Key Points
-
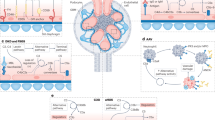
The complement system is primarily a host defence mechanism, but it causes injury to tissue cells through the formation of the membrane attack complex (C5b–C9), which leads to cell lysis and cell death, and through the stimulation of inflammation and antigen-specific immunity.
-
A central pool of circulating complement components (mainly produced by hepatic cells) is distinct from a peripheral pool of extracellular complement secreted by various tissue-resident cells and migratory leukocytes.
-
In most vital organs, ischaemia–reperfusion injury is mediated by complement components C5a and C5b–C9 and, as shown in a mouse kidney transplant model, this is strongly dependent on tissue-mediated production of C3.
-
The lectin pathway of complement activation is a common trigger of ischaemia–reperfusion injury in several organ models (initiated either by direct binding of the lectin to ischaemic tissue or via natural IgM), and the alternative pathway may significantly increase the deposition of complement at the site of activation.
-
Effective priming of CD4+ T cells that mediate graft rejection requires C3a and C5a, which are produced by dendritic cells and stimulate the presentation of alloantigens and the differentiation of naive CD4+ T helper (TH) cells to TH1 cells.
-
The reactivity of antigen-primed CD4+ and CD8+ T cells against donor cells is enhanced by the peripheral synthesis of complement and the generation of the effectors C3a, C3b and C5a.
-
Antibody-mediated rejection requires complement (specifically C3) not only for the efficient priming of B cells against alloantigens but also for the induction of inflammation and thrombosis (by C5a and C5b–C9) at the site of antibody binding in the graft.
-
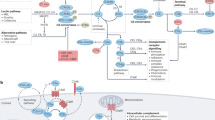
Emerging therapeutic approaches include targeting the donor organ with complement regulators to prevent stress-induced injury and sensitization of the recipient, and treatment of the recipient to prevent complement-mediated vascular injury during antibody-mediated rejection and the recurrence of haemolytic–uraemic syndrome.
-
Imaging ligands that can be used to detect and quantify tissue-bound C3 at the site of tissue injury, as well as biomarkers that predict or associate with complement-mediated damage to the transplant, are allowing the significance of these findings to be addressed in humans.
Abstract
The complement system is a key element of the innate immune system, and the production of complement components can be divided into central (hepatic) and peripheral compartments. Essential complement components such as C3 are produced in both of these compartments, but until recently the functional relevance of the peripheral synthesis of complement was unclear. Here, we review recent findings showing that local peripheral synthesis of complement in a transplanted organ is required for the immediate response of the donor organ to tissue stress and for priming alloreactive T cells that can mediate transplant rejection. We also discuss recent insights into the role of complement in antibody-mediated rejection, and we examine how new treatment strategies that take into account the separation of central and peripheral production of complement are expected to make a difference to transplant outcome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Farrar, C. A., Zhou, W., Lin, T. & Sacks, S. H. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. FASEB J. 20, 217–226 (2006). This paper demonstrated that local synthesis of C3 is essential for post-transplant reperfusion injury in a renal isograft model and that the contribution of this local production depends on the length of cold ischaemic time.
Pratt, J. R., Basheer, S. A. & Sacks, S. H. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nature Med. 8, 582–587 (2002). This study demonstrated that intra-organ complement synthesis can contribute to allograft rejection by enhancing anti-donor T cell responses.
Dorling, A. Transplant accommodation — are the lessons learned from xenotransplantation pertinent for clinical allotransplantation? Am. J. Transplant. 12, 545–553 (2012).
Ekser, B., Gridelli, B., Veroux, M. & Cooper, D. K. Clinical pig liver xenotransplantation: how far do we have to go? Xenotransplantation 18, 158–167 (2011).
Zhou, W., Medof, M. E., Heeger, P. S. & Sacks, S. Graft-derived complement as a mediator of transplant injury. Curr. Opin. Immunol. 19, 569–576 (2007).
Zhou, W. et al. Macrophages from C3-deficient mice have impaired potency to stimulate alloreactive T cells. Blood 107, 2461–2469 (2006).
Peng, Q. et al. Local production and activation of complement up-regulates the allostimulatory function of dendritic cells through C3a–C3aR interaction. Blood 111, 2452–2461 (2008).
