Key Points
-
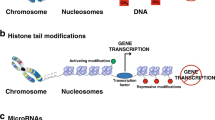
Gene expression is regulated by chromatin structure, with active regions being in an open conformation and repressed genes in a closed form. This structure is determined by DNA methylation, histone modifications, nuclear localization and nucleosome positioning.
-
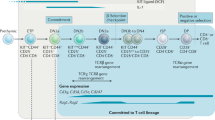
The immune system is generated from self-renewing haematopoietic stem cells that have a plastic chromatin structure and the potential to differentiate. Lineage commitment is associated with a more fixed chromatin structure, as genes associated with alternative lineages assume a closed conformation.
-
Lineage development takes place in distinct stages. During this process, changes in histone modification take place before alterations in gene usage and represent a primary mechanism that allows for the rapid generation of terminally differentiated cells.
-
Lineage commitment in vivo is unidirectional. This process appears to be driven by epigenetic changes (in DNA methylation, for example), which provide long-term stability to decisions made at an earlier stage.
-
Allelic choice in the immune system is controlled in a pre-programmed manner through a process that marks the two alleles separately, probably on the basis of differences in regional replication timing.
Abstract
Cells of the immune system are generated through a developmental cascade that begins in haematopoietic stem cells. During this process, gene expression patterns are programmed in a series of stages that bring about the restriction of cell potential, ultimately leading to the formation of specialized innate immune cells and mature lymphocytes that express antigen receptors. These events involve the regulation of both gene expression and DNA recombination, mainly through the control of chromatin accessibility. In this Review, we describe the epigenetic changes that mediate this complex differentiation process and try to understand the logic of the programming mechanism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bergman, Y. & Cedar, H. Epigenetic control of recombination in the immune system. Semin. Immunol. 22, 323–329 (2010).
Weintraub, H. & Groudine, M. Chromosomal subunits in active genes have an altered conformation. Science 193, 848–856 (1976).
Axel, R., Cedar, H. & Felsenfeld, G. The synthesis of globin RNA from duck reticulocyte chromatin in vitro. Proc. Natl Acad. Sci. USA 70, 2029–2032 (1973).
Jhunjhunwala, S., van Zelm, M. C., Peak, M. M. & Murre, C. Chromatin architecture and the generation of antigen receptor diversity. Cell 138, 435–448 (2009).
Hand, R. Eucaryotic DNA: organization of the genome for replication. Cell 15, 317–325 (1978).
Goren, A. & Cedar, H. Replicating by the clock. Nature Rev. Mol. Cell Biol. 4, 25–32 (2003).
Kerem, B. S., Goitein, R., Diamond, G., Cedar, H. & Marcus, M. Mapping of DNase-I sensitive regions on mitotic chromosomes. Cell 38, 493–499 (1984).
Zhang, J., Feng, X., Hashimshony, T., Keshet, I. & Cedar, H. Establishment of transcriptional competence in early and late S-phase. Nature 420, 198–202 (2002).
Brandeis, M. et al. Sp1 elements protect a CpG island from de novo methylation. Nature 371, 435–438 (1994).
Macleod, D., Charlton, J., Mullins, J. & Bird, A. P. Sp1 sites in the mouse Aprt gene promoter are required to prevent methylation of the CpG island. Genes Dev. 8, 2282–2292 (1994).
Keshet, I., Lieman-Hurwitz, J. & Cedar, H. DNA methylation affects the formation of active chromatin. Cell 44, 535–543 (1986).
Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 16, 6–21 (2002).
Campos, E. I. & Reinberg, D. Histones: annotating chromatin. Annu. Rev. Genet. 43, 559–599 (2009).
Imhof, A. Epigenetic regulators and histone modification. Brief. Funct. Genomic. Proteomic. 5, 222–227 (2006).
Hansen, K. H. et al. A model for transmission of the H3K27me3 epigenetic mark. Nature Cell Biol. 10, 1291–1300 (2008).
Bannister, A. J. et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410, 120–124 (2001).
Margueron, R. et al. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature 461, 762–767 (2009).
Xu, C. et al. Binding of different histone marks differentially regulates the activity and specificity of polycomb repressive complex 2 (PRC2). Proc. Natl Acad. Sci. USA 107, 19266–19271 (2010).
