Key Points
-
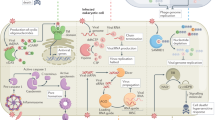
The nematode Caenorhabditis elegans was developed as a model for studying bacterial virulence and innate immunity in 1999. C. elegans does not have circulating cells and seems to rely almost exclusively on epithelial immunity to combat pathogen attack. Several parallel immune response pathways have been identified that activate distinct but partially overlapping sets of immune effectors. Despite its simplicity, the C. elegans immune response is highly pathogen specific and different pathogens activate distinct immune response pathways.
-
Although C. elegans has a single Toll-like receptor (TLR), myeloid differentiation primary-response protein 88 (MYD88) and nuclear factor-κB (NF-κB) are not encoded in the C. elegans genome or in the genomes of other nematode species. Moreover, the single C. elegans TLR does not seem to have an important role in the immune response. Because some cnidaria (such as the sea anemone Nematostella vectensis) have TLRs, MYD88 and NF-κB, it seems that TLR signalling has been lost in the nematode lineage.
-
A highly conserved p38 mitogen-activated protein kinase (MAPK) signalling cascade has a central role in the C. elegans immune response as it does in mammals. The p38 MAPK pathway is required for the activation of a set of immune effectors that are required to maintain a basal level of immune function.
-
The p38 MAPK signalling pathway is active during both infection and wounding and functions in at least the intestine, neurons and epidermis in response to pathogen infection.
-
Several highly conserved metazoan signalling pathways have dual roles, functioning as important components of the C. elegans immune response. It is of interest to determine whether the same pathways function in immune signalling throughout metazoan evolution, including acting in concert with TLR pathways in mammals.
-
In addition to its role in stress resistance, lifespan extension and metabolic regulation in C. elegans, the DAF-2–DAF-16 insulin signalling pathway confers resistance to a wide variety of pathogens when DAF-16 is constitutively activated.
-
The C. elegans β-catenin homologue β-catenin/armadillo-related family member 1 (BAR-1) and the downstream homoebox protein egg laying defective protein 5 (EGL-5) have central roles in activating the C. elegans immune response to infection by Staphylococcus aureus but not Pseudomonas aeruginosa. Roles for β-catenin and homeobox proteins in immune signalling in flies and mammals have also been recently shown.
-
The G protein-coupled receptor FSHR-1 is the first candidate immune receptor to be identified in C. elegans.
Abstract
The genetically tractable model organism Caenorhabditis elegans was first used to model bacterial virulence in vivo a decade ago. Since then, great strides have been made in identifying the host response pathways that are involved in its defence against infection. Strikingly, C. elegans seems to detect, and respond to, infection without the involvement of its homologue of Toll-like receptors, in contrast to the well-established role for these proteins in innate immunity in mammals. What, therefore, do we know about host defence mechanisms in C. elegans and what can they tell us about innate immunity in higher organisms?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hemmrich, G., Miller, D. J. & Bosch, T. C. G. The evolution of immunity: a low-life perspective. Trends Immunol. 28, 449–454 (2007).
Salzet, M. Vertebrate innate immunity resembles a mosaic of invertebrate immune responses. Trends Immunol. 22, 285–288 (2001).
Waterfield, N. R., Wren, B. W. & Ffrench-Constant, R. H. Invertebrates as a source of emerging human pathogens. Nature Rev. Microbiol. 2, 833–841 (2004).
Adams, B. et al. Biodiversity and systematics of nematode–bacterium entomopathogens. Biol. Control 37, 32–49 (2006).
Lee, D. G. et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 7, R90 (2006).
Pedersen, A. L., Nybroe, O., Winding, A., Ekelund, F. & Bjørnlund, L. Bacterial feeders, the nematode Caenorhabditis elegans and the flagellate Cercomonas longicauda, have different effects on outcome of competition among the Pseudomonas biocontrol strains CHA0 and DSS73. Microb. Ecol. 57, 501–509 (2009).
Hilbi, H., Weber, S. S., Ragaz, C., Nyfeler, Y. & Urwyler, S. Environmental predators as models for bacterial pathogenesis. Environ. Microbiol. 9, 563–575 (2007).
Ruvkun, G. & Hobert, O. The taxonomy of developmental control in Caenorhabditis elegans. Science 282, 2033–2041 (1998).