Peng, Q. et al. Dendritic cell function in allostimulation is modulated by C5aR signaling. J. Immunol. 183, 6058–6068 (2009).
Li, K. et al. Functional modulation of human monocytes derived DCs by anaphylatoxins C3a and C5a. Immunobiology 217, 65–73 (2012). References 7–9 show that C3aR or C5aR signalling on bone marrow-derived DCs or monocyte-derived DCs causes cell activation and subsequently enhances the capacity of DCs for allospecific T cell stimulation.
Strainic, M. G. et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 28, 425–435 (2008). This paper demonstrated that the interaction of locally produced C5a or C3a with C5aR or C3aR on APCs and T cells both upstream and downstream of CD28 and CD40L signalling is integrally involved in T cell proliferation and differentiation.
Colvin, R. B. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J. Am. Soc. Nephrol. 18, 1046–1056 (2007).
Shoskes, D. A. & Cecka, J. M. Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation 66, 1697–1701 (1998).
Weisman, H. F. et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 249, 146–151 (1990). A landmark paper showing that therapeutic inhibition of complement activation using a recombinant complement regulator markedly protects rats from myocardial ischaemia–reperfusion injury.
Eppinger, M. J., Deeb, G. M., Bolling, S. F. & Ward, P. A. Mediators of ischemia–reperfusion injury of rat lung. Am. J. Pathol. 150, 1773–1784 (1997).
Atkinson, C. et al. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J. Clin. Invest. 115, 2444–2453 (2005).
Zhang, J. et al. The protective role of CD59 and pathogenic role of complement in hepatic ischemia and reperfusion injury. Am. J. Pathol. 179, 2876–2884 (2011).
von Dobschuetz, E. et al. Soluble complement receptor 1 preserves endothelial barrier function and microcirculation in postischemic pancreatitis in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G791–G796 (2004).
Zhou, W. et al. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J. Clin. Invest. 105, 1363–1371 (2000). This paper demonstrated that complement activation is a crucial mediator of renal ischaemia–reperfusion injury in mice, and that the activation of the alternative pathway and the formation of C5b–C9 complexes on renal tubular epithelial cells represent an important underlying mechanism.
Fondevila, C. et al. The membrane attack complex (C5b-9) in liver cold ischemia and reperfusion injury. Liver Transpl. 14, 1133–1141 (2008).
Turnberg, D. et al. CD59a deficiency exacerbates ischemia–reperfusion injury in mice. Am. J. Pathol. 165, 825–832 (2004).
de Vries, B. et al. Complement factor C5a mediates renal ischemia–reperfusion injury independent from neutrophils. J. Immunol. 170, 3883–3889 (2003).
Lewis, A. G., Köhl, G., Ma, Q., Devarajan, P. & Köhl, J. Pharmacological targeting of C5a receptors during organ preservation improves kidney graft survival. Clin. Exp. Immunol. 153, 117–126 (2008).
Busche, M. N. & Stahl, G. L. Role of the complement components C5 and C3a in a mouse model of myocardial ischemia and reperfusion injury. Ger. Med. Sci. 8, Doc20 (2010).
Fosbrink, M., Niculescu, F., Rus, V., Shin, M. L. & Rus, H. C5b-9-induced endothelial cell proliferation and migration are dependent on Akt inactivation of forkhead transcription factor FOXO1. J. Biol. Chem. 281, 19009–19018 (2006).
Tedesco, F. et al. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J. Exp. Med. 185, 1619–1627 (1997).
Biancone, L. et al. Alternative pathway activation of complement by cultured human proximal tubular epithelial cells. Kidney Int. 45, 451–460 (1994).
David, S. et al. Alternative pathway complement activation induces proinflammatory activity in human proximal tubular epithelial cells. Nephrol. Dial. Transplant. 12, 51–56 (1997).
Qiu, W. et al. Sublytic C5b-9 complexes induce proliferative changes of glomerular mesangial cells in rat Thy-1 nephritis through TRAF6-mediated PI3K-dependent Akt1 activation. J. Pathol. 226, 619–632 (2012).
Williams, J. P. et al. Intestinal reperfusion injury is mediated by IgM and complement. J. Appl. Physiol. 86, 938–942 (1999).
