Key Points
-
Malaria is a disease that kills more than 2 million people per year and there is no vaccine.
-
The greatest challenge is to develop a vaccine to reduce parasite growth during the stage in the life cycle when the parasite resides within red blood cells. Malaria antigens were first cloned 20 years ago, providing a strategy to develop a 'subunit' vaccine.
-
Natural immunity to malaria takes years to develop and depends primarily on the acquisition of antibodies to 'variant' antigens expressed on the surface of infected red cells.
-
Antibodies to the surface of merozoites (the form of the parasite that invades red cells) are known to limit parasite growth in vivo, and such antibodies might also contribute to natural immunity.
-
T-cell-mediated immunity, in the absence of antibodies, can control parasite growth in animal models, and a principal goal of research is to define antigens that stimulate protective T cells.
-
There have been more than15 clinical trials of malaria vaccines focusing on merozoite antigens, which have been very instructive in terms of defining the challenges and allowing the field to move forward.
-
The fact that natural immunity to malaria can develop and that the genome of the parasite is now available provides great optimism that a vaccine to malaria will be developed.
Abstract
Although the malaria parasite was discovered more than 120 years ago, it is only during the past 20 years, following the cloning of malaria genes, that we have been able to think rationally about vaccine design and development. Effective vaccines for malaria could interrupt the life cycle of the parasite at different stages in the human host or in the mosquito. The purpose of this review is to outline the challenges we face in developing a vaccine that will limit growth of the parasite during the stage within red blood cells — the stage responsible for all the symptoms and pathology of malaria. More than 15 vaccine trials have either been completed or are in progress, and many more are planned. Success in current trials could lead to a vaccine capable of saving more than 2 million lives per year.
Similar content being viewed by others
Main
The malaria parasite remains a scourge on human civilization and in recent years the incidence of the disease has been increasing. It is estimated that 1.5–2.5 million people die each year from malaria — mostly young children and pregnant women. Although most of these deaths occur in sub-Saharan Africa, no country is without malaria — either through endemic transmission or through importation of cases from endemic regions of the world.
It is now more than 120 years since the French physician Charles Louis Alphonse Laveran first observed malaria parasites under the microscope, and more than 100 years since Ronald Ross and Giovanni Grassi identified the vector of malaria transmission. Malaria is not, therefore, a newly described disease. Since then, there have been many significant developments in malaria research, which have included: unravelling the complex life cycle of the parasite; the development of anti-malarial drugs and insecticides; the discovery of 'malaria therapy' for tertiary syphilis (in which the fever associated with malaria killed the heat-sensitive Treponema pallidum organism responsible for syphilis); the development of drug resistance; the cloning of malaria genes (by Kemp and Anders and colleagues in Melbourne, and by Ellis, Godson and the Nussenzweig's group in New York); and now early-phase vaccine trials (Box 1). Although there are dozens of species of malaria parasites, those that infect humans are limited to Plasmodium falciparum, P. vivax, P. ovale and P. malariae. P. falciparum and P. vivax are the most common, and P. falciparum is responsible for most of the malaria deaths. Multiple strains of each species exist, differing at crucial antigenic determinants. Although some degree of immunity can develop between strains, there is generally thought to be no inter-species immunity.
Malaria vaccine development
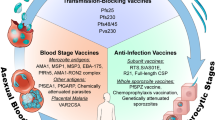
Vaccination is a successful method of disease control and there have been numerous success stories — the most notable being the eradication of smallpox and the virtual elimination of polio. Many factors conspire to make the development of a malaria vaccine an incredibly difficult challenge. An important factor impeding vaccine development is the complex biology of the life cycle of the parasite, which exists in different forms (and each form with a different pattern of antigen expression) in different tissues of the body and the mosquito (Fig. 1). In these various forms the parasite is susceptible to immune attack, although the type of immune response required is very different for each form. Several vaccine strategies, therefore, need to be used.
The life cycle in the mammalian host commences with the inoculation of sporozoites by an infected anopheline mosquito that travel by the circulation to the liver. After about 1 week (depending on the species of malaria) parasites have multiplied intracellularly and merozoites rupture from infected hepatocytes to invade red blood cells (RBCs). For Plasmodium falciparum and P. vivax there is a 48-hour period inside the RBCs during which merozoites multiply and approximately 16 fresh merozoites are released from ruptured RBCs to invade fresh cells. Sexual forms (gametocytes) develop within RBCs and are taken up by the mosquito. These emerge in the gut of the mosquito as gametes, which fuse to form an oocyst, and sporozoites develop. Sporozoites are released and travel to the salivary gland of the mosquito.
A second factor that impedes vaccine development is the ability of the malaria parasite to alter itself. ANTIGENIC VARIATION and ALLELIC POLYMORPHISM are important obstacles to SUBUNIT VACCINE development, especially given that many of the sequence alterations in malaria proteins occur in regions that are crucial to immunity. Other factors impeding malaria vaccine development include: immunological non-responsiveness of certain individuals (depending on their human leukocyte antigen and other antigens) to proteins that might comprise a vaccine1; CLONAL IMPRINTING or ORIGINAL ANTIGENIC SIN influenced by stochastic events and prior exposure to other, perhaps crossreactive antigens2,3,4; the difficulties encountered in properly folding recombinant subunit vaccines so as to maintain their immunogenic properties; the lack of suitably potent adjuvants necessary to induce high-titre antibody responses; and the lack of animal/parasite systems which adequately model the situation with humans and malaria parasites in terms of disease pathogenesis and immunological responses.
Most of the vaccines that have been developed for various diseases have relied on the 'simple' approach of presenting the entire antigenic compartment of the organism in the form of killed or living, but attenuated, organisms5. Such an approach is not possible at present for malaria as the organisms grow within red blood cells (RBCs) in the mammalian host (Fig. 1), and although in vitro culture is possible (for example, for P. falciparum, the most virulent of the human plasmodia), a source of RBCs is required. However, it is simply impractical and potentially unsafe to consider growing a vaccine in human RBCs for a disease for which 40% of the world's population is potentially at risk. Malaria vaccine development has therefore focused on the development of a subunit vaccine.
