Key Points
-
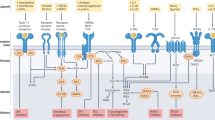
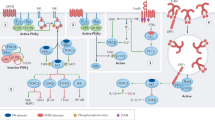
By functioning as a soluble analogue of the membrane lipid phosphatidylinositol-3,4,5-trisphosphate (here termed PtdInsP3), inositol-1,3,4,5-tetrakisphosphate (here termed InsP4) can control the functions of phosphoinositide 3-kinases (PI3Ks), phosphatase and tensin homologue (PTEN), SH2-domain-containing inositol-5-phosphatase 1 (SHIP1) and SHIP2 — the kinases that produce and the phosphatases that metabolize PtdInsP3 in lymphocytes. InsP4 probably has additional functions that might include those of the many InsP4 derivatives. In the best understood mechanism so far, InsP4 regulates the membrane recruitment of proteins by promoting or inhibiting the interactions of their pleckstrin-homology (PH) domains with PtdInsP3.
-
In mouse thymocytes, positive regulation of inducible T cell kinase (ITK) membrane recruitment by InsP4 establishes a feedback loop of phospholipase Cγ1 activation downstream of the T cell receptor that is essential for the production of sufficient amounts of the second messenger lipid diacylglycerol to trigger positive selection and the generation of a mature T cell repertoire. These findings identified the first physiological function of InsP4 as an essential soluble messenger molecule, two decades after its original discovery.
-
Unexpectedly, defective function of the InsP4-producing kinase ITPKC in humans might result in peripheral T cell hyperactivation and contribute to Kawasaki disease, which is the leading cause of acquired heart disease among children in developed countries.
-
The InsP4-producing kinase ITPKB is required for B cell development and functional competence, as shown by the B cell developmental defects and anergy in Itpkb−/− mice. The mechanisms through which ITPKB controls B cell development are unclear but might include inhibition of store-operated Ca2+ entry (SOCE) by InsP4 through an as yet unknown mechanism.
-
ITPKB limits SOCE and PtdInsP3-mediated membrane recruitment of the PI3K effector kinase AKT in neutrophils. Defects in these processes and in the production of opsonizing IgG by B cells probably underlie a complex phenotype of increased neutrophil chemotaxis and superoxide production, but decreased neutrophil viability and bacterial clearance, in an acute peritonitis model in Itpkb−/− mice.
-
As key regulators of PI3K function with essential roles in the adaptive and innate immune system and unique structural features that facilitate the development of selective inhibitors, the InsP4-producing ITPKs are attractive targets for the development of novel anti-inflammatory therapies, improved vaccination strategies or methods to overcome immunodeficiencies. However, more detailed studies in relevant disease models and with proof-of-concept small molecule inhibitors are required to establish indications, evaluate potential side effects and address toxicity concerns.
Abstract
The membrane lipid phosphatidylinositol-3,4,5-trisphosphate (PtdInsP3) regulates membrane receptor signalling in many cells, including immunoreceptor signalling. Here, we review recent data that have indicated essential roles for the soluble PtdInsP3 analogue inositol-1,3,4,5-tetrakisphosphate (InsP4) in T cell, B cell and neutrophil development and function. Decreased InsP4 production in leukocytes causes immunodeficiency in mice and might contribute to inflammatory vasculitis in Kawasaki disease in humans. InsP4-producing kinases could therefore provide attractive drug targets for inflammatory and infectious diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
York, J. D. Regulation of nuclear processes by inositol polyphosphates. Biochim. Biophys. Acta 1761, 552–559 (2006).
Michell, R. H., Heath, V. L., Lemmon, M. A. & Dove, S. K. Phosphatidylinositol 3,5-bisphosphate: metabolism and cellular functions. Trends Biochem. Sci. 31, 52–63 (2006). This notable review proposed a unified standard nomenclature for inositol phosphates.
Streb, H., Irvine, R. F., Berridge, M. J. & Schulz, I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306, 67–69 (1983). This seminal publication first described that Ins(1,4,5)P 3 functions as a second messenger that mediates Ca2+ release in mammalian cells.
Skwarek, L. C. & Boulianne, G. L. Great expectations for PIP: phosphoinositides as regulators of signaling during development and disease. Dev. Cell 16, 12–20 (2009).
Fruman, D. A. & Bismuth, G. Fine tuning the immune response with PI3K. Immunol. Rev. 228, 253–272 (2009).
Buitenhuis, M. & Coffer, P. J. The role of the PI3K–PKB signaling module in regulation of hematopoiesis. Cell Cycle 8, 560–566 (2009).
Rommel, C., Camps, M. & Ji, H. PI3Kδ and PI3Kγ: partners in crime in inflammation in rheumatoid arthritis and beyond? Nature Rev. Immunol. 7, 191–201 (2007).
Hawkins, P. T. & Stephens, L. R. PI3Kγ is a key regulator of inflammatory responses and cardiovascular homeostasis. Science 318, 64–66 (2007).
