Key Points
-
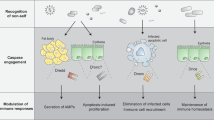
Apoptosis and programmed necrosis are common host defence signalling pathways that contribute to the elimination of intracellular pathogens.
-
Caspase 8 promotes apoptosis and suppresses programmed necrosis in complex with FAS-associated death domain protein (FADD) and cellular FLICE-like inhibitory protein (cFLIP).
-
Many viruses encode inhibitors of caspase 8 activity to suppress apoptosis. Caspase 8 inhibition unveils programmed necrosis, a pathway that may also be suppressed by viral inhibitors of cell death.
-
Programmed necrosis has only recently been recognized as a host-defence pathway through the characterization of cell death suppressors encoded by murine cytomegalovirus (MCMV).
-
Programmed necrosis is dependent on receptor-interacting protein 3 (RIP3) and can be either RIP1-dependent (necroptosis) or RIP1-independent (MCMV-induced programmed necrosis).
-
Pathogen-associated dysregulation of caspase 8 can drive both inflammation and programmed necrosis, contributing to disease outcomes.
Abstract
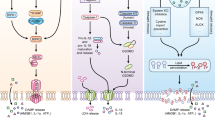
Pathogens specifically target both the caspase 8-dependent apoptotic cell death pathway and the necrotic cell death pathway that is dependent on receptor-interacting protein 1 (RIP1; also known as RIPK1) and RIP3 (also known as RIPK3). The fundamental co-regulation of these two cell death pathways emerged when the midgestational death of mice deficient in FAS-associated death domain protein (FADD) or caspase 8 was reversed by elimination of RIP1 or RIP3, indicating a far more entwined relationship than previously appreciated. Thus, mammals require caspase 8 activity during embryogenesis to suppress the kinases RIP1 and RIP3 as part of the dialogue between two distinct cell death processes that together fulfil reinforcing roles in the host defence against intracellular pathogens such as herpesviruses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Strasser, A., O'Connor, L. & Dixit, V. M. Apoptosis signaling. Annu. Rev. Biochem. 69, 217–245 (2000).
Hengartner, M. O. The biochemistry of apoptosis. Nature 407, 770–776 (2000).
Yuan, J. & Kroemer, G. Alternative cell death mechanisms in development and beyond. Genes Dev. 24, 2592–2602 (2010).
Holler, N. et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature Immunol. 1, 489–495 (2000). A seminal study that demonstrated that the kinase RIP1 is a mediator of necrosis induced by the death receptor FAS and that caspase 8 is a crucial negative regulator of the pathway.
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137, 1100–1111 (2009).
Zhang, D. W. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009). References 5–7 demonstrated that a RHIM-dependent RIP1–RIP3 kinase complex mediates necrosis induced by death receptors.
Sun, X., Yin, J., Starovasnik, M. A., Fairbrother, W. J. & Dixit, V. M. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J. Biol. Chem. 277, 9505–9511 (2002).
Chipuk, J. E., Moldoveanu, T., Llambi, F., Parsons, M. J. & Green, D. R. The BCL-2 family reunion. Mol. Cell 37, 299–310 (2010).
Danial, N. N. & Korsmeyer, S. J. Cell death: critical control points. Cell 116, 205–219 (2004).
Thornberry, N. A. & Lazebnik, Y. Caspases: enemies within. Science 281, 1312–1316 (1998).
Pop, C. & Salvesen, G. S. Human caspases: activation, specificity, and regulation. J. Biol. Chem. 284, 21777–21781 (2009).
Kantari, C. & Walczak, H. Caspase-8 and Bid: caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta 1813, 558–563 (2011).
Vandenabeele, P., Galluzzi, L., Vanden Berghe, T. & Kroemer, G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature Rev. Mol. Cell Biol. 11, 700–714 (2010).
Kang, T. B. et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J. Immunol. 173, 2976–2984 (2004).
Kang, T. B. et al. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J. Immunol. 181, 2522–2532 (2008). This study formally demonstrated that the role of caspase 8 in mediating extrinsic apoptosis is distinct from its essential role during mammalian embryogenesis.
Sakamaki, K. et al. Ex vivo whole-embryo culture of caspase-8-deficient embryos normalize their aberrant phenotypes in the developing neural tube and heart. Cell Death Differ. 9, 1196–1206 (2002).