The purpose of this review is to discuss the different strategies for developing a vaccine to the blood stage of malaria. Why is it important to target the blood stage of the parasite? There are different phases in the life cycle of the parasite (Fig. 1): the stage inside the Anopheline mosquito vector, the 'pre-erythrocytic stage' and the erythrocytic or blood stage. The pre-erythrocytic stage, during which the sporozoites travel in the blood after inoculation, and then invade and develop within hepatocytes, is perhaps the best understood in terms of immunity6,7, and vaccine development is further advanced for this stage than others. The mechanism of immunity required to block parasite transmission by the mosquito is also well understood — antibodies taken up by the mosquito during its blood meal can prevent gamete fertilization or zygote development8. This type of vaccine protects only at the population level and does not protect the vaccinee from malaria, and it will be suitable for only certain populations. The blood stage is the stage for which immunity is least well understood and which arguably presents the greatest challenges in terms of vaccine development. It is also the stage that is responsible for all the symptoms and pathology of malaria, the most serious of which are anaemia and cerebral malaria9, and is therefore an important target for vaccine development. In spite of much effort, a blood-stage malaria vaccine remains elusive, although several vaccines have been trialled or are now in clinical trials (Table 1).
What do we want a blood-stage vaccine to do? Because the level of parasitaemia is in general proportional to the severity of disease9, a vaccine must limit parasite growth. Most would argue, however, that it is not necessary to induce sterile immunity after vaccination (that is, no parasites present within a vaccinated individual). It is well recognized that most adults in malaria-endemic settings are clinically immune (that is, they do not suffer symptoms associated with malaria, but they nevertheless have parasites at low density in their blood). The challenge for developing a malaria vaccine might be less if sterile immunity is not required, but until the immunological and other factors that control parasite growth are properly understood, and until their potential is tested in vaccine trials, it is too early to say what type of immunity (sterile or otherwise) should be the goal. Certainly, if sterile immunity could be achieved, then transmission of malaria between individuals would cease.
Immunity to malaria
Before considering the current approaches for development of a subunit vaccine, it is instructive to consider what we know (or think we know) about immunity to malaria. When considering what we know, it can be helpful to distinguish between 'natural' immunity and 'non-natural' immunity. Natural immunity is induced by multiple exposure to parasites and takes many years of endemic exposure to develop, although in some populations this period can be shortened10. Theoretically, a vaccine could mimic, but accelerate, this process. Non-natural immunity refers to immune mechanisms that might not be induced to any great extent by natural exposure, but that could theoretically be induced by a vaccine, and that could be highly effective. Examples are given below in which these different approaches are being taken.
Variant antigens
Natural immunity and variant antigens. Several years ago Marsh and Howard observed that the sera of convalescent children were able to agglutinate the strains of P. falciparum parasite to which the child was recently exposed, but not other strains circulating in the same village11. By contrast, sera from immune adults in the same village were mostly able to agglutinate all strains. It was subsequently shown that only one strain was present within each agglutinate12. These data indicated that 'natural' immunity (as held by adults in a malaria-endemic setting) was due to the development of AGGLUTINATING ANTIBODIES that could recognize each of the different strains in a community. Because each agglutinate contained only parasites of the one strain, it seemed that the immune antibodies within an individual consisted of multiple specificities, as opposed to a monospecific antibody recognizing an antigenic determinant shared by all strains. It is not surprising, therefore, that 'natural' immunity takes several years of endemic parasite exposure to develop13.
During infection of humans with P. falciparum there is a cyclic recrudescence of parasites to peak levels approximately every 20 days (Fig. 2; W. E. Collins and G. M. Jeffrey, unpublished observations referred to in Ref 9). This waxing and waning of parasite density in the blood is likely to be due to the selective pressure on the surface of infected RBCs by antibodies to 'variant' antigens that arise when a single clone changes phenotype as a result of gene switching14. The principal antigen is P. falciparum erythrocyte membrane protein (PfEMP1) (Refs 15–17), for which there are ∼50 variant copies represented in the genome. It is generally believed, but technically unproven, that after one of the variant antigens is clonally expressed, antibodies develop against the expanding clone, which is then eliminated. However, a different variant, not recognized by the antibodies, emerges and the cycling continues. This provides a partial explanation at the molecular level for the above-mentioned observations11. However, the peaks of parasitaemia with each wave gradually diminish, indicating that other immunological responses (other antigens or other types of immune response) also contribute to the development of natural immunity. The relative contribution of each of these is yet to be determined.
The sequential peaks in parasite density over the course of an infection are shown. The waning of each peak is thought to be largely due to antibodies to variant antigens expressed on the red-cell surface. However, other factors might contribute to this and the gradual diminution in the height of the parasite peaks over time. Adapted with permission from Ref. 9 © (1994) American Association for the Advancement of Science.
The fact that natural immunity to malaria (as acquired by individuals living in malaria-endemic regions) takes several years to develop is thought to depend largely on the time taken to acquire antibodies to the multiple PfEMP1 variants. Antibodies to PfEMP1 prevent adherence of the more mature forms of the parasite to the small blood vessels of various organs and tissues, and promote agglutination of parasitized RBCs. Many of the circulating parasites are thought to be removed from the blood in the spleen.
Non-natural immunity and variant antigens. One approach to vaccine development would be to identify and combine multiple variant epitopes of PfEMP1, analogous with one of the approaches being taken to develop a vaccine for Streptococcus pyogenes18. A related, but distinct, approach is to define a conserved epitope on PfEMP1 that is not normally recognized after infection, but which nevertheless could be a target for agglutinating antibodies. Such an epitope would be defined as 'cryptic'19. Although crytpic epitopes are poorly immunogenic after infection, they can be recognized by antibodies (or T cells) raised artificially to the isolated epitope. Examples of such epitopes are found in malaria20,21 and other organisms22,23,24,25. Because cryptic epitopes are only immunogenic when presented out of context in a non-native form (for example, as peptides), variants would not be selected by immune pressure, and they often have conserved sequences. Of interest is the observation that immune responses to cryptic epitopes can often recognize the native protein or organism21. Recently described PfEMP1-specific monoclonal antibodies that can agglutinate multiple strains26 are likely to be targeting cryptic epitopes. Such epitopes could be the basis for an exciting approach to malaria vaccine development, which could induce a form of non-natural immunity.