Schulenburg, H., Hoeppner, M. P., Weiner, J. & Bornberg-Bauer, E. Specificity of the innate immune system and diversity of C-type lectin domain (CTLD) proteins in the nematode Caenorhabditis elegans. Immunobiology 213, 237–250 (2008).
Zhang, X. & Zhang, Y. Neural–immune communication in Caenorhabditis elegans. Cell Host Microbe 5, 425–429 (2009).
Shivers, R. P., Youngman, M. J. & Kim, D. H. Transcriptional responses to pathogens in Caenorhabditis elegans. Curr. Opin. Microbiol. 11, 251–256 (2008).
Brenner, S. In the beginning was the worm. Genetics 182, 413–415 (2009).
Powell, J. R. & Ausubel, F. M. Models of Caenorhabditis elegans infection by bacterial and fungal pathogens. Methods Mol. Biol. 415, 403–427 (2008).
Aballay, A. & Ausubel, F. M. Caenorhabditis elegans as a host for the study of host–pathogen interactions. Curr. Opin. Microbiol. 5, 97–101 (2002).
Couillault, C. & Ewbank, J. J. Diverse bacteria are pathogens of Caenorhabditis elegans. Infect. Immun. 70, 4705–4707 (2002).
Kurz, C. L. & Ewbank, J. J. Caenorhabditis elegans for the study of host–pathogen interactions. Trends Microbiol. 8, 142–144 (2000).
Sifri, C. D., Begun, J. & Ausubel, F. M. The worm has turned — microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 13, 119–127 (2005).
Tan, M. W., Mahajan-Miklos, S. & Ausubel, F. M. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl Acad. Sci. USA 96, 715–720 (1999).
Hodgkin, J., Kuwabara, P. E. & Corneliussen, B. A novel bacterial pathogen, Microbacterium nematophilum, induces morphological change in the nematode C. elegans. Curr. Biol. 10, 1615–1618 (2000).
Pujol, N. et al. A reverse genetic analysis of components of the Toll signaling pathway in Caenorhabditis elegans. Curr. Biol. 11, 809–821 (2001). Reverse genetic study of the Toll signalling pathway in C. elegans , showing that diverse mutants do not have defective survival after infection with Serratia marcescens but have a pathogen avoidance phenotype.
Zhang, Y., Lu, H. & Bargmann, C. I. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 438, 179–184 (2005).
Aballay, A., Yorgey, P. & Ausubel, F. M. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr. Biol. 10, 1539–1542 (2000).
Troemel, E. R. et al. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2, e183 (2006). Extensive global transcriptional profiling of an early infection with P. aeruginosa , showing the relationship between p38 MAPK signalling and insulin signalling.
Irazoqui, J. E., Ng, A., Xavier, R. J. & Ausubel, F. M. Role for β-catenin and HOX transcription factors in Caenorhabditis elegans and mammalian host epithelial–pathogen interactions. Proc. Natl Acad. Sci. USA 105, 17469–17474 (2008).
Tan, M. W., Rahme, L. G., Sternberg, J. A., Tompkins, R. G. & Ausubel, F. M. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc. Natl Acad. Sci. USA 96, 2408–2413 (1999).
Mahajan-Miklos, S., Tan, M. W., Rahme, L. G. & Ausubel, F. M. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa–Caenorhabditis elegans pathogenesis model. Cell 96, 47–56 (1999).
Darby, C., Cosma, C. L., Thomas, J. H. & Manoil, C. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA 96, 15202–15207 (1999).
DeNardo, D. G., Johansson, M. & Coussens, L. M. Inflaming gastrointestinal oncogenic programming. Cancer Cell 14, 7–9 (2008).
Karin, M. The IκB kinase — a bridge between inflammation and cancer. Cell Res. 18, 334–342 (2008).
Ferrandon, D., Imler, J.-L., Hetru, C. & Hoffmann, J. A. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nature Rev. Immunol. 7, 862–874 (2007).
Anderson, K. V., Jürgens, G. & Nüsslein-Volhard, C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42, 779–789 (1985).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Vallabhapurapu, S. & Karin, M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 (2009).
Gottar, M. et al. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 416, 640–644 (2002).
Carty, M. et al. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nature Immunol. 7, 1074–1081 (2006).
Liberati, N. T. et al. Requirement for a conserved Toll/interleukin-1 resistance domain protein in the Caenorhabditis elegans immune response. Proc. Natl Acad. Sci. USA 101, 6593–6598 (2004).
Couillault, C. et al. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nature Immunol. 5, 488–494 (2004).