Naesens, M. et al. Expression of complement components differs between kidney allografts from living and deceased donors. J. Am. Soc. Nephrol. 20, 1839–1851 (2009). This clinical study demonstrated that the expression of C3 and other complement molecules is markedly increased in kidneys from deceased donors before reperfusion. This local complement expression correlates significantly with the length of cold ischaemic injury and with renal allograft function.
Thurman, J. M. et al. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. J. Clin. Invest. 116, 357–368 (2006).
Damman, J. et al. Local renal complement C3 induction by donor brain death is associated with reduced renal allograft function after transplantation. Nephrol. Dial. Transplant. 26, 2345–2354 (2011).
Brooimans, R. A. et al. Interleukin 2 mediates stimulation of complement C3 biosynthesis in human proximal tubular epithelial cells. J. Clin. Invest. 88, 379–384 (1991).
Gerritsma, J. S., Gerritsen, A. F., De, L. M., van Es, L. A. & Daha, M. R. Interferon-γ induces biosynthesis of complement components C2, C4 and factor H by human proximal tubular epithelial cells. Cytokine 9, 276–283 (1997).
Busche, M. N., Pavlov, V., Takahashi, K. & Stahl, G. L. Myocardial ischemia and reperfusion injury is dependent on both IgM and mannose-binding lectin. Am. J. Physiol. Heart Circ. Physiol. 297, H1853–H1859 (2009).
Hart, M. L. et al. Gastrointestinal ischemia–reperfusion injury is lectin complement pathway dependent without involving C1q. J. Immunol. 174, 6373–6380 (2005).
Moller-Kristensen, M. et al. Mannan-binding lectin recognizes structures on ischaemic reperfused mouse kidneys and is implicated in tissue injury. Scand. J. Immunol. 61, 426–434 (2005).
Schwaeble, W. J. et al. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc. Natl Acad. Sci. USA 108, 7523–7528 (2011). This study identified a previously unrecognized C4-independent, MASP2-dependent route of complement activation, demonstrating a pivotal role for the lectin pathway in mediating cardiac and intestinal ischaemia–reperfusion injury.
de Vries, B. et al. The mannose-binding lectin-pathway is involved in complement activation in the course of renal ischemia–reperfusion injury. Am. J. Pathol. 165, 1677–1688 (2004).
Weiser, M. R. et al. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J. Exp. Med. 183, 2343–2348 (1996).
Williams, J. P. et al. Intestinal reperfusion injury is mediated by IgM and complement. J. Appl. Physiol. 86, 938–942 (1999).
Zhang, M. et al. The role of natural IgM in myocardial ischemia–reperfusion injury. J. Mol. Cell. Cardiol. 41, 62–67 (2006).
Fleming, S. D. et al. Anti-phospholipid antibodies restore mesenteric ischemia/reperfusion-induced injury in complement receptor 2/complement receptor 1-deficient mice. J. Immunol. 173, 7055–7061 (2004).
Thurman, J. M., Ljubanovic, D., Edelstein, C. L., Gilkeson, G. S. & Holers, V. M. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J. Immunol. 170, 1517–1523 (2003).
Baldwin, W. M. et al. Complement deposition in early cardiac transplant biopsies is associated with ischemic injury and subsequent rejection episodes. Transplantation 68, 894–900 (1999).
Khan, M. A. et al. CD4+ T cells and complement independently mediate graft ischemia in the rejection of mouse orthotopic tracheal transplants. Circ. Res. 109, 1290–1301 (2011).
Damman, J. et al. Systemic complement activation in deceased donors is associated with acute rejection after renal transplantation in the recipient. Transplantation 92, 163–169 (2011).
Muller, T. F., Kraus, M., Neumann, C. & Lange, H. Detection of renal allograft rejection by complement components C5A and TCC in plasma and urine. J. Lab. Clin. Med. 129, 62–71 (1997).
Welch, T. R., Beischel, L. S. & Witte, D. P. Differential expression of complement C3 and C4 in the human kidney. J. Clin. Invest. 92, 1451–1458 (1993).
Andrews, P. A., Finn, J. E., Mathieson, P. W. & Sacks, S. H. Molecular analysis of C3 allotypes related to transplant outcome in human renal allografts. Transplantation 60, 1342–1346 (1995).
Tang, S., Zhou, W., Sheerin, N. S., Vaughan, R. W. & Sacks, S. H. Contribution of renal secreted complement C3 to the circulating pool in humans. J. Immunol. 162, 4336–4341 (1999).
