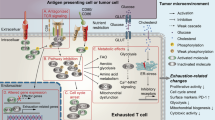
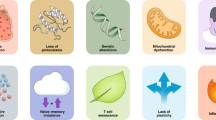
Abstract
Can the immune system be reactivated continuously throughout the lifetime of an organism or is there a finite point at which repeated antigenic challenge leads to the loss of lymphocyte function or the cells themselves or both? Replicative senescence and exhaustion are processes that control T cell proliferative activity and function; however, there is considerable confusion over the relationship between these two intrinsic cellular control mechanisms. In this Opinion article, we compare the molecular regulation of senescence and exhaustion in T cells. Available data suggest that both processes are regulated independently of each other and that it may be safer to block exhaustion than senescence to enhance immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Blackburn, S. D. et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature Immunol. 10, 29–37 (2009).
Freeman, G. J., Wherry, E. J., Ahmed, R. & Sharpe, A. H. Reinvigorating exhausted HIV-specific T cells via PD-1–PD-1 ligand blockade. J. Exp. Med. 203, 2223–2227 (2006).
Day, C. L. et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354 (2006).
Akbar, A. N. & Vukmanovic-Stejic, M. Telomerase in T lymphocytes: use it and lose it? J. Immunol. 178, 6689–6694 (2007).
Hodes, R. J., Hathcock, K. S. & Weng, N. P. Telomeres in T and B cells. Nature Rev. Immunol. 2, 699–706 (2002).
Effros, R. B., Dagarag, M., Spaulding, C. & Man, J. The role of CD8+ T-cell replicative senescence in human aging. Immunol. Rev. 205, 147–157 (2005).
Voehringer, D. et al. Viral infections induce abundant numbers of senescent CD8 T cells. J. Immunol. 167, 4838–4843 (2001).
Fletcher, J. M. et al. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J. Immunol. 175, 8218–8225 (2005).
Appay, V., Almeida, J. R., Sauce, D., Autran, B. & Papagno, L. Accelerated immune senescence and HIV-1 infection. Exp. Gerontol. 42, 432–437 (2007).
d'Adda di Fagagna, F. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003).
Passos, J. F. et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 6, 347 (2010).
Akbar, A. N., Beverley, P. C. & Salmon, M. Will telomere erosion lead to a loss of T-cell memory? Nature Rev. Immunol. 4, 737–743 (2004).
Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 (1965).
Blackburn, E. H. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 579, 859–862 (2005).
Campisi, J. & d'Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nature Rev. Mol. Cell Biol. 8, 729–740 (2007).
d'Adda di Fagagna, F. Living on a break: cellular senescence as a DNA-damage response. Nature Rev. Cancer 8, 512–522 (2008).
Passos, J. F. & Von Zglinicki, T. Oxygen free radicals in cell senescence: are they signal transducers? Free Radic. Res. 40, 1277–1283 (2006).
Rufer, N. et al. Ex vivo characterization of human CD8+ T subsets with distinct replicative history and partial effector functions. Blood 102, 1779–1787 (2003).
Plunkett, F. J. et al. The loss of telomerase activity in highly differentiated CD8+CD28−CD27− T cells is associated with decreased Akt (Ser473) phosphorylation. J. Immunol. 178, 7710–7719 (2007).
Plunkett, F. J. et al. The impact of telomere erosion on memory CD8+ T cells in patients with X-linked lymphoproliferative syndrome. Mech. Ageing Dev. 126, 855–865 (2005).
Migliaccio, M., Raj, K., Menzel, O. & Rufer, N. Mechanisms that limit the in vitro proliferative potential of human CD8+ T lymphocytes. J. Immunol. 174, 3335–3343 (2005).
Akbar, A. N., Soares, M. V., Plunkett, F. J. & Salmon, M. Differential regulation of CD8+ T cell senescence in mice and men. Mech. Ageing Dev. 121, 69–76 (2000).
Itahana, K., Campisi, J. & Dimri, G. P. Mechanisms of cellular senescence in human and mouse cells. Biogerontology 5, 1–10 (2004).
Herbig, U., Jobling, W. A., Chen, B. P., Chen, D. J. & Sedivy, J. M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol. Cell 14, 501–513 (2004).
Iwasa, H., Han, J. & Ishikawa, F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells 8, 131–144 (2003).
Shiloh, Y. ATM and related protein kinases: safeguarding genome integrity. Nature Rev. Cancer 3, 155–168 (2003).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Reed, J. R. et al. Telomere erosion in memory T cells induced by telomerase inhibition at the site of antigenic challenge in vivo. J. Exp. Med. 199, 1433–1443 (2004).