Shivers, R. P., Kooistra, T., Chu, S. W., Pagano, D. J. & Kim, D. H. Tissue-specific activities of an immune signaling module regulate physiological responses to pathogenic and nutritional bacteria in C. elegans. Cell Host Microbe 6, 321–330 (2009).
Zugasti, O. & Ewbank, J. J. Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-β signaling pathway in Caenorhabditis elegans epidermis. Nature Immunol. 10, 249–256 (2009). An excellent example of characterization of tissue-specific signalling pathways involved in defence against fungal infection.
Tenor, J. L. & Aballay, A. A conserved Toll-like receptor is required for Caenorhabditis elegans innate immunity. EMBO Rep. 9, 103–109 (2008).
Zhong, J. et al. GCK is essential to systemic inflammation and pattern recognition receptor signaling to JNK and p38. Proc. Natl Acad. Sci. USA 106, 4372–4377 (2009).
Dong, C., Davis, R. J. & Flavell, R. A. MAP kinases in the immune response. Annu. Rev. Immunol. 20, 55–72 (2002).
Lu, H. T. et al. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 18, 1845–1857 (1999).
Tobiume, K. et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2, 222–228 (2001).
Kim, D. H. et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297, 623–626 (2002). This report identified the PMK-1 p38 MAPK pathway using forward genetic identification and showed that the pathway is essential for defence against P. aeruginosa.
Kim, D. H. et al. Integration of Caenorhabditis elegans MAPK pathways mediating immunity and stress resistance by MEK-1 MAPK kinase and VHP-1 MAPK phosphatase. Proc. Natl Acad. Sci. USA 101, 10990–10994 (2004).
Mizuno, T. et al. The Caenorhabditis elegans MAPK phosphatase VHP-1 mediates a novel JNK-like signaling pathway in stress response. EMBO J. 23, 2226–2234 (2004).
Takeda, K., Noguchi, T., Naguro, I. & Ichijo, H. Apoptosis signal-regulating kinase 1 in stress and immune response. Annu. Rev. Pharmacol. Toxicol. 48, 199–225 (2008).
Aballay, A. & Ausubel, F. M. Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc. Natl Acad. Sci. USA 98, 2735–2739 (2001).
Kim, D. H. & Ausubel, F. M. Evolutionary perspectives on innate immunity from the study of Caenorhabditis elegans. Curr. Opin. Immunol. 17, 4–10 (2005).
Putnam, N. H. et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317, 86–94 (2007).
Miller, D. J. et al. The innate immune repertoire in cnidaria — ancestral complexity and stochastic gene loss. Genome Biol. 8, R59 (2007). A phylogenetic perspective on the evolution of innate immune signalling pathways in primitive invertebrates. Representatives of the Toll or TLR and IL-1R signalling pathways are identified, as well as a complement system.
Abad, P. et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nature Biotechnol. 26, 909–915 (2008).
Wong, D., Bazopoulou, D., Pujol, N., Tavernarakis, N. & Ewbank, J. J. Genome-wide investigation reveals pathogen-specific and shared signatures in the response of Caenorhabditis elegans to infection. Genome Biol. 8, R194 (2007).
Chen, R. E. & Thorner, J. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1773, 1311–1340 (2007).
Bischof, L. J. et al. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog. 4, e1000176 (2008). This report describes UPR activation during exposure to the bacterial pore-forming toxin Cry5B.
Huffman, D. L. et al. Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proc. Natl Acad. Sci. USA 101, 10995–11000 (2004).
Huffman, D. L., Bischof, L. J., Griffitts, J. S. & Aroian, R. V. Pore worms: using Caenorhabditis elegans to study how bacterial toxins interact with their target host. Int. J. Med. Microbiol. 293, 599–607 (2004).
Gal-Mor, O. & Finlay, B. B. Pathogenicity islands: a molecular toolbox for bacterial virulence. Cell. Microbiol. 8, 1707–1719 (2006).
Jansson, H. B. Adhesion of conidia of Drechmeria coniospora to Caenorhabditis elegans wild type and mutants. J. Nematol. 26, 430–435 (1994).
Pujol, N. et al. Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Curr. Biol. 18, 481–489 (2008).
Ren, M., Feng, H., Fu, Y., Land, M. & Rubin, C. S. Protein kinase D is an essential regulator of C. elegans innate immunity. Immunity 30, 521–532 (2009). A good example of the use of genetics to identify new components of innate immune signalling pathways, followed by the biochemical characterization of PKD.