Keslar, K., Rodriguez, E. R., Tan, C. D., Starling, R. C. & Heeger, P. S. Complement gene expression in human cardiac allograft biopsies as a correlate of histologic grade of injury. Transplantation 86, 1319–1321 (2008).
Andrews, P. A. et al. Expression and tissue localization of donor-specific complement C3 synthesized in human renal allografts. Eur. J. Immunol. 25, 1087–1093 (1995).
Naughton, M. A. et al. Extrahepatic secreted complement C3 contributes to circulating C3 levels in humans. J. Immunol. 156, 3051–3056 (1996).
Pavlov, V. et al. Donor deficiency of decay-accelerating factor accelerates murine T cell-mediated cardiac allograft rejection. J. Immunol. 181, 4580–4589 (2008).
Vieyra, M. et al. Complement regulates CD4 T-cell help to CD8 T cells required for murine allograft rejection. Am. J. Pathol. 179, 766–774 (2011).
Raedler, H., Yang, M., Lalli, P. N., Medof, M. E. & Heeger, P. S. Primed CD8+ T-cell responses to allogeneic endothelial cells are controlled by local complement activation. Am. J. Transplant. 9, 1784–1795 (2009).
Peng, Q., Li, K., Patel, H., Sacks, S. H. & Zhou, W. Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J. Immunol. 176, 3330–3341 (2006).
Li, K. et al. Cyclic AMP plays a critical role in C3a-receptor-mediated regulation of dendritic cells in antigen uptake and T-cell stimulation. Blood 112, 5084–5094 (2008).
Lalli, P. N. et al. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood 112, 1759–1766 (2008).
Li, K. et al. Expression of complement components, receptors and regulators by human dendritic cells. Mol. Immunol. 48, 1121–1127 (2011).
Li, Q. et al. Deficiency of C5aR prolongs renal allograft survival. J. Am. Soc. Nephrol. 21, 1344–1353 (2010).
Li, K. et al. Complement activation regulates the capacity of proximal tubular epithelial cell to stimulate alloreactive T cell response. J. Am. Soc. Nephrol. 15, 2414–2422 (2004).
Marsh, J. E. et al. The allogeneic T and B cell response is strongly dependent on complement components C3 and C4. Transplantation 72, 1310–1318 (2001).
Pepys, M. B. Role of complement in induction of antibody production in vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J. Exp. Med. 140, 126–145 (1974).
Fang, Y., Xu, C., Fu, Y. X., Holers, V. M. & Molina, H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J. Immunol. 160, 5273–5279 (1998).
Dempsey, P. W., Allison, M. E., Akkaraju, S., Goodnow, C. C. & Fearon, D. T. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 271, 348–350 (1996).
Carroll, M. C. The complement system in regulation of adaptive immunity. Nature Immunol. 5, 981–986 (2004).
Verschoor, A., Brockman, M. A., Knipe, D. M. & Carroll, M. C. Cutting edge: myeloid complement C3 enhances the humoral response to peripheral viral infection. J. Immunol. 167, 2446–2451 (2001).
Verschoor, A., Brockman, M. A., Gadjeva, M., Knipe, D. M. & Carroll, M. C. Myeloid C3 determines induction of humoral responses to peripheral herpes simplex virus infection. J. Immunol. 171, 5363–5371 (2003).
Solez, K. et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am. J. Transplant. 8, 753–760 (2008).
Park, W. D. et al. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am. J. Transplant. 3, 952–960 (2003).
Tan, C. D. et al. Correlation of donor-specific antibodies, complement and its regulators with graft dysfunction in cardiac antibody-mediated rejection. Am. J. Transplant. 9, 2075–2084 (2009).
Gonzalez-Stawinski, G. V., Tan, C. D., Smedira, N. G., Starling, R. C. & Rodriguez, E. R. Decay-accelerating factor expression may provide immunoprotection against antibody-mediated cardiac allograft rejection. J. Heart Lung Transplant. 27, 357–361 (2008).
Griesemer, A. D. et al. Upregulation of CD59: potential mechanism of accommodation in a large animal model. Transplantation 87, 1308–1317 (2009).