Kaplan, N. et al. The DNA-encoded nucleosome organization of a eukaryotic genome. Nature 458, 362–366 (2009).
Zhang, Y. et al. Intrinsic histone–DNA interactions are not the major determinant of nucleosome positions in vivo. Nature Struct. Mol. Biol. 16, 847–852 (2009).
Gao, H. et al. Opposing effects of SWI/SNF and Mi-2/NuRD chromatin remodeling complexes on epigenetic reprogramming by EBF and Pax5. Proc. Natl Acad. Sci. USA 106, 11258–11263 (2009).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Lin, Y. C. et al. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nature Immunol. 11, 635–643 (2010).
Treiber, T. et al. Early B cell factor 1 regulates B cell gene networks by activation, repression, and transcription-independent poising of chromatin. Immunity 32, 714–725 (2010).
Li, L. et al. Nuclear adaptor Ldb1 regulates a transcriptional program essential for the maintenance of hematopoietic stem cells. Nature Immunol. 12, 129–136 (2011). References 22–25 described the genome-wide localization of transcription factors that are key determinants in the haematopoietic system.
Feldman, N. et al. G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nature Cell Biol. 8, 188–194 (2006).
Epsztejn-Litman, S. et al. De novo DNA methylation promoted by G9a prevents reprogramming of embryonically silenced genes. Nature Struct. Mol. Biol. 15, 1176–1183 (2008).
Kim, J. B. et al. Direct reprogramming of human neural stem cells by OCT4. Nature 461, 649–643 (2009).
Chao, M. P., Seita, J. & Weissman, I. L. Establishment of a normal hematopoietic and leukemia stem cell hierarchy. Cold Spring Harb. Symp. Quant. Biol. 73, 439–449 (2008).
Morrison, S. J. & Weissman, I. L. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity 1, 661–673 (1994).
Christensen, J. L. & Weissman, I. L. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Natl Acad. Sci. USA 98, 14541–14546 (2001).
Serwold, T., Ehrlich, L. I. & Weissman, I. L. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood 113, 807–815 (2009).
Karsunky, H., Inlay, M. A., Serwold, T., Bhattacharya, D. & Weissman, I. L. Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages. Blood 111, 5562–5570 (2008).
Kondo, M., Weissman, I. L. & Akashi, K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91, 661–672 (1997).
Akashi, K., Traver, D., Miyamoto, T. & Weissman, I. L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000).
Orkin, S. H. & Zon, L. I. SnapShot: hematopoiesis. Cell 132, 712 (2008).
Luc, S., Buza-Vidas, N. & Jacobsen, S. E. Delineating the cellular pathways of hematopoietic lineage commitment. Semin. Immunol. 20, 213–220 (2008).
Zandi, S., Bryder, D. & Sigvardsson, M. Load and lock: the molecular mechanisms of B-lymphocyte commitment. Immunol. Rev. 238, 47–62 (2010).
Inlay, M. A. et al. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 23, 2376–2381 (2009).
Adolfsson, J. et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential: a revised road map for adult blood lineage commitment. Cell 121, 295–306 (2005).
Pronk, C. J. et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell 1, 428–442 (2007).
Kiel, M. J., Yilmaz, O. H., Iwashita, T., Terhorst, C. & Morrison, S. J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Challen, G. A., Boles, N. C., Chambers, S. M. & Goodell, M. A. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-β1. Cell Stem Cell 6, 265–278 (2010).
Arinobu, Y. et al. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell 1, 416–427 (2007).
Laslo, P., Pongubala, J. M., Lancki, D. W. & Singh, H. Gene regulatory networks directing myeloid and lymphoid cell fates within the immune system. Semin. Immunol. 20, 228–235 (2008).
Mandel, E. M. & Grosschedl, R. Transcription control of early B cell differentiation. Curr. Opin. Immunol. 22, 161–167 (2010).
Novershtern, N. et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell 144, 296–309 (2011). This paper reported a comprehensive transcriptome analysis of 38 distinct purified populations of human haematopoietic cells. These data, together with sophisticated bioinformatics analysis and high-throughput DNA binding data for multiple transcription factors, provided a resource for studying gene regulation in haematopoiesis.