Shirai, Y. & Saito, N. Activation mechanisms of protein kinase C: maturation, catalytic activation, and targeting. J. Biochem. 132, 663–668 (2002).
Ziegler, K. et al. Antifungal innate immunity in C. elegans: PKCδ links G protein signaling and a conserved p38 MAPK cascade. Cell Host Microbe 5, 341–352 (2009). An excellent study of the signalling pathways upstream of PMK-1 in the epidermis of C. elegans during fungal infection.
Pujol, N. et al. Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 4, e1000105 (2008).
Tong, A. et al. Negative regulation of Caenorhabditis elegans epidermal damage responses by death-associated protein kinase. Proc. Natl Acad. Sci. USA 106, 1457–1461 (2009).
Zeuthen, L. H., Fink, L. N. & Frokiaer, H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-β. Immunology 123, 197–208 (2008).
Wahl, S. M. Transforming growth factor-β: innately bipolar. Curr. Opin. Immunol. 19, 55–62 (2007).
Wolkow, C. A., Kimura, K. D., Lee, M. S. & Ruvkun, G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290, 147–150 (2000).
Garsin, D. A. et al. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300, 1921 (2003).
Antebi, A. Genetics of aging in Caenorhabditis elegans. PLoS Genet. 3, 1565–1571 (2007).
Lee, S. S., Kennedy, S., Tolonen, A. C. & Ruvkun, G. DAF-16 target genes that control C. elegans life-span and metabolism. Science 300, 644–647 (2003).
Murphy, C. T. et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424, 277–283 (2003).
Miyata, S., Begun, J., Troemel, E. R. & Ausubel, F. M. DAF-16-dependent suppression of immunity during reproduction in Caenorhabditis elegans. Genetics 178, 903–918 (2008).
Evans, E. A., Chen, W. C. & Tan, M.-W. The DAF-2 insulin-like signaling pathway independently regulates aging and immunity in C. elegans. Aging Cell 7, 879–893 (2008).
McElwee, J. J., Schuster, E., Blanc, E., Thomas, J. H. & Gems, D. Shared transcriptional signature in Caenorhabditis elegans dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J. Biol. Chem. 279, 44533–44543 (2004).
Evans, E. A., Kawli, T. & Tan, M.-W. Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog. 4, e1000175 (2008).
Anyanful, A., Easley, K. A., Benian, G. M. & Kalman, D. Conditioning protects C. elegans from lethal effects of enteropathogenic E. coli by activating genes that regulate lifespan and innate immunity. Cell Host Microbe 5, 450–462 (2009). The first identification that DAF-16 activation is involved in the 'conditioned' phenotype resulting from previous exposure to attenuated pathogenic E. coli.
Shapira, M. et al. A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc. Natl Acad. Sci. USA 103, 14086–14091 (2006).
O'Rourke, D., Baban, D., Demidova, M., Mott, R. & Hodgkin, J. Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res. 16, 1005–1016 (2006). An excellent study of the transcriptional response to infection with the nematode-specific bacterial pathogen M. nematophilum.
Eisenmann, D. M. Wnt signaling. WormBook 25, 1–17 (2005).
Eisenmann, D. M. & Kim, S. K. Protruding vulva mutants identify novel loci and Wnt signaling factors that function during Caenorhabditis elegans vulva development. Genetics 156, 1097–1116 (2000).
Eisenmann, D. M., Maloof, J. N., Simske, J. S., Kenyon, C. & Kim, S. K. The β-catenin homolog BAR-1 and LET-60 Ras coordinately regulate the Hox gene lin-39 during Caenorhabditis elegans vulval development. Development 125, 3667–3680 (1998).
Nicholas, H. R. & Hodgkin, J. The C. elegans Hox gene egl-5 is required for correct development of the hermaphrodite hindgut and for the response to rectal infection by Microbacterium nematophilum. Dev. Biol. 329, 16–24 (2009).
Gravato-Nobre, M. J. et al. Multiple genes affect sensitivity of Caenorhabditis elegans to the bacterial pathogen Microbacterium nematophilum. Genetics 171, 1033–1045 (2005).
Nicholas, H. R. & Hodgkin, J. The ERK MAP kinase cascade mediates tail swelling and a protective response to rectal infection in C. elegans. Curr. Biol. 14, 1256–1261 (2004).
