Key Points
-
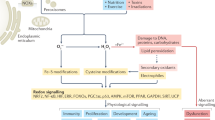
Reactive oxygen species (ROS) include superoxide, hydrogen peroxide, singlet oxygen, ozone, hypohalous acids and organic peroxides. They interact with and share some of the actions of other classes of small, reactive, endogenous signalling molecules — reactive nitrogen species such as NO• and NO2•; H2S or its anion, HS−; and carbon monoxide.
-
ROS can both promote and prevent cell death, cancer, ageing and inflammation. For example, ROS mediate inflammasome activation, but patients with chronic granulomatous disease, who lack a functional form of a principal ROS-producing enzyme, NADPH oxidase 2 (NOX2), demonstrate considerable susceptibility to infection, as well as non-resolving inflammation.
-
The numerous enzymatic sources of ROS include mitochondria and multiple isoforms of NOXs. The first NOX, now called NOX2, was discovered in neutrophils, but NOXs contribute to signal transduction in diverse cell types. ROS are produced following B and T cell receptor stimulation and can dictate whether T cell activation is fostered or impeded.
-
Many antioxidant systems contribute to the regulation of ROS, including superoxide dismutases, catalases and the enzymes of the glutathione redox cycle, which reflects the widespread functional effects of ROS.
-
Reactions involving ROS demonstrate atomic rather than molecular specificity. That is, ROS preferentially react with certain types of atoms and most readily with a subset of those atoms, but the atomic targets of ROS are distributed in many different macromolecules. For example, ROS preferentially react with the sulphur atom in some but not other cysteine residues; the cysteine thiols that are most susceptible include many that participate in enzyme active sites, such as in phosphatases. This kind of specificity equips ROS to influence many different signalling pathways simultaneously.
-
The immunosuppressive capacity of myeloid-derived suppressor cells and regulatory T cells results in part from their production of ROS. Tumour cells also produce ROS, which can contribute to their immunosuppressive and metastatic potential.
Abstract
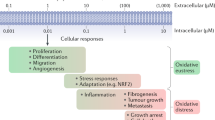
Reactive oxygen species (ROS) react preferentially with certain atoms to modulate functions ranging from cell homeostasis to cell death. Molecular actions include both inhibition and activation of proteins, mutagenesis of DNA and activation of gene transcription. Cellular actions include promotion or suppression of inflammation, immunity and carcinogenesis. ROS help the host to compete against microorganisms and are also involved in intermicrobial competition. ROS chemistry and their pleiotropy make them difficult to localize, to quantify and to manipulate — challenges we must overcome to translate ROS biology into medical advances.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nathan, C. & Ding, A. Snapshot: reactive oxygen intermediates (ROI). Cell 140, 951–951.e2 (2010).
Nishida, M. et al. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nature Chem. Biol. 8, 714–724 (2012).
Finkel, T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci. Signal. 5, pe10 (2012).
Paul, B. D. & Snyder, S. H. H2S signalling through protein sulfhydration and beyond. Nature Rev. Mol. Cell. Biol. 13, 499–507 (2012).
Wink, D. A. et al. Nitric oxide and redox mechanisms in the immune response. J. Leuk. Biol. 89, 873–891 (2011).
Steinhubl, S. R. Why have antioxidants failed in clinical trials? Am. J. Cardiol. 101, 14D–19D (2008).
Brennan, M. L. & Hazen, S. L. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr. Opin. Lipidol. 14, 353–359 (2003).
Bae, Y. S., Oh, H., Rhee, S. G. & Yoo, Y. D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 32, 491–509 (2011).
Finkel, T. Signal transduction by mitochondrial oxidants. J. Biol. Chem. 287, 4434–4440 (2012).
Jiang, F., Zhang, Y. & Dusting, G. J. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol. Rev. 63, 218–242 (2011).
Lambeth, J. D. NOX enzymes and the biology of reactive oxygen. Nature Rev. Immunol. 4, 181–189 (2004).
Aguirre, J. & Lambeth, J. D. Nox enzymes from fungus to fly to fish and what they tell us about Nox function in mammals. Free Radic. Biol. Med. 49, 1342–1353 (2010).
Imlay, J. A. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77, 755–776 (2008).
Yazdanpanah, B. et al. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature 460, 1159–1163 (2009).