Varfolomeev, E. E. et al. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9, 267–276 (1998).
Yeh, W. C. et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279, 1954–1958 (1998).
Zhang, J., Cado, D., Chen, A., Kabra, N. H. & Winoto, A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392, 296–300 (1998).
Yeh, W. C. et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity 12, 633–642 (2000).
Kaiser, W. J. et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 (2011). This study showed that mice lacking both Casp8 and Rip3 are viable, fertile and immunocompetent, and sustain antiviral T cell immunity. Thus, dysregulation of RIP3 underlies the embryonic lethality, as well as the defects in T cell function, in caspase 8-deficient mice. Like FAS-deficient mice, Casp8−/−Rip3−/− mice fail to eliminate excess T cells as they age.
Oberst, A. et al. Catalytic activity of the caspase-8–FLIPL complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011). This study showed that mice lacking both Casp8 and Rip3 develop normally into adults but fail to eliminate excess T cells as they age, similarly to FAS-deficient mice. The regulation of RIP1–RIP3 activity was attributed to proteolytic activity of the caspase 8–cFLIP L complex.
Zhang, H. et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471, 373–376 (2011). This study showed that mice lacking both Fadd and Rip1 complete embryonic development. Thus, dysregulation of RIP1 underlies the embryonic lethality, as well as the defects in T cell function, in FADD-deficient mice.
Degterev, A. & Yuan, J. Expansion and evolution of cell death programmes. Nature Rev. Mol. Cell Biol. 9, 378–390 (2008).
Moquin, D. & Chan, F. K. The molecular regulation of programmed necrotic cell injury. Trends Biochem. Sci. 35, 434–441 (2010).
Upton, J. W., Kaiser, W. J. & Mocarski, E. S. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7, 302–313 (2010). A virus-encoded RHIM-containing suppressor of programmed necrosis was identified in this study, unveiling a natural RIP3-dependent, RIP1-independent death pathway in mammalian host defence.
Kalai, M. et al. Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell Death Differ. 9, 981–994 (2002).
Feoktistova, M. et al. cIAPs block ripoptosome formation, a RIP1/Caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 43, 449–463 (2011). This study showed that TLR3 signalling engages a cytosolic caspase 8-activating complex (the ripoptosome) that requires RIP1 kinase activity, is repressed by cIAP and is differentially controlled by cFLIP L and cFLIP s.
Tenev, T. et al. The ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell 43, 432–448 (2011). This study identified that cellular responses to genotoxic stress involve the formation of a cytosolic caspase 8-activating complex (the ripoptosome), with a requirement for RIP1 kinase activity and repression by cIAP.
Wallach, D., Kovalenko, A. & Kang, T. B. 'Necrosome'-induced inflammation: must cells die for it? Trends Immunol. 32, 505–509 (2011).
Bonnet, M. C. et al. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity 35, 572–582 (2011).
Welz, P. S. et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477, 330–334 (2011).
Gunther, C. et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 477, 335–339 (2011).
Gerlach, B. et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 471, 591–596 (2011).
Wilson, N. S., Dixit, V. & Ashkenazi, A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nature Immunol. 10, 348–355 (2009).
Walczak, H. TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol. Rev. 244, 9–28 (2011).
Silke, J. The regulation of TNF signalling: what a tangled web we weave. Curr. Opin. Immunol. 23, 620–626 (2011).
Wajant, H. & Scheurich, P. TNFR1-induced activation of the classical NF-κB pathway. FEBS J. 278, 862–876 (2011).
Benedict, C. A., Norris, P. S. & Ware, C. F. To kill or be killed: viral evasion of apoptosis. Nature Immunol. 3, 1013–1018 (2002).
Kischkel, F. C. et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 14, 5579–5588 (1995).
Muzio, M. et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85, 817–827 (1996).
Sprick, M. R. et al. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12, 599–609 (2000).
Ashkenazi, A. & Dixit, V. M. Death receptors: signaling and modulation. Science 281, 1305–1308 (1998).
Irmler, M. et al. Inhibition of death receptor signals by cellular FLIP. Nature 388, 190–195 (1997).