Powell, J. R., Kim, D. H. & Ausubel, F. M. The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immune response. Proc. Natl Acad. Sci. USA 106, 2782–2787 (2009).
Haskins, K. A., Russell, J. F., Gaddis, N., Dressman, H. K. & Aballay, A. Unfolded protein response genes regulated by CED-1 are required for Caenorhabditis elegans innate immunity. Dev. Cell 15, 87–97 (2008).
Sieburth, D. et al. Systematic analysis of genes required for synapse structure and function. Nature 436, 510–517 (2005).
Cho, S., Rogers, K. W. & Fay, D. S. The C. elegans glycopeptide hormone receptor ortholog, FSHR-1, regulates germline differentiation and survival. Curr. Biol. 17, 203–212 (2007).
Means, T. K. et al. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J. Exp. Med. 206, 637–653 (2009).
Chung, S., Gumienny, T. L., Hengartner, M. O. & Driscoll, M. A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nature Cell Biol. 2, 931–937 (2000).
Mohri-Shiomi, A. & Garsin, D. A. Insulin signaling and the heat shock response modulate protein homeostasis in the Caenorhabditis elegans intestine during infection. J. Biol. Chem. 283, 194–201 (2008).
Singh, V. & Aballay, A. Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proc. Natl Acad. Sci. USA 103, 13092–13097 (2006).
Medzhitov, R. Approaching the asymptote: 20 years later. Immunity 30, 766–775 (2009).
Moy, T. I. et al. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem. Biol. 4, 527–533 (2009). This is an example of the use of C. elegans infection models for the identification of new antimicrobial compounds.
Boutros, M. & Ahringer, J. The art and design of genetic screens: RNA interference. Nature Rev. Genet. 9, 554–566 (2008).
Trivedi, C. M., Patel, R. C. & Patel, C. V. Differential regulation of HOXA9 expression by nuclear factor κB (NF-κB) and HOXA9. Gene 408, 187–195 (2008).
Trivedi, C. M., Patel, R. C. & Patel, C. V. Homeobox gene HOXA9 inhibits nuclear factor-κB dependent activation of endothelium. Atherosclerosis 195, e50–e60 (2007).
Sun, J. et al. Crosstalk between NF-κB and β-catenin pathways in bacterial-colonized intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G129–G137 (2005).
Sun, J., Hobert, M. E., Rao, A. S., Neish, A. S. & Madara, J. L. Bacterial activation of β-catenin signaling in human epithelia. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G220–G227 (2004).
Franco, A. T. et al. Activation of β-catenin by carcinogenic Helicobacter pylori. Proc. Natl Acad. Sci. USA 102, 10646–10651 (2005).
Ye, Z., Petrof, E. O., Boone, D., Claud, E. C. & Sun, J. Salmonella effector AvrA regulation of colonic epithelial cell inflammation by deubiquitination. Am. J. Pathol. 171, 882–892 (2007).
Deng, J. et al. Crossregulation of NF-κB by the APC/GSK-3β/β-catenin pathway. Mol. Carcinog. 39, 139–146 (2004).
Pereira, C., Schaer, D. J., Bachli, E. B., Kurrer, M. O. & Schoedon, G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler. Thromb. Vasc. Biol. 28, 504–510 (2008).
Kumar, A. et al. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 26, 4457–4466 (2007).
Blumenthal, A. et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 108, 965–973 (2006).
Gregorieff, A. et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 129, 626–638 (2005).
Koslowski, M. J. et al. Genetic variants of Wnt transcription factor TCF-4 (TCF7L2) putative promoter region are associated with small intestinal Crohn's disease. PLoS ONE 4, e4496 (2009).
Wehkamp, J. et al. The Paneth cell α-defensin deficiency of ileal Crohn's disease is linked to Wnt/Tcf-4. J. Immunol. 179, 3109–3118 (2007).
van Es, J. H. et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nature Cell Biol. 7, 381–386 (2005).
Andreu, P. et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development 132, 1443–1451 (2005).
Simms, L. A. et al. Reduced α-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn's disease. Gut 57, 903–910 (2008).
Lengerke, C. et al. BMP and Wnt specify hematopoietic fate by activation of the Cdx–Hox pathway. Cell Stem Cell 2, 72–82 (2008).
Lickert, H. et al. Wnt/β-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine. Development 127, 3805–3813 (2000).