Mailloux, R. J. & Harper, M. E. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 51, 1106–1115 (2011).
Corzo, C. A. et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182, 5693–5701 (2009).
Radi, R., Beckman, J. S., Bush, K. M. & Freeman, B. A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 266, 4244–4250 (1991).
Gonzalez-Nieto, D. et al. Connexin-43 in the osteogenic BM niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors. Blood 119, 5144–5154 (2012).
Holmgren, A. & Lu, J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem. Biophys. Res. Commun. 396, 120–124 (2010).
Weissbach, H. et al. Peptide methionine sulfoxide reductase: structure, mechanism of action, and biological function. Arch. Biochem. Biophys. 397, 172–178 (2002).
Bryk, R., Griffin, P. & Nathan, C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407, 211–215 (2000).
Morgan, B. et al. Multiple glutathione disulfide removal pathways mediate cytosolic redox homeostasis. Nature Chem. Biol. 9, 119–125 (2012).
Anastasiou, D. et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278–1283 (2011).
O'Donnell-Tormey, J., Nathan, C. F., Lanks, K., DeBoer, C. J. & de la Harpe, J. Secretion of pyruvate. An antioxidant defense of mammalian cells. J. Exp. Med. 165, 500–514 (1987).
Bertini, R. et al. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J. Exp. Med. 189, 1783–1789 (1999).
Shichita, T. et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nature Med. 18, 911–917 (2012).
Seifert, U. et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 142, 613–624 (2010).
Scherz-Shouval, R. & Elazar, Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 36, 30–38 (2011).
Thorpe, G. W., Fong, C. S., Alic, N., Higgins, V. J. & Dawes, I. W. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Proc. Natl Acad. Sci. USA 101, 6564–6569 (2004).
Nathan, C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J. Clin. Invest. 111, 769–778 (2003).
Ferrer-Sueta, G. et al. Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chem. Res. Toxicol. 24, 434–450 (2011).
Winterbourn, C. C. & Hampton, M. B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 45, 549–561 (2008).
Kuiper, J. W., Sun, C., Magalhaes, M. A. & Glogauer, M. Rac regulates PtdInsP3 signaling and the chemotactic compass through a redox-mediated feedback loop. Blood 118, 6164–6171 (2011).
Paulsen, C. E. et al. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nature Chem. Biol. 8, 57–64 (2012).
Wani, R. et al. Isoform-specific regulation of Akt by PDGF-induced reactive oxygen species. Proc. Natl Acad. Sci. USA 108, 10550–10555 (2011).
Guo, Z., Kozlov, S., Lavin, M. F., Person, M. D. & Paull, T. T. ATM activation by oxidative stress. Science 330, 517–521 (2010).
Erickson, J. R. et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133, 462–474 (2008).
Burgoyne, J. R. et al. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 317, 1393–1397 (2007).
Kroncke, K. D. & Klotz, L. O. Zinc fingers as biologic redox switches? Antioxid. Redox Signal. 11, 1015–1027 (2009).
de Keizer, P. L., Burgering, B. M. & Dansen, T. B. Forkhead box O as a sensor, mediator, and regulator of redox signaling. Antioxid. Redox Signal. 14, 1093–1106 (2011).
Wang, Y., Yang, J. & Yi, J. Redox sensing by proteins: oxidative modifications on cysteines and the consequent events. Antioxid. Redox Signal. 16, 649–657 (2012).
Fu, X., Kassim, S. Y., Parks, W. C. & Heinecke, J. W. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J. Biol. Chem. 276, 41279–41287 (2001).
Taggart, C. et al. Oxidation of either methionine 351 or methionine 358 in α1-antitrypsin causes loss of anti-neutrophil elastase activity. J. Biol. Chem. 275, 27258–27265 (2000).
Reddy, V. Y. et al. Oxidative dissociation of human α2-macroglobulin tetramers into dysfunctional dimers. J. Biol. Chem. 269, 4683–4691 (1994).
Carp, H. & Janoff, A. Inactivation of bronchial mucous proteinase inhibitor by cigarette smoke and phagocyte-derived oxidants. Exp. Lung Res. 1, 225–237 (1980).
Lando, D., Peet, D. J., Whelan, D. A., Gorman, J. J. & Whitelaw, M. L. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295, 858–861 (2002).