Merozoite surface proteins
Most blood-stage vaccine research is focused on antigens that are expressed not on the surface of the infected RBC, but on the surface of the MEROZOITE27. Merozoites are released from a rupturing RBC and quickly invade other RBCs. Merozoite surface-protein (MSP)-specific antibodies therefore have only a brief period of time in which to be active. The most studied MSPs are MSP1 (Refs 28,29) and apical membrane antigen 1 (AMA1)30,31,32. Both antigens are highly polymorphic and have complex folding patterns. Immunization with antigens from P. falciparum or their homologues from monkey or rodent malaria parasites have been shown to protect animals from challenge with a defined strain of the parasite33,34,35,36,37,38,39. Both MSP1 and AMA1, or parts of them, are being used in clinical trials (Table 1).
AMA1 appears on the merozoite surface after its release from organelles of the parasite, referred to as rhoptries. There is extensive allelic diversity of the protein as a result of point mutations, although some regions are conserved. The external amino-terminal domain of the protein has a complex folding pattern. Purified native and recombinant proteins from simian and rodent malaria parasites have been shown to protect against challenge with homologous strains. An extensive review of AMA1 studies has been published33.
P. falciparum MSP1 is a large protein of ∼200 kDa28,29,40. During release from infected RBCs, the protein undergoes a series of proteolytic digestions, such that only the small 19-kDa carboxy-terminal tail, MSP119 (with a conserved sequence) is carried on the surface of the merozoite into fresh RBCs41. MSP119 and a precursor, MSP142, are the principal MSP1-derived vaccine targets, although a larger amino-terminal fragment, 190L, has shown promise in monkey trials42 and early-stage human vaccine trials43,44. An amino-terminal peptide epitope from MSP1 (not MSP119 or MSP142) was also a component in a multideterminant synthetic vaccine (SPf66), which underwent extensive human trials, resulting overall in negative results — some trials showing efficacy and the more convincing showing none45,46,47,48,49. It has been shown that invasion-inhibitory antibodies can prevent MSP119 processing and invasion, and that blocking antibodies can inhibit these antibodies28,29,50. Epitopes for inhibitory and blocking monoclonal antibodies could be defined on MSP1, and mutagenized proteins that bound inhibitory but not blocking antibodies could be designed, providing a novel, non-natural approach to malaria vaccines based on MSP1 (Ref. 51). An additional region of MSP1 (block 2) has also been identified as a potential target of protective antibodies52. Although MSP119 is considered highly conserved in nature, there are several defined point mutations in the molecule (reviewed in Ref. 53), and it is not known what effect, if any, these will have on the efficacy of an MSP119 vaccine based on a single strain. The molecule has a complex folding pattern40,54 with two epidermal growth factor (EGF)-like domains. Proper folding is essential to the ability of a recombinant MSP119 to induce immunity36.
Rodent studies indicate that for MSP119, high-titre antibodies present at the time of challenge are required for immunity37,39,55. This is not surprising given that such antibodies have to act quickly during the inter-RBC phase in the life cycle. Anti-MSP119-specific antibodies do not require Fc function for activity, as shown by the ability of transferred antibodies to reduce parasite density in Fc receptor-deficient mice56,57 and by the ability of recombinant single light-chain antibodies to delay parasite growth (P. Vukovic, M. Foley and M.F.G., unpublished observations). Although Fc function might be important for some malaria-specific antibodies58,59, this does not seem to be the case for MSP119. Although earlier studies with the native MSP1 molecule indicated that antibody-independent cell-mediated immunity is important in protection (that is, immunity mediated by T cells in the absence of antibodies)60, it has been shown that vaccination with recombinant MSP119 cannot protect B-cell-deficient mice, nor can defined synthetic helper CD4+ T-cell epitopes from the molecule induce any protection in normal mice39,61. It is curious and unexplained why MSP119 does not seem to be a target of protective T cells, whereas immunity induced by the larger MSP1 molecule seems to be mediated, at least in part, by T cells. Furthermore, the ability of cultured T-cell clones and polyclonal populations (cell lines) to adoptively transfer protection is well known (see below), although the antigenic targets of these protective T cells have not been defined.
MSP119-specific antibodies at the time of challenge are required to reduce parasite burden; however, in the mouse at least, such antibodies seem incapable of eradicating all parasites. Passively transferred antibodies, even at very high titre, seem incapable of eradicating all parasites in recipient mice that lack either CD4+ T cells, B cells or both62. The observation that such antibodies can both reduce parasite burden and clear all parasites from immunocompetent mice indicates that an active immune response involving both B cells and CD4+ T cells is required for protection even in mice that contain high-titre MSP119-specific antibodies at the time of challenge. The principal effect that MSP119 vaccination has is likely to be the induction of antibodies that significantly reduce, but do not eliminate, parasite burden post-challenge (Fig. 3) This would provide the vaccinee with the additional time necessary to mount a protective immune response to the parasite independent of the vaccine. The specificity of the immune response that develops post-challenge is not known, but it need not be to MSP1 (Ref. 62). Recent data indicate that it might target multiple antigens (W. Zhang and M.F.G., unpublished observations).
The main effect of MSP119-specific antibodies is to delay the prepatent period of immunity (the period after infection when parasitaemia is so low that parasites cannot be detected by microscopy). In normal mice, that were passively transfused with MSP119-specific antibodies and later challenged with Plasmodium yoelii parasites, the parasitaemia rises and then eventually declines, indicating the induction of an active immune response at a time post-challenge. MSP119, 19-kDa carboxy-terminal tail of merozoite surface-protein 1.
It is not yet known whether the requirements for immunity observed in the mouse will also be relevant in humans. Although some population-based studies have argued for an association between natural immunity and immune responses to MSP1 (Refs 63–65), others have not66. The association would seem less than that between natural immunity and the presence of antibodies to several variants67. As mentioned above, however, there is evidence for inhibitory and blocking antibodies with specificity for MSP1. The different studies that have examined the correlation of protection with the presence of MSP1-specific antibodies have not looked at the fine specificity of these antibodies, and the discrepancies in the data might possibly be explained by the presence of antibodies of different fine specificities in the different populations. It is of interest, also, that antibodies to MSP119 seem to be an important component of the invasion-inhibitory repertoire of malaria parasite-specific antibodies. Elegant studies with transgenic parasites have shown that immune sera (human or rodent) were significantly less capable of blocking invasion of parasites that expressed heterologous versus homologous MSP119 sequences68. Although a strong correlation between invasion-inhibitory antibodies and clinical immunity has not been proven, there is reason to think that such antibodies must contribute to a reduction in parasite load, and as such this study highlights the potential importance of MSP119-specific antibodies.