Ji, H. et al. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature 467, 338–342 (2010). This study was the first to provide comprehensive DNA methylation maps for MPPs, CLPs, CMPs, GMPs and thymocyte progenitors, revealing a programme of DNA methylation changes during lineage-specific differentiation.
Borgel, J. et al. Targets and dynamics of promoter DNA methylation during early mouse development. Nature Genet. 42, 1093–1100 (2010).
Kirillov, A. et al. A role for nuclear NF-κB in B-cell-specific demethylation of the Igκ locus. Nature Genet. 13, 435–441 (1996).
Tahiliani, M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 (2009).
Wu, S. C. & Zhang, Y. Active DNA demethylation: many roads lead to Rome. Nature Rev. Mol. Cell Biol. 11, 607–620 (2010).
Guo, J. U., Su, Y., Zhong, C., Ming, G. L. & Song, H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 145, 423–434 (2011).
Langemeijer, S. M. et al. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nature Genet. 41, 838–842 (2009).
Delhommeau, F. et al. Mutation in TET2 in myeloid cancers. N. Engl. J. Med. 360, 2289–2301 (2009).
Levine, R. L. & Carroll, M. A common genetic mechanism in malignant bone marrow diseases. N. Engl. J. Med. 360, 2355–2357 (2009).
Ko, M. et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature 468, 839–843 (2010).
Figueroa, M. E. et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell 17, 13–27 (2010).
Pillay, L. M., Forrester, A. M., Erickson, T., Berman, J. N. & Waskiewicz, A. J. The Hox cofactors Meis1 and Pbx act upstream of gata1 to regulate primitive hematopoiesis. Dev. Biol. 340, 306–317 (2010).
Vire, E. et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 439, 871–874 (2006).
Broske, A. M. et al. DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nature Genet. 41, 1207–1215 (2009).
Trowbridge, J. J., Snow, J. W., Kim, J. & Orkin, S. H. DNA methyltransferase 1 is essential for and uniquely regulates haematopoietic stem and progenitor cells. Cell Stem Cell 5, 442–449 (2009). References 61 and 62 showed a crucial role for DNMT1 in controlling the self-renewal of HSCs and commitment to lymphoid versus myeloid differentiation.
Gaudet, F. et al. Induction of tumors in mice by genomic hypomethylation. Science 300, 489–492 (2003).
Tadokoro, Y., Ema, H., Okano, M., Li, E. & Nakauchi, H. De novo DNA methyltransferase is essential for self-renewal, but not for differentiation, in hematopoietic stem cells. J. Exp. Med. 204, 715–722 (2007).
Schwartz, Y. B. & Pirrotta, V. Polycomb complexes and epigenetic states. Curr. Opin. Cell Biol. 20, 266–273 (2008).
Pietersen, A. M. & van Lohuizen, M. Stem cell regulation by polycomb repressors: postponing commitment. Curr. Opin. Cell Biol. 20, 201–207 (2008).
Muller, J. & Kassis, J. A. Polycomb response elements and targeting of Polycomb group proteins in Drosophila. Curr. Opin. Genet. Dev. 16, 476–484 (2006).
Sing, A. et al. A vertebrate Polycomb response element governs segmentation of the posterior hindbrain. Cell 138, 885–897 (2009).
Woo, C. J., Kharchenko, P. V., Daheron, L., Park, P. J. & Kingston, R. E. A region of the human HOXD cluster that confers polycomb-group responsiveness. Cell 140, 99–110 (2010).
Landeira, D. & Fisher, A. G. Inactive yet indispensable: the tale of Jarid2. Trends Cell Biol. 21, 74–80 (2011).
Zhao, J. et al. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol. Cell 40, 939–953 (2010).
Kanhere, A. et al. Short RNAs are transcribed from repressed polycomb target genes and interact with polycomb repressive complex-2. Mol. Cell 38, 675–688 (2010).
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006). This study identified 'bivalent domains' as regions marked with H3K27me3 and H3K4me3 that coincide with silenced developmental genes in ES cells, and suggested that these genes are poised for activation following differentiation.
Pan, G. et al. Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell 1, 299–312 (2007).
