood, K. & Brink, R. EBI2 operates independently of but in cooperation with CXCR5 and CCR7 to direct B cell migration and organization in follicles and the germinal center. J. Immunol. 187, 4621–4628 (2011). By removing the influence of two major chemokines, this report reveals additional influences of EBI2 in B cell positioning in the spleen.
Chan, T. D. et al. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J. Immunol. 183, 3139–3149 (2009).
MacLennan, I. & Vinuesa, C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity 17, 235–238 (2002).
Xu, W. & Banchereau, J. The antigen presenting cells instruct plasma cell differentiation. Front. Immunol. 4, 504 (2014).
Mueller, S. N. & Germain, R. N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nature Rev. Immunol. 9, 618–629 (2009).
Coffey, F., Alabyev, B. & Manser, T. Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles. Immunity 30, 599–609 (2009).
Cyster, J. G. B cell follicles and antigen encounters of the third kind. Nature Immunol. 11, 989–996 (2010).
Shaffer, A. L. et al. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 13, 199–212 (2000).
Turqueti-Neves, A. et al. B-cell-intrinsic STAT6 signaling controls germinal center formation. Eur. J. Immunol. 44, 2130–2138 (2014).
Kawamoto, S. et al. Foxp3+ T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 41, 152–165 (2014).
Coelho, F. M. et al. Naive B-cell trafficking is shaped by local chemokine availability and LFA-1-independent stromal interactions. Blood 121, 4101–4109 (2013).
Barroso, R. et al. EBI2 regulates CXCL13-mediated responses by heterodimerization with CXCR5. FASEB J. 26, 4841–4854 (2012).
Lund, R., Aittokallio, T., Nevalainen, O. & Lahesmaa, R. Identification of novel genes regulated by IL-12, IL-4, or TGF-β during the early polarization of CD4+ lymphocytes. J. Immunol. 171, 5328–5336 (2003).
Kroenke, M. A. et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J. Immunol. 188, 3734–3744 (2012).
Kraal, G. & Mebius, R. New insights into the cell biology of the marginal zone of the spleen. Int. Rev. Cytol. 250, 175–215 (2006).
Kabashima, K. et al. Intrinsic lymphotoxin-β receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity 22, 439–450 (2005).
Heinig, M. et al. A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature 467, 460–464 (2010).
Wallace, C. et al. Statistical colocalization of monocyte gene expression and genetic risk variants for type 1 diabetes. Hum. Mol. Genet. 21, 2815–2824 (2012).
Jostins, L. et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Chiang, E. Y., Johnston, R. J. & Grogan, J. EBI2 is a negative regulator of type I interferons in plasmacytoid and myeloid dendritic cells. PLoS ONE 8, e83457 (2013).
Nau, G. J. et al. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl Acad. Sci. USA 99, 1503–1508 (2002).
Preuss, I. et al. Transcriptional regulation and functional characterization of the oxysterol/EBI2 system in primary human macrophages. Biochem. Biophys. Res. Commun. 446, 663–668 (2014).
Cantor, R. M. et al. Systemic lupus erythematosus genome scan: support for linkage at 1q23, 2q33, 16q12–13, and 17q21–23 and novel evidence at 3p24, 10q23–24, 13q32, and 18q22–23. Arthritis Rheumatism 50, 3203–3210 (2004).
Moser, K. L. et al. Genome scan of human systemic lupus erythematosus: evidence for linkage on chromosome 1q in African-American pedigrees. Proc. Natl Acad. Sci. USA 95, 14869–14874 (1998).
Ye, S. et al. Protein interaction for an interferon-inducible systemic lupus associated gene, IFIT1. Rheumatology 42, 1155–1163 (2003).
Pascual, V., Chaussabel, D. & Banchereau, J. A genomic approach to human autoimmune diseases. Annu. Rev. Immunol. 28, 535–571 (2010).
Wilson, L. E., Widman, D., Dikman, S. H. & Gorevic, P. D. Autoimmune disease complicating antiviral therapy for hepatitis C virus infection. Semin. Arthritis Rheum. 32, 163–173 (2002).
Niedobitek, G. et al. Patterns of Epstein-Barr virus infection in non-neoplastic lymphoid tissue. Blood 79, 2520–2526 (1992).
Kurth, J. et al. EBV-infected B cells in infectious mononucleosis: viral strategies for spreading in the B cell compartment and establishing latency. Immunity 13, 485–495 (2000).
Hanlon, P., Avenell, A., Aucott, L. & Vickers, M. A. Systematic review and meta-analysis of the sero-epidemiological association between Epstein-Barr virus and systemic lupus erythematosus. Arthritis Res. Ther. 16, R3 (2014).
Lin, C. Y. & Morel, D. W. Esterification of oxysterols in human serum: effects on distribution and cellular uptake. J. Lipid Res. 37, 168–178 (1996).
Frederico, B., Chao, B., May, J. S., Belz, G. T. & Stevenson, P. G. A murid gamma-herpesviruses exploits normal splenic immune communication routes for systemic spread. Cell Host Microbe 15, 457–470 (2014).