Weichhart, T. & Saemann, M. D. The PI3K/Akt/mTOR pathway in innate immune cells: emerging therapeutic applications. Ann. Rheum. Dis. 67, 70–74 (2008).
Harris, S. J., Parry, R. V., Westwick, J. & Ward, S. G. Phosphoinositide lipid phosphatases: natural regulators of phosphoinositide 3-kinase signaling in T lymphocytes. J. Biol. Chem. 283, 2465–2469 (2008).
Irvine, R. F., Lloyd-Burton, S. M., Yu, J. C., Letcher, A. J. & Schell, M. J. The regulation and function of inositol 1,4,5-trisphosphate 3-kinases. Adv. Enzyme Regul. 46, 314–323 (2006).
Pattni, K. & Banting, G. Ins(1,4,5)P3 metabolism and the family of IP3-3Kinases. Cell Signal. 16, 643–654 (2004).
Nalaskowski, M. M. & Mayr, G. W. The families of kinases removing the Ca2+ releasing second messenger Ins(1,4,5)P3 . Curr. Mol. Med. 4, 277–290 (2004).
Schell, M. J. Inositol trisphosphate 3-kinases: focus on immune and neuronal signaling. Cell. Mol. Life Sci. 12 Jan 2010 (doi: 10.1007/s00018-009-0238-0235).
Xia, H. J. & Yang, G. Inositol 1,4,5-trisphosphate 3-kinases: functions and regulations. Cell Res. 15, 83–91 (2005).
Miller, A. T. et al. Production of Ins(1,3,4,5)P4 mediated by the kinase Itpkb inhibits store-operated calcium channels and regulates B cell selection and activation. Nature Immunol. 8, 514–521 (2007). References 16 and 21 describe the effects of Itpkb deficiency on B cell development and function in a non-BCR-transgenic background. Reference 16 showed that increased BCR-induced SOCE and B cell anergy are key components of the Itpkb−/− mouse phenotype.
Huang, Y. H. et al. Positive regulation of Itk PH domain function by soluble IP4. Science 316, 886–889 (2007). Reference 17 described the identification of ITK as the first physiological InsP 4 effector. InsP 4 promoted ITK PH domain binding to the membrane-bound lipid PtdInsP 3 , probably through a cooperative allosteric mechanism involving ITK PH domain oligomerization. This is essential for thymocyte positive selection.
Wen, B. G. et al. Inositol (1,4,5) trisphosphate 3 kinase B controls positive selection of T cells and modulates Erk activity. Proc. Natl Acad. Sci. USA 101, 5604–5609 (2004).
Miller, A. T., Beisner, D. R., Liu, D. & Cooke, M. P. Inositol 1,4,5-trisphosphate 3-kinase B is a negative regulator of BCR signaling that controls B cell selection and tolerance induction. J. Immunol. 182, 4696–4704 (2009). This study follows up on references 16 and 21 and described the effects of Itpkb deficiency on B cell selection in BCR-transgenic mice.
Jia, Y. et al. Inositol trisphosphate 3-kinase B (InsP3KB) as a physiological modulator of myelopoiesis. Proc. Natl Acad. Sci. USA 105, 4739–4744 (2008). This study showed that Itpkb deficiency results in granulocyte–monocyte progenitor accumulation and increased neutrophil production, probably through mechanisms involving AKT hyperactivation secondary to impaired InsP 4 -mediated inhibition of interactions between the AKT PH domain and PtdInsP 3.
Marechal, Y. et al. Inositol 1,3,4,5-tetrakisphosphate controls proapoptotic Bim gene expression and survival in B cells. Proc. Natl Acad. Sci. USA 104, 13978–13983 (2007).
Jia, Y. et al. Inositol 1,3,4,5-tetrakisphosphate negatively regulates phosphatidylinositol-3,4,5- trisphosphate signaling in neutrophils. Immunity 27, 453–467 (2007). This study described, for the first time, alterations in neutrophil signalling and function in Itpkb−/− mice. The underlying mechanism probably includes impaired InsP 4 -mediated antagonism of AKT PH domain membrane recruitment through PtdIns(3,4,5)P 3 and/or PtdIns(3,4)P 2 binding in neutrophils.
Pouillon, V. et al. Inositol 1,3,4,5-tetrakisphosphate is essential for T lymphocyte development. Nature Immunol. 4, 1136–1143 (2003). This study and reference 18 showed that Itpkb deficiency blocks thymocyte positive selection. Both studies showed that TCR-induced Ca2+ mobilization in Itpkb−/− thymocytes is normal. Reference 18 also showed that the block is associated with impaired TCR-induced ERK activation.
Stokes, A. J. et al. FcɛRI control of Ras via inositol (1,4,5) trisphosphate 3-kinase and inositol tetrakisphosphate. Cell Signal. 18, 640–651 (2006). This publication used results obtained with small molecule ITPK inhibitors to suggest potential functions of ITPKs in mast cells.
Sauer, K., Huang, Y. H., Ying, H., Sandberg, M. & Mayr, G. W. Phosphoinositide analysis in lymphocyte activation. Curr. Protoc. Immunol. 11, 11.1 (2009).