As mentioned earlier, data from mouse models for MSP119 indicate that very high antibody levels might be required39. If that applies to humans, then novel adjuvants, such as SBAS2 (Refs 69,70), or novel vaccine-delivery schedules, such as those targeting DNA-based vaccines to antigen-presenting cells71, might enable human vaccinees to generate significantly higher titre antibodies following vaccination and be protected from challenge. Human clinical trials with MSP119 have not yet been undertaken. However, a recent trial of a trivalent falciparum vaccine that combines MSP1 (190L), RESA (ring-infected erythrocyte surface antigen) and MSP2 (Ref. 33) has recently been completed. There was a reported 62% reduction in parasite densities in the blood of vaccinated children33,44. Although the correlates of immunity have not been defined, it is entirely possible that the type of immunity induced by MSP119 will be different to that induced by the large amino-terminal fragment of MSP1, 190L.
Other multicomponent vaccines are also under development (for example, see Refs 72,73). A multicomponent vaccine would offer theoretical advantages over a single-component vaccine: the percentage of immunological non-responders would be reduced and the effect of antigenic polymorphism would also be reduced. However, a drawback to a multicomponent recombinant protein vaccine will be the difficulty in producing and properly folding each of the component parts. The cost of such a vaccine would obviously be significantly greater than for a single-component vaccine. However, multicomponent DNA vaccines for malaria74 would be far less expensive. A challenge with DNA-based vaccines will be to induce a sufficiently high-titre antibody response to the encoded antigens71. Another challenge relates to whether the encoded polypeptide folds appropriately, which might be affected by the ordering of the epitopes on the DNA vaccine.
Although this section has focused primarily on MSP1 and AMA1, several other merozoite vaccine candidates are progressing towards clinical trial. Many of these are referred to in Table 1 and in other references33,75,76.
Cellular immunity and vaccine strategies
A third general strategy for developing a vaccine for malaria is to identify and use antigens that are targets for antibody-independent cell-mediated immunity. This term refers to the ability of CD4+ T cells that express αβ T-cell receptors to act, in the absence of antibody, and limit parasite growth and is referred to from here on as 'T-cell immunity'. Early studies elegantly showed the importance of T cells in malaria immunity and indicated that T-cell responses can be effective in controlling parasite densities in the absence of B cells (in mice treated with anti-μ-chain antibodies77). These experiments have now been repeated and extended using B-cell-deficient mice78,79. Data from several papers show that CD4+ T cells, in the apparent absence of antibody, can significantly limit parasite growth; although, in some situations at least, parasite eradication was not achieved79. Adoptive-transfer studies with CD4+ T-cell lines and clones also show that effector T cells of limited antigenic specificity are able to reduce parasite density and, in some cases, to eradicate parasites80,81,82.
Research into vaccines that stimulate T-cell immunity to malaria has been impeded, however, by two factors: a lack of clear understanding of how T cells could kill parasites that are hidden inside RBCs that lack expression of major histocompatibility complex (MHC) molecules; and lack of information of the target antigens of T cells. The most generally accepted model of how parasites might be killed is outlined in Fig. 4, commencing with activation of CD4+ T cells in the spleen, after antigen presentation by dendritic cells (DCs) and ending with the death of parasites (probably intracellularly) after phagocytosis by macrophages and by small inflammatory molecules (oxygen and nitric oxide radicals) in the spleen81,83,84. Evidence from elegant rodent studies indicates that T-cell immunity is regulated by interleukin-12 (IL-12), involves further cytokines (IFN-γ and TNF-α), and might operate finally through nitric oxide83,85. Human studies also support a role for IFN-γ in resistance to malaria86. Immunity to malaria is largely species specific, even though there is obviously no specificity in the action of molecules such as nitric oxide. It would seem that memory CD4+ T cells are reactivated specifically after reinfection, but the parasites are then killed non-specifically. The activation of T cells by parasites, however, might be far more complex. A recent study87 showed that parasitized RBCs might inhibit the maturation of immature DCs, possibly by interaction of CD36 and PfEMP1.The size of the population of already matured DCs at the time of infection might be crucial to the outcome. A further population of γδ T cells (those expressing γδ T-cell receptors) might also be important in immunity, possibly independently of αβ CD4+ T cells and antibody88,89.
Some of the factors that are thought to give rise to antibody-independent (T-cell) immunity are shown. This commences with the activation of CD4+ T cells by mature dendritic cells leading to macrophage activation, phagocytosis of parasitized red blood cells (pRBC), and elaboration of cytokines and small inflammatory molecules (such as nitric oxide and oxygen redicals). T-cell immunity is thought to occur largely, but not entirely, in the spleen. IFN-γ, interferon-γ; IL-12, interleukin-12; MHC II, major histocompatibility complex class II; TCR, T-cell receptor; TNF-α, tumour-necrosis factor-α.
When considering the role of CD4+ T cells in human malaria immunity it is instructive to consider the impact of human immunodeficiency virus (HIV)/AIDS on malaria prevalence. Data from several earlier studies90,91,92,93,94,95,96,97 indicated that HIV infection was not associated with a loss of malaria immunity. A recent comprehensive study by Whitworth et al.98, however, found a statistically significant association between the HIV status of adults (mean age 35 years) and the presence of parasites in the blood, and between HIV status and clinical malaria. However, the reported parasite densities were low throughout the study and there were no deaths due to malaria. The overall effects, although statistically significant, would seem small. Furthermore, as noted by Verhoef et al.99, the regression lines of parasite density versus CD4+ T-cell count were not parallel in the HIV-positive cohort and the HIV-negative cohort, as would be expected if HIV was acting directly through a mechanism that involves CD4+ T cells. An earlier study in Malawi100, however, found a significant association between HIV status and the presence of parasitaemia in multigravida women during pregnancy and at delivery. However, little or no difference was observed between parasite burdens of primigravidae with and without HIV. Furthermore, and in contrast with the Whitworth et al.98 study, the prevalence and density of malaria parasitaemia in women more than 60 days after delivery did not differ significantly by HIV status or gravidity. The pregnancy data indicate that HIV specifically interferes with the development of pregnancy-specific immunity, which is thought in large part to be due to the development of antibodies to PfEMP1 epitopes that bind receptors on the placenta101,102,103. The impact of HIV on non-pregnancy-associated malaria immunity is, however, still a controversial area, and further studies are required to clarify the situation and provide insight into the role of T cells in human malaria immunity.