Zhao, X. D. et al. Whole-genome mapping of histone H3 Lys4 and 27 trimethylations reveals distinct genomic compartments in human embryonic stem cells. Cell Stem Cell 1, 286–298 (2007).
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007).
Barski, A. et al. High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837 (2007).
Cui, K. et al. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell 4, 80–93 (2009).
Weishaupt, H., Sigvardsson, M. & Attema, J. L. Epigenetic chromatin states uniquely define the developmental plasticity of murine hematopoietic stem cells. Blood 115, 247–256 (2010). References 78 and 79 identified genome-wide changes in gene expression and histone modification during haematopoiesis, emphasizing that developmentally regulated genes are specifically epigenetically primed in HSCs for subsequent activation or repression during lineage commitment.
Adli, M., Zhu, J. & Bernstein, B. E. Genome-wide chromatin maps derived from limited numbers of hematopoietic progenitors. Nature Methods 7, 615–618 (2010).
Iwama, A. et al. Enhanced self-renewal of hematopoietic stem cells mediated by the polycomb gene product Bmi-1. Immunity 21, 843–851 (2004).
Oguro, H. et al. Poised lineage specification in multipotential hematopoietic stem and progenitor cells by the polycomb protein Bmi1. Cell Stem Cell 6, 279–286 (2010). This paper reported that BMI1 is crucial for the multipotency of HSCs and MPPs.Loss of BMI1 results in accelerated lymphoid specification owing to premature expression of Ebf1 and Pax5 , which are bivalently marked in HSCs.
Attema, J. L. et al. Epigenetic characterization of hematopoietic stem cell differentiation using miniChIP and bisulfite sequencing analysis. Proc. Natl Acad. Sci. USA 104, 12371–12376 (2007).
Orford, K. et al. Differential H3K4 methylation identifies developmentally poised hematopoietic genes. Dev. Cell 14, 798–809 (2008).
Maës, J. et al. Lymphoid-affiliated genes are associated with active histone modifications in human hematopoietic stem cells. Blood 112, 2722–2729 (2008).
Pekowska, A., Benoukraf, T., Ferrier, P. & Spicuglia, S. A unique H3K4me2 profile marks tissue-specific gene regulation. Genome Res. 20, 1493–1502 (2010).
Ramirez, J., Lukin, K. & Hagman, J. From hematopoietic progenitors to B cells: mechanisms of lineage restriction and commitment. Curr. Opin. Immunol. 22, 177–184 (2010).
Wei, G. et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009).
Decker, T. et al. Stepwise activation of enhancer and promoter regions of the B cell commitment gene Pax5 in early lymphopoiesis. Immunity 30, 508–520 (2009).
Walter, K., Bonifer, C. & Tagoh, H. Stem cell-specific epigenetic priming and B cell-specific transcriptional activation at the mouse Cd19 locus. Blood 112, 1673–1682 (2008).
Xu, J. et al. Pioneer factor interactions and unmethylated CpG dinucleotides mark silent tissue-specific enhancers in embryonic stem cells. Proc. Natl Acad. Sci. USA 104, 12377–12382 (2007).
Xu, J. et al. Transcriptional competence and the active marking of tissue-specific enhancers by defined transcription factors in embryonic and induced pluripotent stem cells. Genes Dev. 23, 2824–2838 (2009). This study demonstrated that enhancer sequences are already epigenetically pre-marked in ES cells.These marks may be essential for maintaining competence for transcriptional activation.
Yancopoulos, G. D., Blackwell, T. K., Suh, H., Hood, L. & Alt, F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell 44, 251–259 (1986). This study was the first to suggest that the control of V(D)J recombination is mediated by regulating the accessibility of the different immune receptor loci.
Maës, J. et al. Chromatin remodeling at the Ig loci prior to V(D)J recombination. J. Immunol. 167, 866–874 (2001).
Chowdhury, D. & Sen, R. Stepwise activation of the immunoglobulin μ heavy chain gene locus. EMBO J. 20, 6394–6403 (2001).
Hesslein, D. G. et al. Pax5 is required for recombination of transcribed, acetylated, 5′ IgH V gene segments. Genes Dev. 17, 37–42 (2003).