Zhong, X. P., Guo, R., Zhou, H., Liu, C. & Wan, C. K. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol. Rev. 224, 249–264 (2008).
Readinger, J. A., Mueller, K. L., Venegas, A. M., Horai, R. & Schwartzberg, P. L. Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk. Immunol. Rev. 228, 93–114 (2009).
Prince, A. L., Yin, C. C., Enos, M. E., Felices, M. & Berg, L. J. The Tec kinases Itk and Rlk regulate conventional versus innate T-cell development. Immunol. Rev. 228, 115–131 (2009).
Feske, S. Calcium signalling in lymphocyte activation and disease. Nature Rev. Immunol. 7, 690–702 (2007).
Starr, T. K., Jameson, S. C. & Hogquist, K. A. Positive and negative selection of T cells. Annu. Rev. Immunol. 21, 139–176 (2003).
Lemmon, M. A. Membrane recognition by phospholipid-binding domains. Nature Rev. Mol. Cell Biol. 9, 99–111 (2008).
Juntilla, M. M. & Koretzky, G. A. Critical roles of the PI3K/Akt signaling pathway in T cell development. Immunol. Lett. 116, 104–110 (2008).
Huang, Y. H., Hoebe, K. & Sauer, K. New therapeutic targets in immune disorders: ItpkB, Orai1 and UNC93B. Expert Opin. Ther. Targets 12, 391–413 (2008).
Irvine, R. F. Inositide evolution — towards turtle domination? J. Physiol. 566, 295–300 (2005).
Otto, J. C. et al. Biochemical analysis of inositol phosphate kinases. Methods Enzymol. 434, 171–185 (2007).
Chang, S. C., Miller, A. L., Feng, Y., Wente, S. R. & Majerus, P. W. The human homolog of the rat inositol phosphate multikinase is an inositol 1,3,4,6-tetrakisphosphate 5-kinase. J. Biol. Chem. 277, 43836–43843 (2002).
Saiardi, A. et al. Mammalian inositol polyphosphate multikinase synthesizes inositol 1,4,5-trisphosphate and an inositol pyrophosphate. Proc. Natl Acad. Sci. USA 98, 2306–2311 (2001).
Nalaskowski, M. M., Deschermeier, C., Fanick, W. & Mayr, G. W. The human homologue of yeast ArgRIII protein is an inositol phosphate multikinase with predominantly nuclear localization. Biochem. J. 366, 549–556 (2002).
Seeds, A. M. & York, J. D. Inositol polyphosphate kinases: regulators of nuclear function. Biochem. Soc. Symp. 74, 183–197 (2007).
Jun, K. et al. Enhanced hippocampal CA1 LTP but normal spatial learning in inositol 1,4,5-trisphosphate 3-kinase(A)-deficient mice. Learn. Mem. 5, 317–330 (1998).
Kim, I. H. et al. Inositol 1,4,5-trisphosphate 3-kinase A functions as a scaffold for synaptic Rac signaling. J. Neurosci. 29, 14039–14049 (2009).
Onouchi, Y. et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nature Genet. 40, 35–42 (2008). This intriguing study described a human ITPKC polymorphism that may associate with Kawasaki disease — the first genetic implication of an ITPK in a human disease. It also showed that ITPKC might negatively regulate Jurkat T cell activation.
Muller, P., Kuttenkeuler, D., Gesellchen, V., Zeidler, M. P. & Boutros, M. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature 436, 871–875 (2005).
Irvine, R. F. & Schell, M. J. Back in the water: the return of the inositol phosphates. Nature Rev. Mol. Cell Biol. 2, 327–338 (2001).
Irvine, R. Inositol phosphates: does IP4 run a protection racket? Curr. Biol. 11, R172–R174 (2001).
Miller, A. T., Chamberlain, P. P. & Cooke, M. P. Beyond IP3: roles for higher order inositol phosphates in immune cell signaling. Cell Cycle 7, 463–467 (2008).
Jia, Y., Schurmans, S. & Luo, H. R. Regulation of innate immunity by inositol 1,3,4,5-tetrakisphosphate. Cell Cycle 7, 2803–2808 (2008).
Cullen, P. J. et al. Identification of a specific Ins(1,3,4,5)P4-binding protein as a member of the GAP1 family. Nature 376, 527–530 (1995).
Cozier, G. E. et al. GAP1IP4BP contains a novel group I pleckstrin homology domain that directs constitutive plasma membrane association. J. Biol. Chem. 275, 28261–28268 (2000).
Bird, G. S. & Putney, J. W. Jr. Effect of inositol 1,3,4,5-tetrakisphosphate on inositol trisphosphate-activated Ca2+ signaling in mouse lacrimal acinar cells. J. Biol. Chem. 271, 6766–6770 (1996).
Hermosura, M. C. et al. InsP4 facilitates store-operated calcium influx by inhibition of InsP3 5-phosphatase. Nature 408, 735–740 (2000).