Ding, J. W. et al. Expression of complement regulatory proteins in accommodated xenografts induced by anti-α-Gal IgG1 in a rat-to-mouse model. Am. J. Transplant. 8, 32–40 (2008).
Salama, A. D. et al. Transplant accommodation in highly sensitized patients: a potential role for Bcl-xL and alloantibody. Am. J. Transplant. 1, 260–269 (2001).
Kinderlerer, A. R. et al. Heme oxygenase-1 expression enhances vascular endothelial resistance to complement-mediated injury through induction of decay-accelerating factor: a role for increased bilirubin and ferritin. Blood 113, 1598–1607 (2009).
Ekser, B. et al. Clinical xenotransplantation: the next medical revolution? Lancet 379, 672–683 (2012).
Markiewski, M. M., Nilsson, B., Ekdahl, K. N., Mollnes, T. E. & Lambris, J. D. Complement and coagulation: strangers or partners in crime? Trends Immunol. 28, 184–192 (2007).
Krarup, A., Wallis, R., Presanis, J. S., Gal, P. & Sim, R. B. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS ONE 2, e623 (2007).
Amara, U. et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 185, 5628–5636 (2010).
Lewis, E. C. et al. α1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc. Natl Acad. Sci. USA 105, 16236–16241 (2008).
Zheng, X. et al. Preventing renal ischemia–reperfusion injury using small interfering RNA by targeting complement 3 gene. Am. J. Transplant. 6, 2099–2108 (2006).
Zheng, X. et al. Gene silencing of complement C5a receptor using siRNA for preventing ischemia/reperfusion injury. Am. J. Pathol. 173, 973–980 (2008).
Smith, R. A. Targeting anticomplement agents. Biochem. Soc. Trans. 30, 1037–1041 (2002).
Patel, H., Smith, R. A., Sacks, S. H. & Zhou, W. Therapeutic strategy with a membrane-localizing complement regulator to increase the number of usable donor organs after prolonged cold storage. J. Am. Soc. Nephrol. 17, 1102–1111 (2006). This study provided the proof-of-concept for a new strategy that increases the number of surviving post-ischaemic grafts through intragraft delivery of a membrane-localizing complement regulator.
Pratt, J. R. et al. Nontransgenic hyperexpression of a complement regulator in donor kidney modulates transplant ischemia/reperfusion damage, acute rejection, and chronic nephropathy. Am. J. Pathol. 163, 1457–1465 (2003).
Smith, R. A. G. et al. Membrane-localising complement inhibitors — clinical progress. Mol. Immunol. 44, 3915 (2007).
Hillmen, P. et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood 110, 4123–4128 (2007).
Stegall, M. D. et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am. J. Transplant. 11, 2405–2413 (2011).
Locke, J. E. et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am. J. Transplant. 9, 231–235 (2009).
Al-Akash, S. I., Almond, P. S., Savell, V. H. Jr, Gharaybeh, S. I. & Hogue, C. Eculizumab induces long-term remission in recurrent post-transplant HUS associated with C3 gene mutation. Pediatr. Nephrol. 26, 613–619 (2011).
Caprioli, J. et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 108, 1267–1279 (2006).
Bresin, E. et al. Outcome of renal transplantation in patients with non-Shiga toxin-associated hemolytic uremic syndrome: prognostic significance of genetic background. Clin. J. Am. Soc. Nephrol. 1, 88–99 (2006).
Nurnberger, J. et al. Eculizumab for atypical hemolytic-uremic syndrome. N. Engl. J. Med. 360, 542–544 (2009).
Chatelet, V. et al. Eculizumab: safety and efficacy after 17 months of treatment in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome: case report. Transplant. Proc. 42, 4353–4355 (2010).
Davin, J. C. et al. Maintenance of kidney function following treatment with eculizumab and discontinuation of plasma exchange after a third kidney transplant for atypical hemolytic uremic syndrome associated with a CFH mutation. Am. J. Kidney Dis. 55, 708–711 (2010).
Cheong, H. I. et al. Attempted treatment of factor H deficiency by liver transplantation. Pediatr. Nephrol. 19, 454–458 (2004).
Atkinson, C. et al. Targeted complement inhibitors protect against posttransplant cardiac ischemia and reperfusion injury and reveal an important role for the alternative pathway of complement activation. J. Immunol. 185, 7007–7013 (2010).