It is difficult to reconcile the existing epidemiological data with the data from animal models that indicate that T cells can be highly efficacious in their anti-parasite effect. A possible explanation is that effector T cells are not activated during infection with P. falciparum, or that, if activated, they are quickly deleted. There is evidence to support this in humans with data showing apoptosis of mononuclear cells taken from patients with acute malaria and placed in vitro104. The specificity of these T cells, however, could not be determined. An alternative hypothesis is that lymphopaenia observed in malaria patients might be due to reallocation of CD4+ T cells to other sites, particularly during severe malaria105. However, we have examined the effect of malaria infection on adoptively transferred CD4+ T cells labelled with a fluorescent dye and specific for whole rodent parasites (P. berghei, P. yoelii or P. chabaudi). This dye labels cytoplasmic proteins and enables one to track and enumerate the labelled cells in recipient mice and to estimate the number of cellular divisions that have occurred (judged by the sequential halving of the fluorescent signal at each division). This technique, in conjunction with other methodologies to quantify cells and cell death, enables one to determine whether cells are expanded in number or deleted from the recipient as a result of infection. We found that infection specifically deletes CD4+ T cells that are specific for the parasite, although it spares CD4+ T cells specific for an irrelevant antigen, ovalbumin (Ref. 106 and H. Xu et al., unpublished observations). The epidemiological data with respect to HIV/AIDS and malaria might reflect a possible deletion of malaria-specific effector CD4+ T cells after exposure to malaria, which would not be further affected by infection with HIV. An alternative explanation for the lack of a pronounced worsening of malaria resistance (in the non-pregnant human) in the face of HIV exposure is that severe disease in malaria might, in part, be T-cell mediated107. However, this is a controversial area, and it is difficult at this stage to reconcile all the epidemiological and experimental data.
If effector CD4+ T cells do not contribute greatly to natural immunity to malaria then antigens that would otherwise be targeted by these cells might not be under immune pressure. Antigenic variation and allelic polymorphism are the hallmarks of target antigens on the surface of infected RBCs or merozoites recognized by protective antibodies (see above). If target antigens of T cells could be defined they could be ideal vaccine candidates for a vaccine designed to stimulate a non-natural form of immunity. By boosting the number of parasite-specific CD4+ effector T cells, the ability of the parasite to multiply might be severely impeded. CD4+ T cells might get the 'upper hand' before the parasite leads to their apoptotic deletion. Evidence to support this concept comes from studies in rodents in which it has been shown that it is possible to passively transfer immunity to malaria (that is, lower parasite densities in the blood after challenge) with clones or lines of cultured T cells80,81,82.
The future
So the question that is posed in the title of this article remains — are we following all the leads? We are, but progress is slow. When malaria antigens were first cloned, there was a belief that it would be a relatively short time until a vaccine was produced. The 'average' vaccine takes 10–15 years to develop. Against the many challenges, there have been some very optimistic signs. The resilience and dedication of the scientific community to pursue this vaccine are noteworthy. Over the years we have therefore accumulated a lot of knowledge about the pathogenesis of malaria and the nature of immunological responses. The P. falciparum genome sequence is now nearly complete (see link to PlasmoDB: The Plasmodium Genome Resource) and this will provide new insights into pathogenesis and vaccine development. Governments are continuing to invest in the more basic aspects of malaria vaccine research and important philanthropic organizations (such as the Bill & Melinda Gates Foundation) have pledged significant funding to further study vaccine development and to enable trials of the main vaccine candidates to proceed.With continuing perseverance, an effective vaccine will be developed.
References
Good, M. F., Kumar, S. & Miller, L. H. The real difficulties for malaria sporozoite vaccine development. Nonresponsiveness and antigenic variation. Immunol. Today 9, 351–355 (1988).
Good, M. F., Zevering, Y., Currier, J. & Bilsborough, J. 'Original antigenic sin', T cell memory, and malaria sporozoite immunity: an hypothesis for immune evasion. Parasite Immunol. 15, 187–193 (1993).
Taylor, R. R. et al. Selective recognition of malaria antigens by human serum antibodies is not genetically determined but demonstrates some features of clonal imprinting. Int. Immunol. 8, 905–915 (1996).
Riley, E. M. The role of MHC- and non-MHC-associated genes in determining the human immune response to malaria antigens. Parasitology 112, S39–S51 (1996).
Plotkin, S. L. & Plotkin, S. A. in Vaccines (eds Plotkin, S. A. & Mortimer, E. A.) 1–7 (W. B. Saunders Co., Philadelphia, 1988).
Good, M. F. & Doolan, D. L. Immune effector mechanisms in malaria. Curr. Opin. Immunol. 11, 412–419 (1999).
Doolan, D. L. & Hoffman, S. L. The complexity of protective immunity against liver-stage malaria. J. Immunol. 165, 1453–1462 (2000).
Kaslow, D. C. Transmission-blocking vaccines: uses and current status of development. Int. J. Parasitol. 27, 183–189 (1997).
Miller, L. H., Good, M. F. & Milon, G. Disease pathogenesis in malaria. Science 264, 1878–1883 (1994).This paper provides a concise overview of the pathogenesis of malaria.
Baird, J. K. Age-dependent characteristics of protection v. susceptibility to Plasmodium falciparum. Ann. Trop. Med. Parasitol. 92, 367–390 (1998).
Marsh, K. & Howard, R. J. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231, 150–153 (1986).This paper provides some of the best early insights into the nature of natural immunity to malaria and provides an explanation for why prolonged exposure to the parasite is required for this to develop.
Newbold, C. I., Pinches, R., Roberts, D. J. & Marsh, K. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp. Parasitol. 75, 281–292 (1992).
Greenwood, B. M. et al. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans. R. Soc. Trop. Med. Hyg. 81, 478–486 (1987).
Biggs, B. A. et al. Antigenic variation in Plasmodium falciparum. Proc. Natl Acad. Sci. USA 88, 9171–9174 (1991).
Baruch, D. I. et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82, 77–87 (1995).
Su, X. Z. et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82, 89–100 (1995).
Smith, J. D. et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82, 101–110 (1995).References 15–17 describe the cloning of the variant antigens of P. falciparum , which enables us to develop rational molecular approaches to understanding pathogenesis and natural immunity to malaria.
Brandt, E. R. et al. New multi-determinant strategy for a group A streptococcal vaccine designed for the Australian Aboriginal population. Nature Med. 6, 455–459 (2000).
Sercarz, E. E. et al. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 11, 729–766 (1993).