Klein, C. & Malviya, A. N. Mechanism of nuclear calcium signaling by inositol 1,4,5-trisphosphate produced in the nucleus, nuclear located protein kinase C and cyclic AMP-dependent protein kinase. Front. Biosci. 13, 1206–1226 (2008).
Walker, S. A. et al. Analyzing the role of the putative inositol 1,3,4,5-tetrakisphosphate receptor GAP1IP4BP in intracellular Ca2+ homeostasis. J. Biol. Chem. 277, 48779–48785 (2002).
Szinyei, C., Behnisch, T., Reiser, G. & Reymann, K. G. Inositol 1,3,4,5-tetrakisphosphate enhances long-term potentiation by regulating Ca2+ entry in rat hippocampus. J. Physiol. 516, 855–868 (1999).
Luckhoff, A. & Clapham, D. E. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca2+-permeable channel. Nature 355, 356–358 (1992).
Tsubokawa, H., Oguro, K., Robinson, H. P., Masuzawa, T. & Kawai, N. Intracellular inositol 1,3,4,5-tetrakisphosphate enhances the calcium current in hippocampal CA1 neurones of the gerbil after ischaemia. J. Physiol. 497, 67–78 (1996).
Hashii, M. et al. Bradykinin B2 receptor-induced and inositol tetrakisphosphate-evoked Ca2+ entry is sensitive to a protein tyrosine phosphorylation inhibitor in ras-transformed NIH/3T3 fibroblasts. Biochem. J. 319, 649–656 (1996).
Guse, A. H., Greiner, E., Emmrich, F. & Brand, K. Mass changes of inositol 1,3,4,5,6-pentakisphosphate and inositol hexakisphosphate during cell cycle progression in rat thymocytes. J. Biol. Chem. 268, 7129–7133 (1993).
Guse, A. H., Roth, E., Broker, B. M. & Emmrich, F. Complex inositol polyphosphate response induced by co-cross-linking of CD4 and Fc γ receptors in the human monocytoid cell line U937. J. Immunol. 149, 2452–2458 (1992).
Guse, A. H. & Emmrich, F. Determination of inositol polyphosphates from human T-lymphocyte cell lines by anion-exchange high-performance liquid chromatography and post-column derivatization. J. Chromatogr. 593, 157–163 (1992).
Guse, A. H. & Emmrich, F. T-cell receptor-mediated metabolism of inositol polyphosphates in Jurkat T-lymphocytes. Identification of a D-myo-inositol 1,2,3,4,6-pentakisphosphate-2-phosphomonoesterase activity, a D-myo-inositol 1,3,4,5,6-pentakisphosphate-1/3-phosphatase activity and a D/L-myo-inositol 1,2,4,5,6-pentakisphosphate-1/3-kinase activity. J. Biol. Chem. 266, 24498–24502 (1991).
Imboden, J. B. & Pattison, G. Regulation of inositol 1,4,5-trisphosphate kinase activity after stimulation of human T cell antigen receptor. J. Clin. Invest. 79, 1538–1541 (1987).
Zilberman, Y., Howe, L. R., Moore, J. P., Hesketh, T. R. & Metcalfe, J. C. Calcium regulates inositol 1,3,4,5-tetrakisphosphate production in lysed thymocytes and in intact cells stimulated with concanavalin A. EMBO J. 6, 957–962 (1987).
Stewart, S. J., Kelley, L. L. & Powers, F. S. Production of inositol pentakisphosphate in a human T lymphocyte cell line. Biochem. Biophys. Res. Commun. 145, 895–902 (1987).
Stewart, S. J. et al. Perturbation of the human T-cell antigen receptor-T3 complex leads to the production of inositol tetrakisphosphate: evidence for conversion from inositol trisphosphate. Proc. Natl Acad. Sci. USA 83, 6098–6102 (1986).
Imboden, J. B. & Stobo, J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J. Exp. Med. 161, 446–456 (1985). References 62, 63, 65 and 66 showed for the first time that lymphocyte antigen-receptor stimulation induces the accumulation of InsPs, including InsP 3 and InsP 4 , and Ca2+ release from intracellular stores.
Ooms, L. M. et al. The role of the inositol polyphosphate 5-phosphatases in cellular function and human disease. Biochem. J. 419, 29–49 (2009).
Maehama, T. & Dixon, J. E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375–13378 (1998).
Caffrey, J. J., Darden, T., Wenk, M. R. & Shears, S. B. Expanding coincident signaling by PTEN through its inositol 1,3,4,5,6-pentakisphosphate 3-phosphatase activity. FEBS Lett. 499, 6–10 (2001).
Pesesse, X. et al. The SH2 domain containing inositol 5-phosphatase SHIP2 displays phosphatidylinositol 3,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate 5-phosphatase activity. FEBS Lett. 437, 301–303 (1998).
Erneux, C., Govaerts, C., Communi, D. & Pesesse, X. The diversity and possible functions of the inositol polyphosphate 5-phosphatases. Biochim. Biophys. Acta 1436, 185–199 (1998).