Brown, K. M. et al. Influence of donor C3 allotype on late renal-transplantation outcome. N. Engl. J. Med. 354, 2014–2023 (2006).
Varagunam, M., Yaqoob, M. M., Dohler, B. & Opelz, G. C3 polymorphisms and allograft outcome in renal transplantation. N. Engl. J. Med. 360, 874–880 (2009).
Damman, J. et al. Association of complement C3 gene variants with renal transplant outcome of deceased cardiac dead donor kidneys. Am. J. Transplant. 12, 660–668 (2012).
Wahrmann, M. et al. Clinical relevance of preformed C4d-fixing and non-C4d-fixing HLA single antigen reactivity in renal allograft recipients. Transpl. Int. 22, 982–989 (2009).
de Rooij, B. J. et al. Lectin complement pathway gene profile of donor and recipient determine the risk of bacterial infections after orthotopic liver transplantation. Hepatology 52, 1100–1110 (2010).
de Rooij, B. J. et al. Mannose-binding lectin and ficolin-2 gene polymorphisms predispose to cytomegalovirus (re)infection after orthotopic liver transplantation. J. Hepatol. 55, 800–807 (2011).
Berger, S. P. et al. Low pretransplantation mannose-binding lectin levels predict superior patient and graft survival after simultaneous pancreas–kidney transplantation. J. Am. Soc. Nephrol. 18, 2416–2422 (2007).
Sargsyan, S. A. et al. Detection of glomerular complement C3 fragments by magnetic resonance imaging in murine lupus nephritis. Kidney Int. 81, 152–159 (2012).
Badar, A. et al. Recombinant complement receptor 2 radiolabeled with [99mTc(CO)3]+: a potential new radiopharmaceutical for imaging activated complement. PLoS ONE 6, e18275 (2011).
Springall, T. et al. Epithelial secretion of C3 promotes colonization of the upper urinary tract by Escherichia coli. Nature Med. 7, 801–806 (2001).
Li, K., Feito, M. J., Sacks, S. H. & Sheerin, N. S. CD46 (membrane cofactor protein) acts as a human epithelial cell receptor for internalization of opsonized uropathogenic Escherichia coli. J. Immunol. 177, 2543–2551 (2006).
Lambris, J. D., Ricklin, D. & Geisbrecht, B. V. Complement evasion by human pathogens. Nature Rev. Microbiol. 6, 132–142 (2008).
Monk, N. J. et al. Fc-dependent depletion of activated T cells occurs through CD40L-specific antibody rather than costimulation blockade. Nature Med. 9, 1275–1280 (2003).
Baruah, P. et al. Mice lacking C1q or C3 show accelerated rejection of minor H disparate skin grafts and resistance to induction of tolerance. Eur. J. Immunol. 40, 1758–1767 (2010).
Ezzelarab, M., Ayares, D. & Cooper, D. K. Carbohydrates in xenotransplantation. Immunol. Cell Biol. 83, 396–404 (2005).
Pruitt, S. K. et al. The effect of soluble complement receptor type 1 on hyperacute rejection of porcine xenografts. Transplantation 57, 363–370 (1994).
Houser, S. L. et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation 11, 416–425 (2004).
Kuwaki, K. et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nature Med. 11, 29–31 (2005).
Cooper, D. K., Tseng, Y. L. & Saidman, S. L. Alloantibody and xenoantibody cross-reactivity in transplantation. Transplantation 77, 1–5 (2004).
McGregor, C. G. et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation 93, 686–692 (2012).
Ekser, B., Rigotti, P., Gridelli, B. & Cooper, D. K. Xenotransplantation of solid organs in the pig-to-primate model. Transpl. Immunol. 21, 87–92 (2009).
Diamond, L. E. et al. A human CD46 transgenic pig model system for the study of discordant xenotransplantation. Transplantation 71, 132–142 (2001).
Menoret, S. et al. Characterization of human CD55 and CD59 transgenic pigs and kidney xenotransplantation in the pig-to-baboon combination. Transplantation 77, 1468–1471 (2004).
Morgan, B. P., Berg, C. W. & Harris, C. L. “Homologous restriction” in complement lysis: roles of membrane complement regulators. Xenotransplantation 12, 258–265 (2005).
Yang, Y. G. & Sykes, M. Xenotransplantation: current status and a perspective on the future. Nature Rev. Immunol. 7, 519–531 (2007).