Janowski, B. A., Willy, P. J., Devi, T. R., Falck, J. R. & Mangelsdorf, D. J. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 383, 728–731 (1996).
Bensinger, S. J. & Tontonoz, P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 454, 470–477 (2008).
Jin, L. et al. Structural basis for hydroxycholesterols as natural ligands of orphan nuclear receptor RORγ. Mol. Endocrinol. 24, 923–929 (2010).
Soroosh, P. et al. Oxysterols are agonist ligands of RORγt and drive Th1 cell differentiation. Proc. Natl Acad. Sci. USA 111, 12163–12168 (2014).
Huh, J. R. et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 472, 486–490 (2011).
Kidani, Y. et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nature Immunol. 14, 489–499 (2013).
Acknowledgements
The authors thank R. Locksley and D. Russell for comments on the manuscript. Work from the Cyster laboratory discussed in this Review was supported in part by the US National Institutes of Health (grant AI40098) and a grant from the American Asthma Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Isoprenoids
-
Hydrocarbons composed of two or more isoprene units; an isoprene is a five-carbon unit with the formula: CH2 = C(CH3)CH = CH2.
- Oxysterols
-
Derivatives of cholesterol with a hydroxyl group in addition to the 3β-hydroxyl group.
- Bile acids
-
Highly modified forms of cholesterol that are secreted into the intestine to promote the solubilization and uptake of dietary lipids, in addition to functioning as the only known mechanism to excrete cholesterol from the body.
- Sterol response element-binding proteins
-
(SREBPs). Transcription factors that begin as multi-transmembrane endoplasmic reticulum proteins and are cleaved in the Golgi to release the basic helix–loop–helix leucine zipper transcription factor domain that binds to sterol regulatory elements in DNA.
- 3-hydroxy-3-methylglutaryl-CoA reductase
-
(HMGCR). Also known as HMG-CoA reductase. The rate-controlling enzyme of the mevalonate pathway, which is the metabolic pathway that produces isoprenoids and cholesterol.
- SREBP cleavage-activating protein
-
(SCAP). A multi-transmembrane endoplasmic reticulum protein that contains a sterol-sensing domain and that binds to sterol response element-binding proteins (SREBPs) and escorts them to the Golgi when cholesterol or 25-hydroxycholesterol levels are low.
- IFN-stimulated genes
-
(ISGs). Genes that are directly induced by signalling from the type I interferon (IFN) receptor.
- Prenylation
-
A post-translational modification that involves the attachment of an isoprenoid (farnesyl or geranylgeranyl) lipid to a protein.
- Integrated stress response
-
A cellular stress response pathway initiated by one of the four eukaryotic translation initiation factor 2α kinases.
- Oxysterol-binding protein
-
(OSBP). An intracellular protein named because of its ability to bind 25-hydroxycholesterol as well as cholesterol. It functions in the exchange of cholesterol and phosphatidylinositol 4-phosphate between the endoplasmic reticulum and the Golgi.
- Inflammasome
-
A large multiprotein complex consisting of ASC, caspase 1 and at least one activator, such as NOD-, LRR- and pyrin domain-containing 3 (NLRP3), NOD-, LRR- and CARD-containing 4 (NLRC4) or absent in melanoma 2 (AIM2).
- Statins
-
Cholesterol-lowering drugs that function by inhibiting 3-hydroxy-3-methylglutaryl-CoA reductase.
- Experimental autoimmune encephalomyelitis
-
An inflammatory disease of the central nervous system that models human multiple sclerosis and is induced by immunizing with a myelin autoantigen in a strong adjuvant.
- Marginal zone bridging channels
-
Specialized interfollicular regions that connect the T cell zone and red pulp in the spleen.
- Retinoic acid receptor-related orphan receptor-γt
-
(RORγt). A nuclear hormone receptor that is important for the development of interleukin-17-producing T cells, for thymocyte maturation and for the development of lymphoid tissue inducer cells.
Rights and permissions
About this article
Cite this article
Cyster, J., Dang, E., Reboldi, A. et al. 25-Hydroxycholesterols in innate and adaptive immunity. Nat Rev Immunol 14, 731–743 (2014). https://doi.org/10.1038/nri3755
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3755
This article is cited by
-
Single-molecule localization microscopy reveals STING clustering at the trans-Golgi network through palmitoylation-dependent accumulation of cholesterol
Nature Communications (2024)
-
The mechanism of 25-hydroxycholesterol-mediated suppression of atrial β1-adrenergic responses
Pflügers Archiv - European Journal of Physiology (2024)
-
Outsmarting trogocytosis to boost CAR NK/T cell therapy
Molecular Cancer (2023)
-
Molecular basis for the recognition of 24-(S)-hydroxycholesterol by integrin αvβ3
Scientific Reports (2023)
-
An epithelial cell-derived metabolite tunes immunoglobulin A secretion by gut-resident plasma cells
Nature Immunology (2023)