Astoul, E., Edmunds, C., Cantrell, D. A. & Ward, S. G. PI 3-K and T-cell activation: limitations of T-leukemic cell lines as signaling models. Trends Immunol. 22, 490–496 (2001).
Resnick, A. C. et al. Inositol polyphosphate multikinase is a nuclear PI3-kinase with transcriptional regulatory activity. Proc. Natl Acad. Sci. USA 102, 12783–12788 (2005).
Alcazar-Roman, A. R. & Wente, S. R. Inositol polyphosphates: a new frontier for regulating gene expression. Chromosoma 117, 1–13 (2008).
Leyman, A. et al. The absence of expression of the three isoenzymes of the inositol 1,4,5-trisphosphate 3-kinase does not prevent the formation of inositol pentakisphosphate and hexakisphosphate in mouse embryonic fibroblasts. Cell Signal. 19, 1497–1504 (2007).
Shears, S. B. Molecular basis for the integration of inositol phosphate signaling pathways via human ITPK1. Adv. Enzyme Regul. 49, 87–96 (2009).
Frederick, J. P. et al. An essential role for an inositol polyphosphate multikinase, Ipk2, in mouse embryogenesis and second messenger production. Proc. Natl Acad. Sci. USA 102, 8454–8459 (2005).
von Boehmer, H. & Kisielow, P. Negative selection of the T-cell repertoire: where and when does it occur? Immunol. Rev. 209, 284–289 (2006).
McGargill, M. A. et al. Cutting edge: extracellular signal-related kinase is not required for negative selection of developing T cells. J. Immunol. 183, 4838–4842 (2009).
Neilson, J. R., Winslow, M. M., Hur, E. M. & Crabtree, G. R. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity 20, 255–266 (2004).
Priatel, J. J., Teh, S. J., Dower, N. A., Stone, J. C. & Teh, H. S. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity 17, 617–627 (2002).
Dower, N. A. et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nature Immunol. 1, 317–321 (2000).
Qi, Q., Sahu, N. & August, A. Tec kinase Itk forms membrane clusters specifically in the vicinity of recruiting receptors. J. Biol. Chem. 281, 38529–38534 (2006).
Irvine, R. Cell signaling. The art of the soluble. Science 316, 845–846 (2007).
Chamberlain, P. P. et al. Structural insights into enzyme regulation for inositol 1,4,5-trisphosphate 3-kinase B. Biochemistry 44, 14486–14493 (2005).
Lucas, J. A., Felices, M., Evans, J. W. & Berg, L. J. Subtle defects in pre-TCR signaling in the absence of the Tec kinase Itk. J. Immunol. 179, 7561–7567 (2007).
Felices, M., Yin, C. C., Kosaka, Y., Kang, J. & Berg, L. J. Tec kinase Itk in γδT cells is pivotal for controlling IgE production in vivo. Proc. Natl Acad. Sci. USA 106, 8308–8313 (2009).
Cozier, G., Sessions, R., Bottomley, J. R., Reynolds, J. S. & Cullen, P. J. Molecular modelling and site-directed mutagenesis of the inositol 1,3,4,5-tetrakisphosphate-binding pleckstrin homology domain from the Ras GTPase-activating protein GAP1IP4BP. Biochem. J. 349, 333–342 (2000).
Lockyer, P. J. et al. Distinct subcellular localisations of the putative inositol 1,3,4,5-tetrakisphosphate receptors GAP1IP4BP and GAP1m result from the GAP1IP4BPPH domain directing plasma membrane targeting. Curr. Biol. 7, 1007–1010 (1997).
Windhorst, S. et al. Ins(1,4,5)P3 3-kinase-A overexpression induces cytoskeletal reorganization via a kinase-independent mechanism. Biochem. J. 414, 407–417 (2008).
Windhorst, S. et al. Inositol 1, 4, 5-trisphosphate 3-kinase-A is a new cell motility-promoting protein that increases the metastatic potential of tumor cells by two functional activities. J. Biol. Chem. 285, 5541–5554 (2010).
Culton, D. A. et al. Early preplasma cells define a tolerance checkpoint for autoreactive B cells. J. Immunol. 176, 790–802 (2006).
Cambier, J. C., Gauld, S. B., Merrell, K. T. & Vilen, B. J. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nature Rev. Immunol. 7, 633–643 (2007).
Cooke, M. P. et al. Immunoglobulin signal transduction guides the specificity of B cell–T cell interactions and is blocked in tolerant self-reactive B cells. J. Exp. Med. 179, 425–438 (1994).
Benschop, R. J., Brandl, E., Chan, A. C. & Cambier, J. C. Unique signaling properties of B cell antigen receptor in mature and immature B cells: implications for tolerance and activation. J. Immunol. 167, 4172–4179 (2001).
Cozier, G. E., Carlton, J., Bouyoucef, D. & Cullen, P. J. Membrane targeting by pleckstrin homology domains. Curr. Top. Microbiol. Immunol. 282, 49–88 (2004).
DiNitto, J. P. & Lambright, D. G. Membrane and juxtamembrane targeting by PH and PTB domains. Biochim. Biophys. Acta 1761, 850–867 (2006).