Effros, R. B. Replicative senescence of CD8 T cells: effect on human ageing. Exp. Gerontol. 39, 517–524 (2004).
Nikolich-Žugich, J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nature Rev. Immunol. 8, 512–522 (2008).
Akbar, A. N. & Fletcher, J. M. Memory T cell homeostasis and senescence during aging. Curr. Opin. Immunol. 17, 480–485 (2005).
Goronzy, J. J., Lee, W. W. & Weyand, C. M. Aging and T-cell diversity. Exp. Gerontol. 42, 400–406 (2007).
Spaulding, C., Guo, W. & Effros, R. B. Resistance to apoptosis in human CD8+ T cells that reach replicative senescence after multiple rounds of antigen-specific proliferation. Exp. Gerontol. 34, 633–644 (1999).
Pawelec, G., Wagner, W., Adibzadeh, M. & Engel, A. T cell immunosenescence in vitro and in vivo. Exp. Gerontol. 34, 419–429 (1999).
Libri, V. et al. Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+CD45RA+CD27− T cells: the potential involvement of interleukin-7 in this process. Immunology 132, 326–339 (2011).
Appay, V. et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nature Med. 8, 379–385 (2002).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22, 745–763 (2004).
Hamann, D. et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186, 1407–1418 (1997).
Roth, A. et al. Telomerase levels control the lifespan of human T lymphocytes. Blood 102, 849–857 (2003).
Faragher, R. G. & Kipling, D. How might replicative senescence contribute to human ageing? Bioessays 20, 985–991 (1998).
Akbar, A. N. & Salmon, M. Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol. Today 18, 72–76 (1997).
Pawelec, G. et al. Human immunosenescence: is it infectious? Immunol. Rev. 205, 257–268 (2005).
Khan, N. et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J. Immunol. 169, 1984–1992 (2002).
Wills, M. R. et al. Identification of naive or antigen-experienced human CD8+ T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8+ T cell response. J. Immunol. 168, 5455–5464 (2002).
Das, A. et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J. Exp. Med. 205, 2111–2124 (2008).
Hoare, M. et al. CD4+ T-lymphocyte telomere length is related to fibrosis stage, clinical outcome and treatment response in chronic hepatitis C virus infection. J. Hepatol. 53, 252–260 (2010).
Koch, S. et al. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun. Ageing 5, 6 (2008).
Mountz, J. D., Wu, J., Zhou, T. & Hsu, H. C. Cell death and longevity: implications of Fas-mediated apoptosis in T-cell senescence. Immunol. Rev. 160, 19–30 (1997).
Beausejour, C. M. et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 22, 4212–4222 (2003).
Davis, T. et al. Synthesis and in vivo activity of MK2 and MK2 substrate-selective p38αMAPK inhibitors in Werner syndrome cells. Bioorg. Med. Chem. Lett. 17, 6832–6835 (2007).
Ono, K. & Han, J. The p38 signal transduction pathway: activation and function. Cell Signal. 12, 1–13 (2000).
Zajac, A. J. et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998).
Velu, V. et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458, 206–210 (2009).
Urbani, S. et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 80, 11398–11403 (2006).
Nakamoto, N. et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 5, e1000313 (2009).
Islam, S. A. et al. Persistence of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte clones in a subject with rapid disease progression. J. Virol. 75, 4907–4911 (2001).
Lee, P. P. et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nature Med. 5, 677–685 (1999).
Wherry, E. J., Blattman, J. N., Murali-Krishna, K., van der Most, R. & Ahmed, R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003).
Richter, K., Agnellini, P. & Oxenius, A. On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int. Immunol. 22, 13–23 (2010).
Klenerman, P. & Hill, A. T cells and viral persistence: lessons from diverse infections. Nature Immunol. 6, 873–879 (2005).
Sharpe, A. H., Wherry, E. J., Ahmed, R. & Freeman, G. J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nature Immunol. 8, 239–245 (2007).
Wherry, E. J., Barber, D. L., Kaech, S. M., Blattman, J. N. & Ahmed, R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl Acad. Sci. USA 101, 16004–16009 (2004).
Shin, H., Blackburn, S. D., Blattman, J. N. & Wherry, E. J. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204, 941–949 (2007).
Petrovas, C. et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203, 2281–2292 (2006).
Trautmann, L. et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nature Med. 12, 1198–1202 (2006).
Boni, C. et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81, 4215–4225 (2007).