Kemper, C. et al. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature 421, 388–392 (2003).
Cardone, J. et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nature Immunol. 11, 862–871 (2010).
Ghannam, A. et al. Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. J. Immunol. 181, 5158–5166 (2008).
Acknowledgements
This work was supported by the Medical Research Council (grant numbers G0600698 and G1001197). The research was also funded and supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas's NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Steven H. Sacks received a contribution from Alexion Pharmaceuticals to organize a national meeting of Complement UK in 2010. King's College London owns the intellectual property for mirococept.
Supplementary information
Supplementary information S1 (table)
Potential sources of complement during transplantation (and in some other non-transplanted tissues) (PDF 140 kb)
Related links
Glossary
- Complement cascade
-
There are three independent pathways that can lead to the activation of the complement cascade. The classical pathway is activated via C1q binding to immune complexes, the alternative pathway is triggered by direct C3 activation, and the lectin pathway is initiated by mannose-binding lectin (MBL) binding to the surface of microorganisms and other activating surfaces.
- Xenotransplantation
-
The transplantation of organs, tissues or cells from one species to another.
- Allotransplantation
-
The transplantation of organs, tissues or cells from a donor to a genetically non-identical recipient of the same species.
- Ischaemia–reperfusion injury
-
An injury in which the tissue first suffers from hypoxia as a result of severely decreased, or completely arrested, blood flow. Restoration of normal blood flow then triggers inflammation, which exacerbates the tissue damage.
- Mannose-binding lectin
-
(MBL). A protein of the C-type lectin family that binds to carbohydrate structures such as mannose on the surface of many pathogens and other activating surfaces and triggers complement activation. It is composed of a number of identical subunits and is found as a complex in association with several serine protease molecules.
- Major histocompatibility antigens
-
Highly polymorphic cell-surface glycoproteins (termed MHC molecules) that characterize different members of the same species and underlie the rapid rejection of organ, cell or tissue transplants between those individuals.
- Minor histocompatibility antigens
-
Polymorphic peptides that are expressed by the donor tissue and recognized by recipient T cells, even when a transplant donor and recipient have identical major histocompatibility antigens. The amino acid differences in minor antigens can cause the graft to be slowly rejected. An example is the male H-Y antigen, which is recognized by a female recipient of the same mouse stain.
- Minor histocompatibility antigen H-Y
-
H-Y is a protein encoded on the Y chromosome. T cells from females respond to peptides derived from this protein, and so H-Y is a male-specific minor histocompatibility antigen. Furthermore, T cell receptors (TCRs) specific for this antigen have been cloned and used to generate distinct lines of TCR-transgenic mice.
- Small interfering RNAs
-
(siRNAs). Short (∼21-base-pair) double-stranded RNA fragments that can direct RNA-degradative machinery to homologous endogenous RNA sequences when introduced into cells, thereby inhibiting the expression of the targeted genes.
- Paroxysmal nocturnal haemoglobinuria
-
An acquired defect of the membrane-expressed regulators of complement activation CD55 and CD59 that leads to intermittent complement-induced intravascular haemolysis, haemoglobin in the urine and thrombosis.
- Haemolytic–uraemic syndrome
-
A combination of haemolytic anaemia, acute renal failure and thrombocytopenia that typically follows a gastrointestinal infection with the Escherichia coli strain O157:H7. In atypical cases, this syndrome is caused by a defect in complement regulation by a complement-regulatory protein (namely, CD46, factor H or factor I).
Rights and permissions
About this article
Cite this article
Sacks, S., Zhou, W. The role of complement in the early immune response to transplantation. Nat Rev Immunol 12, 431–442 (2012). https://doi.org/10.1038/nri3225
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3225
This article is cited by
-
Induced neural stem cell grafts exert neuroprotection through an interaction between Crry and Akt in a mouse model of closed head injury
Stem Cell Research & Therapy (2021)
-
Immunological considerations and challenges for regenerative cellular therapies
Communications Biology (2021)
-
Fucose as a new therapeutic target in renal transplantation
Pediatric Nephrology (2021)
-
Complement in ischaemia–reperfusion injury and transplantation
Seminars in Immunopathology (2021)
-
Clinical promise of next-generation complement therapeutics
Nature Reviews Drug Discovery (2019)