Doucette, C. D. Schwab, D.J., Wingreen, N. S. & Rabinowitz, J. D. α-Ketoglutarate coordinates carbon and nitrogen utilization via enzyme I inhibition. Nature Chem. Biol. 7, 894–901 (2011).
Leichert, L. I. & Jakob, U. Protein thiol modifications visualized in vivo. PLoS Biol. 2, e333 (2004).
White, A. A., Crawford, K. M., Patt, C. S. & Lad, P. J. Activation of soluble guanylate cyclase from rat lung by incubation or by hydrogen peroxide. J. Biol. Chem. 251, 7304–7312 (1976).
Feng, W., Liu, G., Allen, P. D. & Pessah, I. N. Transmembrane redox sensor of ryanodine receptor complex. J. Biol. Chem. 275, 35902–35907 (2000).
Karisch, R. et al. Global proteomic assessment of the classical protein-tyrosine phosphatome and “Redoxome”. Cell 146, 826–840 (2011).
Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7–15 (2011).
Cosentino, C., Grieco, D. & Costanzo, V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 30, 546–555 (2011).
Ito, K. et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature Med. 12, 446–451 (2006).
Okuno, Y., Nakamura-Ishizu, A., Otsu, K., Suda, T. & Kubota, Y. Pathological neoangiogenesis depends on oxidative stress regulation by ATM. Nature Med. 18, 1208–1216 (2012).
Storz, G., Tartaglia, L. A. & Ames, B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science 248, 189–194 (1990).
Brunelle, J. K. et al. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell. Metab. 1, 409–414 (2005).
Guzy, R. D. et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell. Metab. 1, 401–408 (2005).
Mansfield, K. D. et al. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-α activation. Cell. Metab. 1, 393–399 (2005).
Ruchko, M. V. et al. Hypoxia-induced oxidative base modifications in the VEGF hypoxia-response element are associated with transcriptionally active nucleosomes. Free Radic. Biol. Med. 46, 352–359 (2009).
Al-Mehdi, A. B. et al. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal. 5, ra47 (2012).
Ju, B. G. et al. A topoisomerase IIβ-mediated dsDNA break required for regulated transcription. Science 312, 1798–1802 (2006).
Perillo, B. et al. DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science 319, 202–206 (2008).
Amente, S., Lania, L., Avvedimento, E. V. & Majello, B. DNA oxidation drives Myc mediated transcription. Cell Cycle 9, 3002–3004 (2010).
Niethammer, P., Grabher, C., Look, A. T. & Mitchison, T. J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459, 996–999 (2009).
Yoo, S. K., Starnes, T. W., Deng, Q. & Huttenlocher, A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 480, 109–112 (2011).
Sakai, D. et al. Remodeling of actin cytoskeleton in mouse periosteal cells under mechanical loading induces periosteal cell proliferation during bone formation. PLoS ONE 6, e24847 (2011).
Hattori, H. et al. Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc. Natl Acad. Sci. USA 107, 3546–3551 (2010).
Henderson, W. R. & Klebanoff, S. J. Leukotriene production and inactivation by normal, chronic granulomatous disease and myeloperoxidase-deficient neutrophils. J. Biol. Chem. 258, 13522–13527 (1983).
Segal, B. H., Kuhns, D. B., Ding, L., Gallin, J. I. & Holland, S. M. Thioglycollate peritonitis in mice lacking C5, 5-lipoxygenase, or p47phox: complement, leukotrienes, and reactive oxidants in acute inflammation. J. Leukoc. Biol. 71, 410–416 (2002).
Martinon, F., Chen, X., Lee, A. H. & Glimcher, L. H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nature Immunol. 11, 411–418 (2010).
West, A. P. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 (2011).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Hogquist, K. A., Nett, M. A., Unanue, E. R. & Chaplin, D. D. Interleukin 1 is processed and released during apoptosis. Proc. Natl Acad. Sci. USA 88, 8485–8489 (1991).
Shiloh, M. U. et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10, 29–38 (1999).
Huang, J. et al. Activation of antibacterial autophagy by NADPH oxidases. Proc. Natl Acad. Sci. USA 106, 6226–6231 (2009).
Espey, M. G. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 55, 130–140 (2013).
Kumar, A. et al. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 26, 4457–4466 (2007).
Winter, S. E. et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467, 426–429 (2010).