Park, W. S. et al. Comprehensive identification of PIP3-regulated PH domains from C. elegans to H. sapiens by model prediction and live imaging. Mol. Cell 30, 381–392 (2008).
Dillon, S. B., Murray, J. J., Verghese, M. W. & Snyderman, R. Regulation of inositol phosphate metabolism in chemoattractant-stimulated human polymorphonuclear leukocytes. Definition of distinct dephosphorylation pathways for IP3 isomers. J. Biol. Chem. 262, 11546–11552 (1987).
Feske, S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol. Rev. 231, 189–209 (2009).
Fukuda, M., Kojima, T., Kabayama, H. & Mikoshiba, K. Mutation of the pleckstrin homology domain of Bruton's tyrosine kinase in immunodeficiency impaired inositol 1,3,4,5-tetrakisphosphate binding capacity. J. Biol. Chem. 271, 30303–30306 (1996).
Rameh, L. E. et al. A comparative analysis of the phosphoinositide binding specificity of pleckstrin homology domains. J. Biol. Chem. 272, 22059–22066 (1997).
Kojima, T., Fukuda, M., Watanabe, Y., Hamazato, F. & Mikoshiba, K. Characterization of the pleckstrin homology domain of Btk as an inositol polyphosphate and phosphoinositide binding domain. Biochem. Biophys. Res. Commun. 236, 333–339 (1997).
Marone, R., Cmiljanovic, V., Giese, B. & Wymann, M. P. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim. Biophys. Acta 1784, 159–185 (2008).
Hopkins, A. L. & Groom, C. R. The druggable genome. Nature Rev. Drug Discov. 1, 727–730 (2002).
Gonzalez, B. et al. Structure of a human inositol 1,4,5-trisphosphate 3-kinase: substrate binding reveals why it is not a phosphoinositide 3-kinase. Mol. Cell 15, 689–701 (2004).
Miller, G. J. & Hurley, J. H. Crystal structure of the catalytic core of inositol 1,4,5-trisphosphate 3-kinase. Mol. Cell. 15, 703–711 (2004).
Holmes, W. & Jogl, G. Crystal structure of inositol phosphate multikinase 2 and implications for substrate specificity. J. Biol. Chem. 281, 38109–38116 (2006). References 85 and 106–108 described the crystal structures of ITPKA, ITPKB and IPMK.
Chang, Y. T. et al. Purine-based inhibitors of inositol-1,4,5-trisphosphate-3-kinase. Chembiochem. 3, 897–901 (2002).
Mayr, G. W., Windhorst, S. & Hillemeier, K. Antiproliferative plant and synthetic polyphenolics are specific inhibitors of vertebrate inositol-1,4,5-trisphosphate 3-kinases and inositol polyphosphate multikinase. J. Biol. Chem. 280, 13229–13240 (2005).
Choi, G., Chang, Y.-T., Chung, S.-K. & Choi, K. Y. Molecular interactions of all possible regioisomers of synthetic myo-inositol phosphates with inositol 1,4,5-trisphosphate 3-kinase. Bioorg. Med. Chem. Lett. 7, 2709–2714 (1997).
Poinas, A. et al. Interaction of the catalytic domain of inositol 1,4,5-trisphosphate 3-kinase A with inositol phosphate analogues. Chembiochem. 6, 1449–1457 (2005).
Liu, H. & Pope, R. M. Phagocytes: mechanisms of inflammation and tissue destruction. Rheum. Dis. Clin. North Am. 30, 19–39 (2004).
Resnick, A. C. & Saiardi, A. Inositol polyphosphate multikinase: metabolic architect of nuclear inositides. Front. Biosci. 13, 856–866 (2008).
Cunha-Melo, J. R., Dean, N. M., Moyer, J. D., Maeyama, K. & Beaven, M. A. The kinetics of phosphoinositide hydrolysis in rat basophilic leukemia (RBL-2H3) cells varies with the type of IgE receptor cross-linking agent used. J. Biol. Chem. 262, 11455–11463 (1987).
Morrison, B. H., Bauer, J. A., Kalvakolanu, D. V. & Lindner, D. J. Inositol hexakisphosphate kinase 2 mediates growth suppressive and apoptotic effects of interferon-β in ovarian carcinoma cells. J. Biol. Chem. 276, 24965–24970 (2001).
Burton, A., Hu, X. & Saiardi, A. Are inositol pyrophosphates signalling molecules? J. Cell Physiol. 220, 8–15 (2009).
Mulugu, S. et al. A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science 316, 106–109 (2007).
Barker, C. J., Illies, C., Gaboardi, G. C. & Berggren, P. O. Inositol pyrophosphates: structure, enzymology and function. Cell. Mol. Life Sci. 66, 3851–3871 (2009).
Lee, Y. S., Mulugu, S., York, J. D. & O'Shea, E. K. Regulation of a cyclin–CDK–CDK inhibitor complex by inositol pyrophosphates. Science 316, 109–112 (2007).