Maes, J. et al. Activation of V(D)J recombination at the IgH chain JH locus occurs within a 6-kilobase chromatin domain and is associated with nucleosomal remodeling. J. Immunol. 176, 5409–5417 (2006).
Morshead, K. B., Ciccone, D. N., Taverna, S. D., Allis, C. D. & Oettinger, M. A. Antigen receptor loci poised for V(D)J rearrangement are broadly associated with BRG1 and flanked by peaks of histone H3 dimethylated at lysine 4. Proc. Natl Acad. Sci. USA 100, 11577–11582 (2003).
Chakraborty, T. et al. Repeat organization and epigenetic regulation of the DH-Cμ domain of the immunoglobulin heavy-chain gene locus. Mol. Cell 27, 842–850 (2007).
Goldmit, M. et al. Epigenetic ontogeny of the Igk locus during B cell development. Nature Immunol. 6, 198–203 (2005). This study showed that individual Igk alleles are epigenetically marked prior to recombination, suggesting a molecular basis for allelic exclusion.
Xu, C. R. & Feeney, A. J. The epigenetic profile of Ig genes is dynamically regulated during B cell differentiation and is modulated by pre-B cell receptor signaling. J. Immunol. 182, 1362–1369 (2009).
Fitzsimmons, S. P., Bernstein, R. M., Max, E. E., Skok, J. A. & Shapiro, M. A. Dynamic changes in accessibility, nuclear positioning, recombination, and transcription at the Ig κ locus. J. Immunol. 179, 5264–5273 (2007).
Liu, Y., Subrahmanyam, R., Chakraborty, T., Sen, R. & Desiderio, S. A plant homeodomain in RAG-2 that binds hypermethylated lysine 4 of histone H3 is necessary for efficient antigen-receptor-gene rearrangement. Immunity 27, 561–571 (2007).
Matthews, A. G. et al. RAG2 PHD finger couples histone H3 lysine 4 trimethylation with V(D)J recombination. Nature 450, 1106–1110 (2007). References 103 and 104 showed that RAG2 specifically recognizes H3K4me3. Moreover, mutations that abrogate this recognition (found in patients with immunodeficiency syndrome) severely impair V(D)J recombination in vivo . These findings highlight the link between chromatin structure and immunoglobulin gene rearrangement.
Shimazaki, N., Tsai, A. G. & Lieber, M. R. H3K4me3 stimulates the V(D)J RAG complex for both nicking and hairpinning in trans in addition to tethering in cis: implications for translocations. Mol. Cell 34, 535–544 (2009).
Ji, Y. et al. The in vivo pattern of binding of RAG1 and RAG2 to antigen receptor loci. Cell 141, 419–431 (2010). This is the first study to demonstrate stage- and lineage-specific binding of RAG1 and RAG2 in vivo . These findings provide insights into how recombination takes place during normal and abnormal development.
Degner, S. C., Wong, T. P., Jankevicius, G. & Feeney, A. J. Cutting edge: developmental stage-specific recruitment of cohesin to CTCF sites throughout immunoglobulin loci during B lymphocyte development. J. Immunol. 182, 44–48 (2009).
Garrett, F. E. et al. Chromatin architecture near a potential 3′ end of the Igh locus involves modular regulation of histone modifications during B-cell development and in vivo occupancy at CTCF sites. Mol. Cell. Biol. 25, 1511–1525 (2005).
Phillips, J. E. & Corces, V. G. CTCF: master weaver of the genome. Cell 137, 1194–1211 (2009).
Jhunjhunwala, S. et al. The 3D structure of the immunoglobulin heavy-chain locus: implications for long-range genomic interactions. Cell 133, 265–279 (2008). This study showed that in pro-B cells the Igh locus undergoes conformational changes that position the entire repertoire of V H segments in close proximity to D H elements, thus permitting long-range genomic interactions to occur with high frequency.
Fuxa, M. et al. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 18, 411–422 (2004).
Liu, H. et al. Yin Yang 1 is a critical regulator of B-cell development. Genes Dev. 21, 1179–1189 (2007).
Reynaud, D. et al. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nature Immunol. 9, 927–936 (2008).