Good, M. F., Branigan, J., Smith, G. & Houghten, R. A. Peptide immunization can elicit malaria protein-specific memory helper but not proliferative T cells. Importance of cryptic epitopes in consideration of vaccine design. Pept. Res. 3, 110–115 (1990).
Amante, F. H., Crewther, P. E., Anders, R. F. & Good M. F. A cryptic T cell epitope on the apical membrane antigen 1 of Plasmodium chabaudi adami can prime for an anamnestic antibody response: implications for malaria vaccine design. J. Immunol. 159, 5535–5544 (1997).
Lo-Man, R. et al. A fully synthetic immunogen carrying a carcinoma-associated carbohydrate for active specific immunotherapy. Cancer Res. 59, 1520–1524 (1999).
Raju, R., Diethelm-Okita, B., Okita, D. & Conti-Fine, B. M. Epitope repertoire of human CD4+ lines propagated with tetanus toxoid or with synthetic tetanus toxin sequences. J. Autoimmun. 9, 79–88 (1996).
Bou-Habib, D. C. et al. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J. Virol. 68, 6006–6013 (1994).
Robert-Guroff, M. et al. Alteration of V3 loop context within the envelope of human immunodeficiency virus type 1 enhances neutralization. J. Virol. 68, 3459–3466 (1994).
Gamain, B., Miller, L. H. & Baruch, D. I. The surface variant antigens of Plasmodium falciparum contain cross-reactive epitopes. Proc. Natl Acad. Sci. USA 98, 2664–2669 (2001).
Good, M. F., Kaslow, D. C. & Miller, L. H. Pathways and strategies for developing a malaria blood-stage vaccine. Annu. Rev. Immunol. 16, 57–87 (1998).
Holder, A. A. in Malaria Vaccine Development: A Multi-immune Response Approach (ed. Hoffman, S. L.) 77–104 (American Society for Microbiology Press, Washington, DC, 1996).
Holder, A. A. Malaria vaccines. Proc. Natl Acad. Sci. USA 96, 1167–1169 (1999).
Deans, J. A. et al. Vaccination trials in rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66 kD merozoite antigen. Parasite Immunol. 10, 535–552 (1988).
Collins, W. E. et al. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am. J. Trop. Med. Hyg. 51, 711–719 (1994).
Crewther, P. E., Matthew, M. L., Flegg, R. H. & Anders, R. F. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect. Immun. 64, 3310–3317 (1996).
Anders, R. F. & Saul, A. Malaria vaccines. Parasitol. Today 16, 444–447 (2000).
Kumar, S. et al. Immunogenicity and efficacy in aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 68, 2215–2223 (2000).
Egan, A. F., Blackman, M. J. & Kaslow, D. C. Vaccine efficacy of recombinant Plasmodium falciparum merozoite surface protein 1 in malaria-naive,-exposed, and/or-rechallenged Aotus vociferans monkeys. Infect. Immun. 68, 1418–1427 (2000).
Ling, I. T., Ogun, S. A. & Holder, A. A. Immunization against malaria with a recombinant protein. Parasite Immunol. 16, 63–67 (1994).
Daly, T. M. & Long, C. A. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J. Immunol. 155, 236–243 (1995).
Tian, J.-H. et al. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J. Immunol. 157, 1176–1183 (1996).
Hirunpetcharat, C. et al. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl terminal fragment of the merozoite surface protein-1 (MSP119) of Plasmodium yoelii expressed in Saccharomyves cerevisiae: Correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 159, 3400–3411 (1997).
Chitarra, V., Holm, I., Bentley, G. A., Petres, S. & Longacre, S. The crystal structure of C-terminal merozoite surface protein 1 at 1.8 Å resolution, a highly protective malaria vaccine candidate. Mol. Cell 3, 457–464 (1999).
Blackman, M. J., Heidrich, H. G., Donachie, S., McBride, J. S. & Holder, A. A. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172, 379–382 (1990).
Herrera, M. A. et al. Protection against malaria in Aotus monkeys immunized with a recombinant blood-stage antigen fused to a universal T-cell epitope: correlation of serum γ-interferon levels with protection. Infect. Immun. 60, 154–158 (1992).
Lawrence, G. et al. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine 18, 1925–1931 (2000).
Genton, B. et al. Safety and immunogenicity of a three-component blood-stage malaria vaccine in adults living in an endemic area of Papua New Guinea. Vaccine 18, 2504–2511 (2000).
Alonso, P. L. et al. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Lancet 344, 1175–1181 (1994).
D'Alessandro, U. et al. Efficacy trial of malaria vaccine SPf66 in Gambian infants. Lancet 346, 462–467 (1995).
Nosten, F. et al. Randomised double-blind placebo-controlled trial of SPf66 malaria vaccine in children in northwestern Thailand. Shoklo SPf66 Malaria Vaccine Trial Group. Lancet 348, 701–707 (1996).
Bojang, K. A. et al. An efficacy trial of the malaria vaccine SPf66 in Gambian infants – second year of follow-up. Vaccine 16, 62–67 (1998).
Metzger, W. G. et al. Serological responses of Gambian children to immunization with the malaria vaccine SPf66. Parasite Immunol. 21, 335–340 (1999).
Guevara Patino, J. A., Holder, A. A., McBride, J. S. & Blackman, M. J. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 186, 1689–1699 (1997).
Uthaipibull, C. et al. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 307, 1381–1394 (2001).
Conway, D. J. et al. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nature Med. 6, 689–692 (2000).
Miller, L. H., Roberts, T., Shahabuddin, M. & McCuthchan, T. F. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1). Mol. Biochem. Parasitol. 59, 1–14 (1993).
Blackman, M. J., Ling, I. T., Nicholls, S. C. & Holder, A. A. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 49, 29–33 (1991).
Hirunpetcharat, C. et al. Intranasal immunization with yeast-expressed 19 kD carboxyl terminal fragment of Plasmodium yoelii merozoite surface protein-1 (yMSP119) induces protective immunity to blood stage malaria infection in mice. Parasite Immunol. 20, 413–420 (1998).
Rotman, H. L., Daly, T. M., Clynes, R. & Long, C. A. Fc receptors are not required for antibody-mediated protection against lethal malaria challenge in a mouse model. J. Immunol. 161, 1908–1912 (1998).