Benahmed, F. et al. Multiple regulatory regions control the complex expression pattern of the mouse Cdx2 homeobox gene. Gastroenterology 135, 1238–1247. e3 (2008).
Wang, M.-L. et al. Regulation of RELM/FIZZ isoform expression by Cdx2 in response to innate and adaptive immune stimulation in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1074–G1083 (2005).
Ryu, J.-H. et al. The homeobox gene Caudal regulates constitutive local expression of antimicrobial peptide genes in Drosophila epithelia. Mol. Cell Biol. 24, 172–185 (2004).
Ryu, J.-H. et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 319, 777–782 (2008).
Aris-Brosou, S. & Yang, Z. Bayesian models of episodic evolution support a late Precambrian explosive diversification of the metazoa. Mol. Biol. Evol. 20, 1947–1954 (2003).
Peterson, K. J. et al. Estimating metazoan divergence times with a molecular clock. Proc. Natl Acad. Sci. USA 101, 6536–6541 (2004).
Acknowledgements
The unpublished observations cited in this review were funded by US National Institutes of Health grants R01 AI64332 and PO1 AI44220 awarded to F.M.A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Hermaphrodite
-
An organism that has both male and female reproductive organs.
- RNA interference
-
The silencing of gene expression by the introduction of double-stranded RNA that triggers the specific degradation of a homologous target mRNA, often accompanied by a concomitant decrease in production of the encoded protein.
- Microarray
-
A technique for measuring the transcription of genes. It involves the hybridization of fluorescently labelled cDNA prepared from a cell or tissue of interest to glass slides or other surfaces dotted with oligodeoxynucleotides or cDNA that represents all genes in the species.
- Quantitative reverse transcription PCR
-
(qRT-PCR). A quantitative PCR method that is used to measure relative or absolute mRNA concentrations.
- Scaffold protein
-
A protein that functions as a support to assemble a multiprotein complex.
- Coelomate
-
An animal that has an internal body cavity derived from the mesoderm.
- Bilateria
-
Members of the animal kingdom that have bilateral symmetry — the property of having two similar sides, with definite upper and lower surfaces, and anterior and posterior ends.
- Neuropeptide-like peptides
-
A family of short peptides with sequence homology to YGGW-amide neuropeptides, which can be induced during infection.
- Caenacins
-
A family of peptides related to neuropeptide-like peptides, which can be induced during infection.
- Epistasis
-
An interaction between non-allelic genes, such that one gene masks, interferes with or enhances the expression of the other gene.
- Reverse genetic approach
-
A genetic approach that proceeds from genotype to phenotype by gene manipulation techniques, such as homologous recombination in embryonic stem cells and RNA interference.
- Homeobox transcription factors
-
The genes encoding these factors contain a 180-base pair sequence encoding the homeodomain and are involved in the regulation of animal and plant development. This sequence encodes a DNA-binding helix–turn–helix motif (the homeodomain), indicating that homeodomain-containing gene products function as transcription factors.
- Leucine-rich repeat (LRR) domains
-
Domains that contain LRRs have a conserved solenoid structure, typically 20–29 residues in length and containing an 11 amino acid consensus sequence, LXXLXLXX(N or C)XL, in which X denotes any amino acid. These domains lack considerable identity or similarity in the amino acids surrounding this structure, both between and among families. Sequence substitutions in LRR-containing proteins are associated with changes in specificity and relative affinity towards LRR domain-binding partners.
Rights and permissions
About this article
Cite this article
Irazoqui, J., Urbach, J. & Ausubel, F. Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat Rev Immunol 10, 47–58 (2010). https://doi.org/10.1038/nri2689
Issue Date:
DOI: https://doi.org/10.1038/nri2689
This article is cited by
-
The homeodomain transcription factor CEH-37 regulates PMK-1/p38 MAPK pathway to protect against intestinal infection via the phosphatase VHP-1
Cellular and Molecular Life Sciences (2023)
-
Alternatives to animal models to study bacterial infections
Folia Microbiologica (2023)
-
Lacticaseibacillus rhamnosus Probio-M9 extends the lifespan of Caenorhabditis elegans
Communications Biology (2022)
-
The transcription factor ZIP-1 promotes resistance to intracellular infection in Caenorhabditis elegans
Nature Communications (2022)
-
Lactic acid bacteria that activate immune gene expression in Caenorhabditis elegans can antagonise Campylobacter jejuni infection in nematodes, chickens and mice
BMC Microbiology (2021)