Maly, F. E. et al. The superoxide generating system of B cell lines. Structural homology with the phagocytic oxidase and triggering via surface Ig. J. Immunol. 140, 2334–2339 (1988).
Maly, F. E. et al. Superoxide-dependent nitroblue tetrazolium reduction and expression of cytochrome b-245 components by human tonsillar B lymphocytes and B cell lines. J. Immunol. 142, 1260–1267 (1989).
Singh, D. K. et al. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell 121, 281–293 (2005).
Devadas, S., Zaritskaya, L., Rhee, S. G., Oberley, L. & Williams, M. S. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 195, 59–70 (2002).
Hara-Chikuma, M. et al. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 209 1743–1752 (2012).
Los, M. et al. IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J. 14, 3731–3740 (1995).
Savina, A. et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126, 205–218 (2006).
Mishell, R. I. & Dutton, R. W. Immunization of normal mouse spleen cell suspensions in vitro. Science 153, 1004–1006 (1966).
Nathan, C. F. & Terry, W. D. Differential stimulation of murine lymphoma growth in vitro by normal and BCG-activated macrophages. J. Exp. Med. 142, 887–902 (1975).
Angelini, G. et al. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc. Natl Acad. Sci. USA 99, 1491–1496 (2002).
Sido, B. et al. A prominent role for mucosal cystine/cysteine metabolism in intestinal immunoregulation. Gastroenterology 134, 179–191 (2008).
Yan, Z., Garg, S. K., Kipnis, J. & Banerjee, R. Extracellular redox modulation by regulatory T cells. Nature Chem. Biol. 5, 721–723 (2009).
Fisher, R. I. & Bostick-Bruton, F. Depressed T cell proliferative responses in Hodgkin's disease: role of monocyte-mediated suppression via prostaglandins and hydrogen peroxide. J. Immunol. 129, 1770–1774 (1982).
Efimova, O., Szankasi, P. & Kelley, T. W. Ncf1 (p47phox) is essential for direct regulatory T cell mediated suppression of CD4+ effector T cells. PLoS ONE 6, e16013 (2011).
Gelderman, K. A., Hultqvist, M., Holmberg, J., Olofsson, P. & Holmdahl, R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc. Natl Acad. Sci. USA 103, 12831–12836 (2006).
Mougiakakos, D., Johansson, C. C., Jitschin, R., Bottcher, M. & Kiessling, R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood 117, 857–861 (2011).
Colombo, M. P. & Piconese, S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nature Rev. Cancer 7, 880–887 (2007).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Rev. Immunol. 9, 162–174 (2009).
Muhlebach, T. J. et al. Treatment of patients with chronic granulomatous disease with recombinant human interferon-gamma does not improve neutrophil oxidative metabolism, cytochrome b558 content or levels of four anti-microbial proteins. Clin. Exp. Immunol. 88, 203–206 (1992).
Nagaraj, S. et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nature Med. 13, 828–835 (2007).
Kusmartsev, S. & Gabrilovich, D. I. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J. Leuk. Biol. 74, 186–196 (2003).
Molon, B. et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 208, 1949–1962 (2011).
Nathan, C. & Cohn, Z. Role of oxygen-dependent mechanisms in antibody-induced lysis of tumor cells by activated macrophages. J. Exp. Med. 152, 198–208 (1980).
Nathan, C. F. & Klebanoff, S. J. Augmentation of spontaneous macrophage-mediated cytolysis by eosinophil peroxidase. J. Exp. Med. 155, 1291–1308 (1982).
Nathan, C. F., Silverstein, S. C., Brukner, L. H. & Cohn, Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J. Exp. Med. 149, 100–113 (1979).
Nathan, C. F., Arrick, B. A., Murray, H. W., DeSantis, N. M. & Cohn, Z. A. Tumor cell anti-oxidant defenses. Inhibition of the glutathione redox cycle enhances macrophage-mediated cytolysis. J. Exp. Med. 153, 766–782 (1981).
Nathan, C. F. & Cohn, Z. A. Antitumor effects of hydrogen peroxide in vivo. J. Exp. Med. 154, 1539–1553 (1981).
O'Donnell-Tormey, J., DeBoer, C. J. & Nathan, C. F. Resistance of human tumor cells in vitro to oxidative cytolysis. J. Clin. Invest. 76, 80–86 (1985).