Ting, A. T., Pimentel-Muinos, F. X. & Seed, B. RIP mediates tumor necrosis factor receptor 1 activation of NF-κB but not Fas/APO-1-initiated apoptosis. EMBO J. 15, 6189–6196 (1996).
Hsu, H., Huang, J., Shu, H. B., Baichwal, V. & Goeddel, D. V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 (1996).
Hsu, H., Xiong, J. & Goeddel, D. V. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell 81, 495–504 (1995).
Van Antwerp, D. J., Martin, S. J., Kafri, T., Green, D. R. & Verma, I. M. Suppression of TNF-α-induced apoptosis by NF-κB. Science 274, 787–789 (1996).
Bertrand, M. J. et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 (2008).
Vince, J. E. et al. TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (TNF) to efficiently activate NF-κB and to prevent TNF-induced apoptosis. J. Biol. Chem. 284, 35906–35915 (2009).
Varfolomeev, E. et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J. Biol. Chem. 283, 24295–24299 (2008).
Wang, L., Du, F. & Wang, X. TNF-α induces two distinct caspase-8 activation pathways. Cell 133, 693–703 (2008). This report revealed that TNFR1 pro-death signalling is blocked by the E3 ligase cIAP in part through inhibition of the RIP1-dependent assembly of a caspase 8-activating complex, but that this inhibition is reversed by the deubiquitylating enzyme CYLD.
Ea, C. K., Deng, L., Xia, Z. P., Pineda, G. & Chen, Z. J. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22, 245–257 (2006).
Vanlangenakker, N. et al. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 18, 656–665 (2011).
Biton, S. & Ashkenazi, A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-α feedforward signaling. Cell 145, 92–103 (2011).
Vucic, D., Dixit, V. M. & Wertz, I. E. Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nature Rev. Mol. Cell Biol. 12, 439–452 (2011).
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003).
Boatright, K. M., Deis, C., Denault, J. B., Sutherlin, D. P. & Salvesen, G. S. Activation of caspases-8 and -10 by FLIPL . Biochem. J. 382, 651–657 (2004).
Micheau, O. et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J. Biol. Chem. 277, 45162–45171 (2002).
Oberst, A. et al. Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J. Biol. Chem. 285, 16632–16642 (2010).
Pop, C. et al. FLIPL induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem. J. 433, 447–457 (2011).
Chan, F. K. et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J. Biol. Chem. 278, 51613–51621 (2003). This study presented initial evidence that some mammalian viruses encode suppressors of programmed necrosis.
Lin, Y., Devin, A., Rodriguez, Y. & Liu, Z. G. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13, 2514–2526 (1999).
Lu, J. V. et al. Complementary roles of FADD and RIPK3 in T cell homeostasis and antiviral immunity. Proc. Natl Acad. Sci. USA 108, 15312–15317 (2011).
Feng, S. et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell. Signal. 19, 2056–2067 (2007).
O'Donnell, M. A. et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nature Cell Biol. 13, 1437–1442 (2011). This study identified the RIP1 deubiquitylating enzyme CYLD as a key target of caspase 8 in suppressing necrosis following death receptor signalling.
Ch'en, I. L., Tsau, J. S., Molkentin, J. D., Komatsu, M. & Hedrick, S. M. Mechanisms of necroptosis in T cells. J. Exp. Med. 208, 633–641 (2011).
Hedrick, S. M., Ch'en, I. L. & Alves, B. N. Intertwined pathways of programmed cell death in immunity. Immunol. Rev. 236, 41–53 (2010).
Ch'en, I. L. et al. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc. Natl Acad. Sci. USA 105, 17463–17468 (2008). This study demonstrated that caspase 8-deficient T cells fail to proliferate in response to antigens owing to RIP1-dependent necroptosis.
Gaide, O. et al. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κB activation. Nature Immunol. 3, 836–843 (2002).
Ruefli-Brasse, A. A., French, D. M. & Dixit, V. M. Regulation of NF-κB-dependent lymphocyte activation and development by paracaspase. Science 302, 1581–1584 (2003).
Ruland, J. et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell 104, 33–42 (2001).
Kawadler, H., Gantz, M. A., Riley, J. L. & Yang, X. The paracaspase MALT1 controls caspase-8 activation during lymphocyte proliferation. Mol. Cell 31, 415–421 (2008).