Boettler, T. et al. Expression of the interleukin-7 receptor α chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J. Virol. 80, 3532–3540 (2006).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Kaufmann, D. E. et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nature Immunol. 8, 1246–1254 (2007).
Jones, R. B. et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 205, 2763–2779 (2008).
Fourcade, J. et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 207, 2175–2186 (2010).
Sakuishi, K. et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194 (2010).
Yi, J. S., Cox, M. A. & Zajac, A. J. T-cell exhaustion: characteristics, causes and conversion. Immunology 129, 474–481 (2010).
Greenwald, R. J., Freeman, G. J. & Sharpe, A. H. The B7 family revisited. Annu. Rev. Immunol. 23, 515–548 (2005).
Czesnikiewicz-Guzik, M. et al. T cell subset-specific susceptibility to aging. Clin. Immunol. 127, 107–118 (2008).
Fann, M. et al. Gene expression characteristics of CD28null memory phenotype CD8+ T cells and its implication in T-cell aging. Immunol. Rev. 205, 190–206 (2005).
Cao, J. N., Gollapudi, S., Sharman, E. H., Jia, Z. & Gupta, S. Age-related alterations of gene expression patterns in human CD8+ T cells. Aging Cell 9, 19–31 (2010).
Sharpe, A. H. & Freeman, G. J. The B7–CD28 superfamily. Nature Rev. Immunol. 2, 116–126 (2002).
Rudd, C. E., Taylor, A. & Schneider, H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 229, 12–26 (2009).
Parry, R. V. et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 25, 9543–9553 (2005).
Henson, S. M. et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood 113, 6619–6628 (2009).
Ouyang, Q. et al. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1). Exp. Gerontol. 38, 911–920 (2003).
Voehringer, D., Koschella, M. & Pircher, H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood 100, 3698–3702 (2002).
Grayson, J. M., Weant, A. E., Holbrook, B. C. & Hildeman, D. Role of Bim in regulating CD8+ T-cell responses during chronic viral infection. J. Virol. 80, 8627–8638 (2006).
Lopes, A. R. et al. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J. Clin. Invest. 118, 1835–1845 (2008).
Shin, H. et al. A role for the transcriptional repressor Blimp-1 in CD8+ T cell exhaustion during chronic viral infection. Immunity 31, 309–320 (2009).
Angelosanto, J. M. & Wherry, E. J. Transcription factor regulation of CD8+ T-cell memory and exhaustion. Immunol. Rev. 236, 167–175 (2010).
Fauce, S. R. et al. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J. Immunol. 181, 7400–7406 (2008).
van de Berg, P. J. et al. Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J. Immunol. 184, 3417–3423 (2010).
Lichterfeld, M. et al. Telomerase activity of HIV-1-specific CD8+ T cells: constitutive up-regulation in controllers and selective increase by blockade of PD ligand 1 in progressors. Blood 112, 3679–3687 (2008).
Dagarag, M., Evazyan, T., Rao, N. & Effros, R. B. Genetic manipulation of telomerase in HIV-specific CD8+ T cells: enhanced antiviral functions accompany the increased proliferative potential and telomere length stabilization. J. Immunol. 173, 6303–6311 (2004).
Henson, S. M. & Akbar, A. N. KLRG1—more than a marker for T cell senescence. Age 31, 285–291 (2009).
Medema, R. H., Kops, G. J., Bos, J. L. & Burgering, B. M. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404, 782–787 (2000).
Greenwald, R. J., Latchman, Y. E. & Sharpe, A. H. Negative co-receptors on lymphocytes. Curr. Opin. Immunol. 14, 391–396 (2002).
Acknowledgements
Work leading to this Review was funded by the British Biotechnology and Biological Sciences Research Council and the Wellcome Trust ViP Scheme. We also wish to thank numerous colleagues for extensive discussions that helped in the production of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Akbar, A., Henson, S. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity?. Nat Rev Immunol 11, 289–295 (2011). https://doi.org/10.1038/nri2959
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2959
This article is cited by
-
T-cell lymphocytes’ aging clock: telomeres, telomerase and aging
Biogerontology (2024)
-
Systemic immune response in young and elderly patients after traumatic brain injury
Immunity & Ageing (2023)
-
Immunological factors linked to geographical variation in vaccine responses
Nature Reviews Immunology (2023)
-
Functional T cells are capable of supernumerary cell division and longevity
Nature (2023)
-
CTLA4 protects against maladaptive cytotoxicity during the differentiation of effector and follicular CD4+ T cells
Cellular & Molecular Immunology (2023)