Szatrowski, T. P. & Nathan, C. F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 51, 794–798 (1991).
Liou, G. Y. & Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 44, 479–496 (2010).
Ishikawa, K. et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320, 661–664 (2008).
Tonks, N. K. Redox redux: revisiting PTPs and the control of cell signaling. Cell 121, 667–670 (2005).
Ward, P. S. & Thompson, C. B. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21, 297–308 (2012).
Weitzman, S. A., Weitberg, A. B., Clark, E. P. & Stossel, T. P. Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science 227, 1231–1233 (1985).
Lonkar, P. & Dedon, P. C. Reactive species and DNA damage in chronic inflammation: reconciling chemical mechanisms and biological fates. Int. J. Cancer 128, 1999–2009 (2011).
Kohanski, M. A., DePristo, M. A. & Collins, J. J. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol. Cell 37, 311–320 (2010).
Ishimoto, T. et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc− and thereby promotes tumor growth. Cancer Cell 19, 387–400 (2011).
Gilbertson, R. J. & Rich, J. N. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nature Rev. Cancer 7, 733–736 (2007).
Diehn, M. et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458, 780–783 (2009).
Knoefler, D. et al. Quantitative in vivo redox sensors uncover oxidative stress as an early event in life. Mol. Cell 47, 767–776 (2012).
Gomes, A., Fernandes, E. & Lima, J. L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 65, 45–80 (2005).
Kim, J. H. et al. Single-molecule detection of H2O2 mediating angiogenic redox signaling on fluorescent single-walled carbon nanotube array. ACS Nano 5, 7848–7857 (2011).
Lee, D. et al. Detection of hydrogen peroxide with chemiluminescent micelles. Int. J. Nanomed. 3, 471–476 (2008).
Belousov, V. V. et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nature Methods 3, 281–286 (2006).
Gutscher, M. et al. Real-time imaging of the intracellular glutathione redox potential. Nature Methods 5, 553–559 (2008).
Raj, L. et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 475, 231–234 (2011).
Trachootham, D., Alexandre, J. & Huang, P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature Rev. Drug Discov. 8, 579–591 (2009).
Kohanski, M. A., Dwyer, D. J., Hayete, B., Lawrence, C. A. & Collins, J. J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130, 797–810 (2007).
Foti, J. J., Devadoss, B., Winkler, J. A., Collins, J. J. & Walker, G. C. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science 336, 315–319 (2012).
Nathan, C. Fresh approaches to anti-infective therapies. Sci. Transl. Med. 4, 140sr2 (2012).
Liby, K. T. & Sporn, M. B. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol. Rev. 64, 972–1003 (2012).
Pineda-Molina, E. et al. Glutathionylation of the p50 subunit of NF-κB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry 40, 14134–14142 (2001).
Warburg, O. Beobachtungen über die Oxydationsprozesse im Seeigelei. Z. Physiol. Chem. 57, 1–16 (1908).
Bentley, R. in The Enzymes 2nd edn Vol. 27 Ch. 24 (eds Boyer, P. D., Lardy, H. & Myrbäck, K.) 567–586 (Academic, 1963).
Wilson, R. & Turner, A. P. F. Glucose oxidase: an ideal enzyme. Biosensors & Bioelectronics 7, 165–185 (1992).
Sbarra, A. J. & Karnovsky, M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J. Biol. Chem. 234, 1355–1362 (1959).
Iyer, G. Y. N., Islam, M. F. & Quastel, J. H. Biochemical aspects of phagocytosis. Nature 192, 535–541 (1961).
McCord, J. M. & Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244, 6049–6055 (1969).
Babior, B. M., Kipnes, R. S. & Curnutte, J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 52, 741–744 (1973).
Curnutte, J. T., Whitten, D. M. & Babior, B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N. Engl. J. Med. 290, 593–597 (1974).
Nathan, C. F. & Root, R. K. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J. Exp. Med. 146, 1648–1662 (1977).
Foerder, C. A., Klebanoff, S. J. & Shapiro, B. M. Hydrogen peroxide production, chemiluminescence, and the respiratory burst of fertilization: interrelated events in early sea urchin development. Proc. Natl Acad. Sci. USA 75, 3183–3187 (1978).
Segal, A. W. & Jones, O. T. Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature 276, 515–517 (1978).
Klebanoff, S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann. Intern. Med. 93, 480–489 (1980).