Ebert, A. et al. The distal VH gene cluster of the Igh locus contains distinct regulatory elements with Pax5 transcription factor-dependent activity in pro-B cells. Immunity 34, 175–187 (2011). This study identified a novel set of cis -regulatory sequences in the distal region of the VH locus that supported PAX5-dependent pro-B-specific generation of antisense transcripts. These elements may have a role in Igh locus V(D)J recombination.
Lennon, G. G. & Perry, R. P. Cμ-containing transcripts initiate heterogeneously within the IgH enhancer region and contain a novel 5′-nontranslatable exon. Nature 318, 475–478 (1985).
Thompson, A., Timmers, E., Schuurman, R. K. & Hendriks, R. W. Immunoglobulin heavy chain germ-line JH–Cμ transcription in human precursor B lymphocytes initiates in a unique region upstream of DQ52. Eur. J. Immunol. 25, 257–261 (1995).
Corcoran, A. E., Riddell, A., Krooshoop, D. & Venkitaraman, A. R. Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature 391, 904–907 (1998).
Martin, D. J. & van Ness, B. G. Initiation and processing of two κ immunoglobulin germ line transcripts in mouse B cells. Mol. Cell. Biol. 10, 1950–1958 (1990).
Singh, N., Bergman, Y., Cedar, H. & Chess, A. Biallelic germline transcription at the κ immunoglobulin locus. J. Exp. Med. 197, 743–750 (2003).
Bolland, D. J. et al. Antisense intergenic transcription in V(D)J recombination. Nature Immunol. 5, 630–637 (2004).
Osipovich, O. A., Subrahmanyam, R., Pierce, S., Sen, R. & Oltz, E. M. Cutting edge: SWI/SNF mediates antisense Igh transcription and locus-wide accessibility in B cell precursors. J. Immunol. 183, 1509–1513 (2009).
Featherstone, K., Wood, A. L., Bowen, A. J. & Corcoran, A. E. The mouse immunoglobulin heavy chain V-D intergenic sequence contains insulators that may regulate ordered V(D)J recombination. J. Biol. Chem. 285, 9327–9338 (2010).
Mostoslavsky, R. et al. κchain monoallelic demethylation and the establishment of allelic exclusion. Genes Dev. 12, 1801–1811 (1998).
Goldmit, M., Schlissel, M., Cedar, H. & Bergman, Y. Differential accessibility at the κ chain locus plays a role in allelic exclusion. EMBO J. 21, 5255–5261 (2002).
Fritz, E. L. & Papavasiliou, F. N. Cytidine deaminases: AIDing DNA demethylation? Genes Dev. 24, 2107–2114 (2010).
Wossidlo, M. et al. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nature Commun. 2, 241 (2011).
Coleclough, C. Chance, necessity and antibody gene dynamics. Nature 303, 23–26 (1983).
Sayegh, C., Jhunjhunwala, S., Riblet, R. & Murre, C. Visualization of looping involving the immunoglobulin heavy-chain locus in developing B cells. Genes Dev. 19, 322–327 (2005).
Hewitt, S. L. et al. RAG-1 and ATM coordinate monoallelic recombination and nuclear positioning of immunoglobulin loci. Nature Immunol. 10, 655–664 (2009).
Skok, J. A. et al. Nonequivalent nuclear location of immunoglobulin alleles in B lymphocytes. Nature Immunol. 2, 848–854 (2001).
Liu, Z. et al. A recombination silencer that specifies heterochromatin positioning and ikaros association in the immunoglobulin κ locus. Immunity 24, 405–415 (2006).
Mostoslavsky, R. et al. Asynchronous replication and allelic exclusion in the immune system. Nature 414, 221–225 (2001). This was the first study to report that the immunoglobulin and Tcrb loci replicate asynchronously. This serves as an epigenetic mark for allelic exclusion, as it is almost always the early-replicating allele that is initially selected to undergo rearrangement in B cells.
Schlimgen, R. J., Reddy, K. L., Singh, H. & Krangel, M. S. Initiation of allelic exclusion by stochastic interaction of Tcrb alleles with repressive nuclear compartments. Nature Immunol. 9, 802–809 (2008). This study showed that, in developing thymocytes, germline Tcrb alleles are associated stochastically and at high frequency with the nuclear lamina or with pericentromeric heterochromatin. The authors proposed that these interactions are crucial for the initiation of allelic exclusion.