Vukovic, P., Hogarth, P. M., Barnes, N., Kaslow, D. C. & Good, M. F. Immunoglobulin G3 antibodies specific for the 19-kilodalton carboxyl-terminal fragment of the Plasmodium yoelii merozoite surface protein 1 transfer protection to mice deficient in Fc–γRI receptors. Infect. Immun. 68, 3019–3022 (2000).
Oeuvray, C. et al. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect. Immun. 68, 2617–2620 (2000).
Druilhe, P. & Perignon, J. L. Mechanisms of defence against P. falciparum asexual blood stages in humans. Immunol. Lett. 41, 115–120 (1994).
Freeman, R. R. & Holder, A. A. Characteristics of the protective response of BALB/c mice immunized with a purified Plasmodium yoelii schizont antigen. Clin. Exp. Immunol. 54, 609–616 (1983).
Tian, J. H. et al. Definition of T cell epitopes within the 19 kDa carboxyl terminal fragment of Plasmodium yoelii merozoite surface protein 1 (MSP1) and their role in immunity to malaria. Parasite Immunol. 20, 263–278 (1998).
Hirunpetcharat, C. et al. Absolute requirement for an active immune response involving B cells and TH cells in immunity to Plasmodium yoelii passively acquired with antibodies to the 19 kDa carboxyl terminal fragment of merozoite surface protein-1. J. Immunol. 162, 7309–7314 (1999).
Riley, E. M. et al. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 14, 321–337 (1992).
Branch, O. H. et al. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kiloDalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am. J. Trop. Med. Hyg. 58, 211–219 (1998).
Egan, A. F., Burghaus, P., Druilhe, P., Holder, A. A. & Riley, E. M. Human antibodies to the 19 kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 21, 133–139 (1999).
Dodoo, D. et al. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect. Immun. 67, 2131–2137 (1999).
Marsh, K., Otoo, L., Hayes, R. J., Carson, D. C. & Greenwood, B. M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 83, 293–303 (1989).
O'Donnell, R. A. et al. Antibodies against merozoite surface protein (MSP)-119 are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193, 1403–1412 (2001).
Stoute, J. A. et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N. Engl. J. Med. 336, 86–91 (1997).
Stoute, J. A. et al. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J. Infect. Dis. 178, 1139–1144 (1998).
Boyle, J. S., Barr, I. G. & Lew, A. M. Strategies for improving responses to DNA vaccines. Mol. Med. 5, 1–8 (1999).By targeting antigen-presenting cells, DNA vaccines can induce far greater antibody responses. This might be crucial to developing successful vaccines against organisms such as malaria.
Shi, Y. P. et al. Immunogenicity and in vitro protective efficacy of a recombinant multistage Plasmodium falciparum candidate vaccine. Proc. Natl Acad. Sci. USA 96, 1615–1620 (1999).
Shi, Y. P. et al. Development, expression, and murine testing of a multistage Plasmodium falciparum malaria vaccine candidate. Vaccine 18, 2902–2914 (2000).
Wang, R. et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282, 476–480 (1998).
Engers, H. D. & Godal, T. Malaria vaccine development: current status. Parasitol. Today 14, 56–64 (1998).
James, S. & Miller, L. Malaria vaccine development: Status report, 2000. http://www.nature.com/nm/special_focus/malaria/commentaries/malcom
Grun, J. L. & Weidanz, W. P. Antibody-independent immunity to reinfection malaria in B-cell-deficient mice. Infect. Immun. 41, 1197–1204 (1983).
van der Heyde, H. C., Huszar, D., Woodhouse, C., Manning, D. D. & Weidanz, W. P. The resolution of acute malaria in a definitive model of B cell deficiency, the JHD mouse. J. Immunol. 152, 4557–4562 (1994).
von der Weid, T., Honarvar, N. & Langhorne, J. Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J. Immunol. 156, 2510–2516 (1996).
Brake, D. A., Long, C. A. & Weidanz, W. P. Adoptive protection against Plasmodium chabaudi adami malaria in athymic nude mice by a cloned T cell line. J. Immunol. 140, 1989–1993 (1988).
Taylor-Robinson, A. W., Phillips, R. S., Severn, A., Moncada, S. & Liew, F. Y. The role of TH1 and TH2 cells in a rodent malaria infection. Science 260, 1931–1934 (1993).Indicates that T H 1- and T H 2-type T cells can control malaria infections through different mechanisms.
Amante, F. H. & Good, M. F. Prolonged TH1-like response generated by a Plasmodium yoelii-specific T cell clone allows complete clearance of infection in reconstituted mice. Parasite Immunol. 19, 111–126 (1997).
Stevenson, M. M., Tam, M. F., Wolf, S. F. & Sher, A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-γ and TNF-α and occurs via a nitric oxide-dependent mechanism. J. Immunol. 155, 2545–2556 (1995).
Favila-Castillo, L. et al. Protection of rats against malaria by a transplanted immune spleen. Parasite Immunol. 18, 325–331 (1996).
Su, Z. & Stevenson, M. M. Central role of endogenous γ-interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect. Immun. 68, 4399–4406 (2000).
Luty, A. J. et al. Interferon-γ responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J. Infect. Dis. 179, 980–988 (1999).
Urban, B. C. et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400, 73–77 (1999).Indicates that the maturation of dendritic cells might be impeded by parasitized RBCs, therefore dampening a developing T-cell response to the parasite.
van der Heyde, H. C., Elloso, M. M., Chang, W. L., Kaplan, M., Manning, D. D. &Weidanz, W. P. γδ T cells function in cell-mediated immunity to acute blood-stage Plasmodium chabaudi adami malaria. J. Immunol. 154, 3985–3990 (1995).
Seixas, E. M. & Langhorne, J. γδ T cells contribute to control of chronic parasitemia in Plasmodium chabaudi infections in mice. J. Immunol. 162, 2837–2841 (1999).
Nguyen-Dinh, P. et al. Absence of association between Plasmodium falciparum malaria and human immunodeficiency virus infection in children in Kinshasa, Zaire. Bull. World Health Organ. 65, 607–613 (1987).
Simooya, O. O., Mwendapole, R. M., Siziya, S. & Fleming, A. F. Relation between falciparum malaria and HIV seropositivity in Ndola, Zambia. Br. Med. J. 297, 30–31 (1988). | PubMed |
Muller, O. & Moser, R. The clinical and parasitological presentation of Plasmodium falciparum malaria in Uganda is unaffected by HIV-1 infection. Trans. R. Soc. Trop. Med. Hyg. 84, 336–338 (1990).