Nathan, C. F., Murray, H. W., Wiebe, M. E. & Rubin, B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158, 670–689 (1983).
Nathan, C. F. et al. Local and systemic effects of intradermal recombinant interferon-γ in patients with lepromatous leprosy. N. Engl. J. Med. 315, 6–15 (1986).
Royer-Pokora, B. et al. Cloning the gene for an inherited human disorder — chronic granulomatous disease — on the basis of its chromosomal location. Nature 322, 32–38 (1986).
Ezekowitz, R. A., Dinauer, M. C., Jaffe, H. S., Orkin, S. H. & Newburger, P. E. Partial correction of the phagocyte defect in patients with X-linked chronic granulomatous disease by subcutaneous interferon gamma. N. Engl. J. Med. 319, 146–151 (1988).
Suh, Y. A. et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401, 79–82 (1999).
Grant, S. S., Kaufmann, B. B., Chand, N. S., Haseley, N. & Hung, D. T. Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proc. Natl Acad. Sci. USA 109, 12147–12152 (2012).
Doroshow, J. H. & Davies, K. J. Comparative cardiac oxygen radical metabolism by anthracycline antibiotics, mitoxantrone, bisantrene, 4′-(9-acridinylamino)-methanesulfon-m-anisidide, and neocarzinostatin. Biochem. Pharmacol. 32, 2935–2939 (1983).
Dorr, R.T. Bleomycin pharmacology: mechanism of action and resistance, and clinical pharmacokinetics. Semin. Oncol. 19, 3–8 (1992).
Liu, Y. & Imlay, J. A. Cell death from antibiotics without the involvement of reactive oxygen species. Science 339, 1210 (2013).
Keren, I., Wu, Y., Inocencio, U., Mulcahy, L. R. & Lewis, K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 339, 1213 (2013).
Acknowledgements
A.C.-B. is a member of the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Programme, which is supported by the Medical Scientist Training Program grant (GM07739) from the National Instiute of General Medical Sciences, USA. The Department of Microbiology and Immunology is supported by the William Randolph Hearst Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Iron–sulphur clusters
-
Prosthetic groups that are required for the function of some enzymes. In iron–sulphur clusters two, three or four atoms of iron are attached to the protein through two or four sulphydryl groups.
- Uncoupling proteins
-
Proteins in the mitochondrial inner membrane that can divert the proton gradient away from the formation of ATP, resulting in the generation of heat instead.
- Xenobiotics
-
Small chemical compounds that enter an organism unnaturally, such as drugs or pollutants.
- Acidic dissociation constant
-
(pKa). The equilibrium constant for the dissociation of an acid into its conjugate base and hydrogen ion, expressed as the negative logarithm. The lower the pKa of a sulphydryl group, the greater the likelihood that the sulphur will be anionic at ambient pH.
- Chronic granulomatous disease
-
(CGD). An immunodeficiency state manifested by recurrent, often life-threatening, infections and the excessive formation of granulomas, caused by mutations in any one of four subunits of NADP oxidase 2.
- Granulomas
-
Histological collections of macrophages, usually surrounded by lymphocytes and sometimes fibrocytes. Some of the macrophages might seem to be 'epithelioid' or fuse to become multinucleated giant cells. Granuloma formation is a chronic inflammatory response to various infectious and non-infectious agents.
- Neddylation
-
A process that is analogous to ubiquitylation, in which ubiquitin-like protein NEDD8 is conjugated to a protein substrate.
Rights and permissions
About this article
Cite this article
Nathan, C., Cunningham-Bussel, A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol 13, 349–361 (2013). https://doi.org/10.1038/nri3423
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3423
This article is cited by
-
HS-GC–MS analysis of volatile organic compounds after hyperoxia-induced oxidative stress: a validation study
Intensive Care Medicine Experimental (2024)
-
Molecular characterization and functional analysis of peroxiredoxin 1 (Prx1) from roughskin sculpin (Trachidermus fasciatus)
Fish Physiology and Biochemistry (2024)
-
Antimicrobial activity of metal-based nanoparticles: a mini-review
BioMetals (2024)
-
Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: a narrative review
Cellular & Molecular Biology Letters (2023)
-
Multifaceted role of redox pattern in the tumor immune microenvironment regarding autophagy and apoptosis
Molecular Cancer (2023)