Stricker, R. et al. Interaction of the brain-specific protein p42IP4/centaurin-α with the peptidase nardilysin is regulated by the cognate ligands of p42IP4, PtdIns(3,4,5)P3 and Ins(1,3,4,5)P4, with stereospecificity. J. Neurochem. 98, 343–354 (2006).
Yang, S. N. et al. Inositol hexakisphosphate increases L-type Ca2+ channel activity by stimulation of adenylyl cyclase. FASEB J. 15, 1753–1763 (2001).
Larsson, O. et al. Inhibition of phosphatases and increased Ca2+ channel activity by inositol hexakisphosphate. Science 278, 471–474 (1997).
Mitchell, J. et al. An expanded biological repertoire for Ins(3,4,5,6)P4 through its modulation of ClC-3 function. Curr. Biol. 18, 1600–1605 (2008).
York, J. D., Odom, A. R., Murphy, R., Ives, E. B. & Wente, S. R. A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science 285, 96–100 (1999).
Alcazar-Roman, A. R., Tran, E. J., Guo, S. & Wente, S. R. Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. Nature Cell Biol. 8, 711–716 (2006).
Bolger, T. A., Folkmann, A. W., Tran, E. J. & Wente, S. R. The mRNA export factor Gle1 and inositol hexakisphosphate regulate distinct stages of translation. Cell 134, 624–633 (2008).
Odom, A. R., Stahlberg, A., Wente, S. R. & York, J. D. A role for nuclear inositol 1,4,5-trisphosphate kinase in transcriptional control. Science 287, 2026–2029 (2000).
Macbeth, M. R. et al. Inositol hexakisphosphate is bound in the ADAR2 core and required for RNA editing. Science 309, 1534–1539 (2005).
Steger, D. J., Haswell, E. S., Miller, A. L., Wente, S. R. & O'Shea, E. K. Regulation of chromatin remodeling by inositol polyphosphates. Science 299, 114–116 (2003).
Shen, X., Xiao, H., Ranallo, R., Wu, W. H. & Wu, C. Modulation of ATP-dependent chromatin-remodeling complexes by inositol polyphosphates. Science 299, 112–114 (2003).
Luo, H. R. et al. Inositol pyrophosphates are required for DNA hyperrecombination in protein kinase c1 mutant yeast. Biochemistry 41, 2509–2515 (2002).
Hanakahi, L. A., Bartlet-Jones, M., Chappell, C., Pappin, D. & West, S. C. Binding of inositol phosphate to DNA–PK and stimulation of double-strand break repair. Cell 102, 721–729 (2000).
Saiardi, A., Resnick, A. C., Snowman, A. M., Wendland, B. & Snyder, S. H. Inositol pyrophosphates regulate cell death and telomere length through phosphoinositide 3-kinase-related protein kinases. Proc. Natl Acad. Sci. USA 102, 1911–1914 (2005).
York, S. J., Armbruster, B. N., Greenwell, P., Petes, T. D. & York, J. D. Inositol diphosphate signaling regulates telomere length. J. Biol. Chem. 280, 4264–4269 (2005).
Saiardi, A., Bhandari, R., Resnick, A. C., Snowman, A. M. & Snyder, S. H. Phosphorylation of proteins by inositol pyrophosphates. Science 306, 2101–2105 (2004).
York, J. D. & Hunter, T. Signal transduction. Unexpected mediators of protein phosphorylation. Science 306, 2053–2055 (2004).
Lemmon, M. A. Pleckstrin homology domains: not just for phosphoinositides. Biochem. Soc. Trans. 32, 707–711 (2004).
Medyouf, H. & Ghysdael, J. The calcineurin/NFAT signaling pathway: a novel therapeutic target in leukemia and solid tumors. Cell Cycle 7, 297–303 (2008).
Huang, Y. H., Barouch-Bentov, R., Herman, A., Walker, J. & Sauer, K. Integrating traditional and postgenomic approaches to investigate lymphocyte development and function. Adv. Exp. Med. Biol. 584, 245–276 (2006).
Acknowledgements
We thank N. Gascoigne, G. Mayr and Y. Hsing Huang for critical reading of the manuscript and valuable comments. We apologize to our colleagues for citing reviews instead of many important original references owing to space considerations. K.S. is supported by NIH grants AI070845 and GM088647.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.P.C. is currently a paid employee of the Genomics Institute of the Novartis Research Foundation.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Inositol phosphate
-
(InsP). The consensus term for all phosphorylated D-myo-inositol derivatives. Mammalian cells produce many higher-order InsPs, including multiple phosphatidylinositol (PtdIns) or inositol (Ins) tris-, tetrakis- and pentakisphosphate isomers. To improve readability, we use the non-IUPAC acronyms PtdInsP3 for phosphatidylinositol-3,4,5-trisphosphate (standard abbreviation: PtdIns(3,4,5)P3), InsP3 for inositol-1,4,5-trisphosphate (standard abbreviation: Ins(1,4,5)P3) and InsP4 for inositol-1,3,4,5-tetrakisphosphate (standard abbreviation: Ins(1,3,4,5)P4) in this Review. This does not imply that PtdInsP3, InsP3 and InsP4 are the only, or the most important, InsPs.