Allen, S. et al. Human immunodeficiency virus and malaria in a representative sample of childbearing women in Kigali, Rwanda. J. Infect. Dis. 164, 67–71 (1991).
Greenberg, A. E. et al. Plasmodium falciparum malaria and perinatally acquired human immunodeficiency virus type 1 infection in Kinshasa, Zaire. A prospective, longitudinal cohort study of 587 children. N. Engl. J. Med. 325, 105–109 (1991).
Colebunders, R. et al. Incidence of malaria and efficacy of oral quinine in patients recently infected with human immunodeficiency virus in Kinshasa, Zaire. J. Infect. 21, 167–173 (1990).
Kalyesubula, I. et al. Effects of malaria infection in human immunodeficiency virus type 1-infected Ugandan children. Pediatr. Infect. Dis. J. 16, 876–881 (1997).
Chandramohan, D. & Greenwood, B. M. Is there an interaction between human immunodeficiency virus and Plasmodium falciparum? Int. J. Epidemiol. 27, 296–301 (1998).
Whitworth, J. et al. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet 356, 1051–1056 (2000).
Verhoef, H., Veenemans, J. & West, C. E. HIV-1 infection and malaria parasitaemia. Lancet 357, 232–233 (2001).
Steketee, R. W. et al. Impairment of a pregnant woman's acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am. J. Trop. Med. Hyg. 55, 42–49 (1996).
Fried, M., Nosten, F., Brockman, A., Brabin, B. J. & Duffy, P. E. Maternal antibodies block malaria. Nature 395, 851–852 (1998).
Reeder, J. C. et al. The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1. Proc. Natl Acad. Sci. USA 96, 5198–5202 (1999).
Buffet, P. A. et al. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: a receptor for human placental infection. Proc. Natl Acad. Sci. USA 96, 12743–12748 (1999).
Toure-Balde, A. et al. Plasmodium falciparum induces apoptosis in human mononuclear cells. Infect. Immun. 64, 744–750 (1996).
Hviid, L. et al. Rapid reemergence of T cells into peripheral circulation following treatment of severe and uncomplicated Plasmodium falciparum malaria. Infect. Immun. 65, 4090–4093 (1997).
Hirunpetcharat, C. & Good, M. F. Deletion of Plasmodium berghei-specific CD4+ T cells adoptively transferred into recipient mice after challenge with homologous parasite. Proc. Natl Acad. Sci. USA 95, 1715–1720 (1998).Indicates that malaria infection can cause deletion of parasite-specific CD4+ T cells. This might prove to be a useful parasite-defence mechanism.
Hirunpetcharat, C., Finkelman, F., Clark, I. A. & Good, M. F. Malaria parasite-specific TH1-like T cells simultaneously reduce parasitemia and promote disease. Parasite Immunol. 21, 319–329 (1999).
Laveran, A. A new parasite found in the blood of malarial patients. Parasitic origin of malarial attacks. Bull. Mem. Soc. Med. Hop. Paris 17, 158–164 (1880).
Ross, R. On some peculiar pigmented cells found in two mosquitos fed on malaria blood. Br. Med. J. 2, 1786–1788 (1897).
Russel, P. F., West, L. R., Manwell, R. D. & MacDonald, G. Practical Malariology 13 (Oxford Univ. Press, 1963).
Russel, P. F., West, L. R., Manwell, R. D. & MacDonald, G. Practical Malariology 631 (Oxford Univ. Press, 1963).
Bruce-Chwatt, L. J. Essential Malariology 301–302 (Alden Press, Oxford, 1985).
Fairley, N. H. et al. Sidelights on malaria in man obtained by subinoculation experiments. Trans. R. Soc. Trop. Med. Hyg. 40, 621–676 (1947).
Kemp, D. J. et al. Expression of Plasmodium falciparum blood-stage antigens in Escherichia coli: detection with antibodies from immune humans. Proc. Natl Acad. Sci. USA 80, 3787–3791 (1983).
Ellis, J. et al. Cloning and expression in E. coli of the malarial sporozoite surface antigen gene from Plasmodium knowlesi. Nature 302, 536–538 (1983).
Acknowledgements
I am very grateful to L. Miller, D. Kemp, R. Anders, L. Martin, M. Wykes, S. Elliott and H. Xu for critically reviewing this manuscript. M.F.G. receives research funding support from the National Health and Medical Research Council (Australia) and the United Nations Development Programme/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases.
Author information
Authors and Affiliations
Related links
Glossary
- ANTIGENIC VARIATION
-
The antigenic changes that can occur within a parasite clone through switching the expression of different 'variant' genes.
- ALLELIC POLYMORPHISM
-
Multiple forms of a gene at a single genetic locus.
- SUBUNIT VACCINES
-
Vaccines comprising only a small part of the entire organism, typically a recombinant protein.
- CLONAL IMPRINITNG/ORIGINAL ANTIGENIC SIN
-
Prior exposure to one strain diverts the antibody response following exposure to a second strain to shared epitopes.
- AGGLUTINATING ANTIBODIES
-
Antibodies directed to parasite-encoded antigens that are expressed on the surface of the red blood cell and lead to clumping of infected red cells.
- MEROZOITE
-
The form of the parasite that emerges from infected liver cells and red blood cells (RBCs) and invades fresh RBCs.
Rights and permissions
About this article
Cite this article
Good, M. Towards a blood-stage vaccine for malaria: are we following all the leads?. Nat Rev Immunol 1, 117–125 (2001). https://doi.org/10.1038/35100540
Issue Date:
DOI: https://doi.org/10.1038/35100540
This article is cited by
-
Effect of malaria transmission reduction by insecticide-treated bed nets (ITNs) on the genetic diversity of Plasmodium falciparum merozoite surface protein (MSP-1) and circumsporozoite (CSP) in western Kenya
Malaria Journal (2013)
-
Immunogenicity of bacterial-expressed recombinant Plasmodium knowlesi merozoite surface protein-142 (MSP-142)
Malaria Journal (2013)
-
Polymorphism and epitope sharing between the alleles of merozoite surface protein-1 of Plasmodium falciparum among Indian isolates
Malaria Journal (2007)
-
Similarity searches in genome-wide numerical data sets
Biology Direct (2006)
-
Genetic vaccination approaches against malaria based on the circumsporozoite protein
Wiener klinische Wochenschrift (2006)