Wang, J., Valo, Z., Smith, D. & Singer-Sam, J. Monoallelic expression of multiple genes in the CNS. PLoS ONE 2, e1293 (2007).
Gimelbrant, A., Hutchinson, J. N., Thompson, B. R. & Chess, A. Widespread monoallelic expression on human autosomes. Science 318, 1136–1140 (2007).
Alexander, M. K. et al. Differences between homologous alleles of olfactory receptor genes require the Polycomb Group protein Eed. J. Cell Biol. 179, 269–276 (2007).
Dutta, D., Ensminger, A. W., Zucker, J. P. & Chess, A. Asynchronous replication and autosome-pair non-equivalence in human embryonic stem cells. PLoS ONE 4, e4970 (2009).
Keohane, A. M., Lavender, J. S., O'Neill, L. P. & Turner, B. M. Histone acetylation and X inactivation. Dev. Genet. 22, 65–73 (1998).
Schlesinger, S., Selig, S., Bergman, Y. & Cedar, H. Allelic inactivation of rDNA loci. Genes Dev. 23, 2437–2447 (2009).
Acknowledgements
This work was supported by research grants from the Israel Academy of Sciences (to H.C. and Y.B.), the US National Institutes of Health (to Y.B.), the Israel Cancer Research Foundation (to H.C. and Y.B.) and the European Community 5th Framework Quality of Life Programme (to Y.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Epigenetic mechanisms
-
Mechanisms that confer heritable, but potentially reversible, states of gene activity that are imposed by the structure of chromatin or covalent modifications of DNA and histones.
- Histone acetylation
-
A post-translational modification of histones that is associated with regions of actively transcribed chromatin.
- CpG island
-
A DNA sequence of 0.5–2 kilobases that is rich in CpG dinucleotides. These sequences are located upstream of housekeeping genes and some tissue-specific genes. They are usually constitutively non-methylated in all animal cell types.
- Induced pluripotent stem cells
-
Self-renewing, pluripotent stem cells that are derived by the transient introduction of transgenes encoding transcription factors such as OCT4, SOX2, KLF4 and MYC (or the gene products themselves) into adult somatic cells. Only transient expression of the transgenes is required because the reprogramming factors upregulate expression of their endogenous counterparts, which subsequently maintain pluripotency.
- Polycomb repressive complex
-
A group of proteins that maintain gene expression states throughout development by regulating chromatin structure. In mammals, there are two core Polycomb complexes: Polycomb repressive complex 1 (PRC1) and PRC2. PRC1 catalyses the monoubiquitylation of histone H2A. Both complexes contribute to chromatin compaction. PRC2 harbours the EZH1 and EZH2 methyltransferases that catalyse the methylation of histone H3 at lysine 27. These two complexes are involved in differentiation, the maintenance of cell identity and proliferation, and stem cell plasticity.
- Cohesin
-
A multiprotein complex that maintains the tight association of sister chromatids. Cohesin has also been implicated in the regulation of gene transcription by contributing to DNA loop formation. It stabilizes chromatin loops organized by the binding of factors such as CTCF or the Mediator complex.
Rights and permissions
About this article
Cite this article
Cedar, H., Bergman, Y. Epigenetics of haematopoietic cell development. Nat Rev Immunol 11, 478–488 (2011). https://doi.org/10.1038/nri2991
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2991
This article is cited by
-
Bridging tissue repair and epithelial carcinogenesis: epigenetic memory and field cancerization
Cell Death & Differentiation (2024)
-
Epigenetic reprogramming of Runx3 reinforces CD8 + T-cell function and improves the clinical response to immunotherapy
Molecular Cancer (2023)
-
Mutational synergy during leukemia induction remodels chromatin accessibility, histone modifications and three-dimensional DNA topology to alter gene expression
Nature Genetics (2021)
-
Preterm birth and sustained inflammation: consequences for the neonate
Seminars in Immunopathology (2020)
-
Tracking hematopoietic precursor division ex vivo in real time
Stem Cell Research & Therapy (2018)