- Phosphoinositides
-
A family of phosphorylated derivatives of the membrane lipid phosphatidylinositol that contain a hydrophobic diacylglycerol backbone esterified to a polar inositol headgroup.
- Second messenger
-
A signalling molecule such as InsP3 or DAG that is produced by a primary effector, usually an enzyme, that is in turn activated by a signal transducer downstream of a transmembrane receptor after its engagement by a first messenger ligand (for example, peptide–MHC complexes in the case of the T cell receptor). The second messenger can then activate one or more secondary effectors that further transmit signals into the cell.
- Kawasaki disease
-
(Also known as mucocutaneous lymph node syndrome). An acute, self-limited vasculitis of infants and the leading cause of acquired heart disease among children in developed countries.
- Store-operated Ca2+ entry
-
(SOCE). A mechanism used by many cells to increase intracellular Ca2+ concentrations and transduce signals in response to cell surface receptor ligation. This first results in the release of Ca2+ from intracellular stores. STIM proteins sense this depletion of Ca2+ stores and trigger the opening of plasma membrane Ca2+ channels containing ORAI proteins that mediate capacitative Ca2+ influx. In some cell types, other Ca2+ channels can contribute to SOCE.
- Pleckstrin-homology (PH) domain
-
A structurally conserved protein domain of ∼ 120 amino acids that is found at least 305 times in the human genome. About 60 PH domains mediate protein membrane recruitment or protein–protein interactions by binding to phosphoinositides or to other proteins, respectively. The functions of most PH domains are unknown.
- IL-2-inducible T cell kinase
-
(ITK). A T cell and NK cell expressed Tec family non-receptor protein tyrosine kinase that is involved in T cell receptor signalling.
- Long-term potentiation
-
A long-lasting enhancement of synaptic transmission between two neurons thought to underlie learning and memory.
- Feedback loop
-
A cause and effect circuit in which several processes occur in sequence and in which the output of a downstream process promotes or inhibits an upstream process, forming a circular signalling loop. Inhibitory loops (negative feedback) can increase the stability and accuracy of a signalling system by correcting unwanted changes. Activation loops (positive feedback) can amplify a signal by increasing the gain of the signal-transducing machinery.
- Neonatal thymic organ culture
-
(NTOC). A method whereby neonatal thymic lobes are incubated in media in vitro. It allows monitoring of the effects of adding pharmacological agents on thymocyte development.
- Feedforward loop
-
A cause and effect circuit in which several processes occur in sequence, and in which the output of an upstream process promotes or inhibits a downstream process, usually bypassing one or more intermediate processes. This can set the gain for a signalling circuit or specifically amplify or limit one of several different downstream signalling branches.
- Innate-like CD8+ T cell
-
An αβ T cell subset characterized by high-level expression of CD44, CD122 and natural killer cell markers, indicative of an activated or memory phenotype. In contrast to conventional naive αβT cells, innate-like T cells are interleukin15 dependent and can exert effector functions immediately after stimulation. Whereas most conventional T cells are selected by peptide–MHC complexes on non-haematopoietic cells in the thymus, innate-like T cells are selected by MHC proteins on haematopoietic cells.
- γδ T cell
-
A distinct lineage of T cells, the T cell receptor of which contains a γ and a δ chain instead of an α and a β chain. γδ T cells are not classical MHC restricted. Their antigenic ligands are largely unknown and might include non-peptides. γδ T cells have particularly important functions in epidermal tissues. They contribute to pathogen defence, tumour surveillance, mucosal and skin barrier functions and, in mice, skin wound healing.
- RNA interference
-
(RNAi). An RNA-dependent gene silencing process initiated by short double-stranded RNA (dsRNA) molecules that can either be produced endogenously by the cell (micro RNA pathway) or be exogenously provided by viruses or through laboratory techniques. The cellular ribonuclease Dicer cleaves dsRNAs into 21–25 base pair small interfering RNAs (siRNAs) of which the 'guide strand' binds to complementary regions in cellular mRNAs within the RNA-induced silencing complex (RISC). This results in degradation of the mRNA and/or translational blockade.
Rights and permissions
About this article
Cite this article
Sauer, K., Cooke, M. Regulation of immune cell development through soluble inositol-1,3,4,5-tetrakisphosphate. Nat Rev Immunol 10, 257–271 (2010). https://doi.org/10.1038/nri2745
Issue Date:
DOI: https://doi.org/10.1038/nri2745
This article is cited by
-
Network pharmacology and experimental verification of the potential mechanism of Er-Xian decoction in aplastic anemia
Scientific Reports (2023)
-
Signaling networks in immunometabolism
Cell Research (2020)
-
Relevance of solute carrier family 5 transporter defects to inherited and acquired human disease
Journal of Applied Genetics (2019)
-
The evolutionary landscape of chronic lymphocytic leukemia treated with ibrutinib targeted therapy
Nature Communications (2017)
-
Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis
Nature Medicine (2015)