Misra, R. S. et al. Caspase-8 and c-FLIPL associate in lipid rafts with NF-κB adaptors during T cell activation. J. Biol. Chem. 282, 19365–19374 (2007).
Eimon, P. M. et al. Delineation of the cell-extrinsic apoptosis pathway in the zebrafish. Cell Death Differ. 13, 1619–1630 (2006).
Naitza, S. et al. The Drosophila immune defense against Gram-negative infection requires the death protein dFADD. Immunity 17, 575–581 (2002).
Leulier, F., Rodriguez, A., Khush, R. S., Abrams, J. M. & Lemaitre, B. The Drosophila caspase Dredd is required to resist Gram-negative bacterial infection. EMBO Rep. 1, 353–358 (2000).
Newton, K., Sun, X. & Dixit, V. M. Kinase RIP3 is dispensable for normal NF-κB signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 24, 1464–1469 (2004).
Challa, S. & Chan, F. K. Going up in flames: necrotic cell injury and inflammatory diseases. Cell. Mol. Life Sci. 67, 3241–3253 (2010).
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007).
Kaiser, W. J., Upton, J. W. & Mocarski, E. S. Receptor-interacting protein homotypic interaction motif-dependent control of NF-κB activation via the DNA-dependent activator of IFN regulatory factors. J. Immunol. 181, 6427–6434 (2008).
Rebsamen, M. et al. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-κB. EMBO Rep. 10, 916–922 (2009).
DeFilippis, V. R., Alvarado, D., Sali, T., Rothenburg, S. & Fruh, K. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. J. Virol. 84, 585–598 (2010).
Ishii, K. J. et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451, 725–729 (2008).
Galluzzi, L. et al. Viral strategies for the evasion of immunogenic cell death. J. Intern. Med. 267, 526–542 (2010).
Lamkanfi, M. & Dixit, V. M. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe 8, 44–54 (2010).
Roy, C. R. & Mocarski, E. S. Pathogen subversion of cell-intrinsic innate immunity. Nature Immunol. 8, 1179–1187 (2007).
Vercammen, D. et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187, 1477–1485 (1998).
Clem, R. J., Fechheimer, M. & Miller, L. K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science 254, 1388–1390 (1991).
Chen, P., Tian, J., Kovesdi, I. & Bruder, J. T. Interaction of the adenovirus 14.7-kDa protein with FLICE inhibits Fas ligand-induced apoptosis. J. Biol. Chem. 273, 5815–5820 (1998).
Taylor, J. M. & Barry, M. Near death experiences: poxvirus regulation of apoptotic death. Virology 344, 139–150 (2006).
Lagunoff, M. & Carroll, P. A. Inhibition of apoptosis by the γ-herpesviruses. Int. Rev. Immunol. 22, 373–399 (2003).
McCormick, A. L. Control of apoptosis by human cytomegalovirus. Curr. Top. Microbiol. Immunol. 325, 281–295 (2008).
Li, M. & Beg, A. A. Induction of necrotic-like cell death by tumor necrosis factor α and caspase inhibitors: novel mechanism for killing virus-infected cells. J. Virol. 74, 7470–7477 (2000).
Mocarski, E. S. in Human Herpesviruses: Biology, Therapy and Immunoprophylaxis (eds Arvin, A. et al.) 204–230 (Cambridge Univ. Press, Cambridge, UK, 2007).
Cicin-Sain, L. et al. Dominant-negative FADD rescues the in vivo fitness of a cytomegalovirus lacking an antiapoptotic viral gene. J. Virol. 82, 2056–2064 (2008).
Skaletskaya, A. et al. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl Acad. Sci. USA 98, 7829–7834 (2001).
Brune, W. Inhibition of programmed cell death by cytomegaloviruses. Virus Res. 157, 144–150 (2011).
Manzur, M., Fleming, P., Huang, D. C., Degli-Esposti, M. A. & Andoniou, C. E. Virally mediated inhibition of Bax in leukocytes promotes dissemination of murine cytomegalovirus. Cell Death Differ. 16, 312–320 (2009).
Upton, J. W., Kaiser, W. J. & Mocarski, E. S. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J. Biol. Chem. 283, 16966–16970 (2008). This was the first report of a virus-encoded RHIM-containing inhibitor of RHIM-dependent signalling pathways.
Mack, C., Sickmann, A., Lembo, D. & Brune, W. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc. Natl Acad. Sci. USA 105, 3094–3099 (2008).
Lembo, D. & Brune, W. Tinkering with a viral ribonucleotide reductase. Trends Biochem. Sci. 34, 25–32 (2009).
McCormick, A. L., Skaletskaya, A., Barry, P. A., Mocarski, E. S. & Goldmacher, V. S. Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology 316, 221–233 (2003).
Paterson, S. et al. Antagonistic coevolution accelerates molecular evolution. Nature 464, 275–278 (2010).
Ferguson, T. A., Choi, J. & Green, D. R. Armed response: how dying cells influence T-cell functions. Immunol. Rev. 241, 77–88 (2011).
Matzinger, P. The danger model: a renewed sense of self. Science 296, 301–305 (2002).
Krysko, D. V., D'Herde, K. & Vandenabeele, P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis 11, 1709–1726 (2006).
Strasser, A., Jost, P. J. & Nagata, S. The many roles of FAS receptor signaling in the immune system. Immunity 30, 180–192 (2009).
Watanabe-Fukunaga, R., Brannan, C. I., Copeland, N. G., Jenkins, N. A. & Nagata, S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356, 314–317 (1992).
Nagata, S. & Suda, T. Fas and Fas ligand: lpr and gld mutations. Immunol. Today 16, 39–43 (1995).
Salmena, L. & Hakem, R. Caspase-8 deficiency in T cells leads to a lethal lymphoinfiltrative immune disorder. J. Exp. Med. 202, 727–732 (2005).
Chun, H. J. et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419, 395–399 (2002).
Wang, J. et al. Inherited human caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell 98, 47–58 (1999).
Rosenberg, S., Zhang, H. & Zhang, J. FADD deficiency impairs early hematopoiesis in the bone marrow. J. Immunol. 186, 203–213 (2011).
Beisner, D. R., Ch'en, I. L., Kolla, R. V., Hoffmann, A. & Hedrick, S. M. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J. Immunol. 175, 3469–3473 (2005).
Imtiyaz, H. Z. et al. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J. Immunol. 176, 6852–6861 (2006).
Lemmers, B. et al. Essential role for caspase-8 in Toll-like receptors and NFκB signaling. J. Biol. Chem. 282, 7416–7423 (2007).
Kovalenko, A. et al. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J. Exp. Med. 206, 2161–2177 (2009).
Lee, P. et al. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature 458, 519–523 (2009).
Li, C. et al. Development of atopic dermatitis-like skin disease from the chronic loss of epidermal caspase-8. Proc. Natl Acad. Sci. USA 107, 22249–22254 (2010).
Ben Moshe, T. et al. Role of caspase-8 in hepatocyte response to infection and injury in mice. Hepatology 45, 1014–1024 (2007).
Acknowledgements
The authors thank D. Livingston-Rosanoff and L. Daley-Bauer for their contributions, L. Roback and A. L. McCormick for helping to facilitate this research and C. Benedict for discussions. The research was supported by grants from the US National Institutes of Health (AI030363 and AI020212) and the Georgia Cancer Coalition. We apologize to investigators whose contributions were not cited more extensively owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Apoptosis
-
The most common form of developmental cell death that regulates cell numbers, drives morphogenesis, deletes structures and eliminates unneeded and harmful cells. Apoptosis is a cell-autonomous death pathway mediated by caspases that dismantles the cell but maintains membrane integrity and is characterized by cell shrinkage, membrane blebbing and DNA fragmentation.
- RIP1
-
(Receptor-interacting protein 1; also known as RIPK1). A signalling adaptor and protein kinase that regulates the activation of gene expression via the NF-κB and MAPK pathways and controls the initiation of necroptosis and apoptosis via death domain- and RHIM-dependent complexes.
- RIP3
-
(Receptor-interacting protein 3; also known as RIPK3). A RHIM-containing signalling adaptor and protein kinase that mediates necroptosis and MCMV-induced necrosis.
- Programmed necrosis
-
A cell-autonomous, regulated cell death pathway that is characterized by cell swelling, membrane rupture and cytoplasmic leakage, but not by membrane blebbing.
- Necroptosis
-
A form of programmed necrosis that is executed by the kinase activities of RIP1 and RIP3. This pathway is inhibited by RIP1 kinase inhibitors, such as necrostatin 1 (5-(1H-indol-3-ylmethyl)-3-methyl-2-thioxo-4-imidazolidinone).
- RIP homotypic interaction motif
-
(RHIM). A protein–protein interaction motif containing the core sequence (I/V/L)-(Q/M)-(I/V/L)-G that mediates homophilic interactions between four cellular proteins (namely, RIP1, RIP3, TRIF and DAI) and one viral protein (MCMV vIRA).
- Death receptors
-
A subset of receptors belonging to the TNF receptor superfamily that transmit cell death signals initiated by their cognate ligands. These receptors include TNFR1, FAS, DR3, TRAILR1 and TRAILR2.
- Toll-like receptors
-
(TLRs). A family of pattern recognition receptors that recognize unique structures derived from microorganisms. TLR signalling promotes inflammatory and cytokine responses, as well as cell proliferation or cell death pathways.
- FAS
-
(Also known as CD95). A death receptor of the TNF receptor superfamily. FAS ligand binding induces cell death. FAS signalling controls the homeostatic elimination of T cells.
- Death-inducing signalling complex
-
(DISC). A death receptor-bound complex that contains FADD and caspase 8 (or caspase 10). The DISC assembles following ligand binding and drives autocatalytic caspase 8 (or caspase 10) activation.
- Complex I
-
A TNFR1-bound complex that contains TRADD, TRAF2 or TRAF5, cIAP and RIP1. This complex drives the activation of gene expression via the NF-κB and MAPK pathways.
- Cellular inhibitor of apoptosis proteins
-
(cIAP1 and cIAP2; collectively referred to as cIAP here). Members of a family of functionally and structurally related E3 ubiquitin ligases that regulate canonical and non-canonical activation of NF-κB, as well as MAPK activation by receptors of the TNF receptor superfamily. cIAP polyubiquitylates RIP1 to prevent the formation of the ripoptosome or TNFR1-dependent complex II.
- Complex II
-
A TNFR1- and RIP1-dependent cytosolic complex that contains caspase 8, FADD, cFLIP and RIP1. Within this complex, caspase 8 and cFLIP regulate programmed cell death pathways.
- Necrosome
-
An inducible cytosolic complex that contains oligomerized RIP1 and RIP3. This complex drives RIP1- and RIP3-dependent necroptosis.
- Ripoptosome
-
A RIP1-dependent cytosolic complex that is similar in composition to TNFR1-dependent complex II and that controls programmed cell death pathways.
- TIR domain-containing adaptor protein inducing interferon-β
-
(TRIF). A adaptor protein for TLR3 and TLR4 that organizes downstream signalling cascades leading to IRF3 and NF-κB activation, or cell death. TRIF mediates signalling through a TIR domain, TRAF-binding sites and RHIM-mediated interactions with RIP1 and RIP3.
- CARMA1–BCL-10–MALT1 complex
-
A PKC-dependent specialized signalling complex that is formed during TCR-dependent antigen recognition and that triggers NF-κB activation.
- DNA-dependent activator of interferon regulatory factors
-
(DAI; also known as ZBP1). A cytosolic, RHIM-containing sensor of double-stranded DNA that activates IRF3 and NF-κB.
Rights and permissions
About this article
Cite this article
Mocarski, E., Upton, J. & Kaiser, W. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol 12, 79–88 (2012). https://doi.org/10.1038/nri3131
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3131
This article is cited by
-
Necroptosis does not drive disease pathogenesis in a mouse infective model of SARS-CoV-2 in vivo
Cell Death & Disease (2024)
-
The role of regulated necrosis in diabetes and its complications
Journal of Molecular Medicine (2024)
-
OASL phase condensation induces amyloid-like fibrillation of RIPK3 to promote virus-induced necroptosis
Nature Cell Biology (2023)
-
Effects of toxicants on endoplasmic reticulum stress and hepatic cell fate determination
Toxicological Research (2023)
-
The role of regulated necrosis in endocrine diseases
Nature Reviews Endocrinology (2021)