Abstract
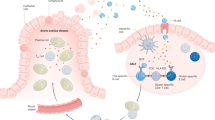
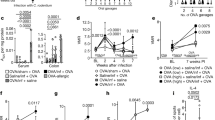
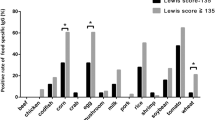
Among gluten-related disorders, gluten sensitivity is an emerging entity that is characterized by a wide array of manifestations. In particular, patients complain of IBS-like symptoms and extraintestinal manifestations that occur shortly after the ingestion of gluten. Symptoms improve or disappear when gluten is withdrawn from the diet, and recur if gluten is reintroduced. Laboratory tests are usually unhelpful for diagnosis, although ∼50% of patients are positive for IgG antigliadin antibodies. The natural history of gluten sensitivity is unknown; in particular, it is still to be clarified whether this disorder is permanent or transient and whether it is linked to autoimmunity. The pathogenesis of gluten sensitivity is unclear; data so far demonstrate a predominant activation of innate immune responses. Further research is necessary to establish the main clinicopathological features of gluten sensitivity, thus enabling physicians to improve their management of the increasing number of patients who are sensitive to dietary gluten.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Catassi, C. et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann. Med. 42, 530–538 (2010).
Troncone, R. & Jabri, B. Coeliac disease and gluten sensitivity. J. Intern. Med. 269, 582–590 (2011).
Blomfeldt, T. O., Kuktaite, R., Johansson, E. & Hedenqvist, M. S. Mechanical properties and network structure of wheat gluten foams. Biomacromolecules 12, 1707–1715 (2011).
Belderok, B. Developments in bread-making processes. Plant Food Hum. Nutr. 55, 1–86 (2000).
Gobbetti, M., Rizzello, C. G., Di Cagno, R. & De Angelis, M. Sourdough lactobacilli and celiac disease. Food Microbiol. 24, 187–196 (2007).
Gibert, A. et al. Consumption of gluten-free products: should the threshold value for trace amounts of gluten be at 20,100 or 200 p.p.m.? Eur. J. Gastroenterol. Hepatol. 18, 1187–1195 (2006).
Fraser, J. S. et al. Coeliac disease: in vivo toxicity of the putative immunodominant epitope. Gut 52, 1698–1702 (2003).
Drago, S. et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 41, 408–419 (2006).
Dolfini, E. et al. Cytoskeleton reorganization and ultrastructural damage induced by gliadin in a three-dimensional in vitro model. World J. Gastroenterol. 11, 7597–7601 (2005).
Caja, S., Mäki, M., Kaukinen, K. & Lindfors, K. Antibodies in coeliac disease: implications beyond diagnostics. Cell. Mol. Immunol. 8, 103–109 (2011).
Verdu, E. F., Armstrong, D. & Murrat, J. A. Between celiac disease and irritable bowel syndrome: the “no man's land” of gluten sensitivity. Am. J. Gastroenterol. 104, 1587–1594 (2009).
Sapone, A. et al. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int. Arch. Allergy Immunol. 152, 75–80 (2010).
Bizzaro, N., Tozzoli, R., Villalta, D., Fabris, M. & Tonutti, E. Cutting-edge issues in celiac disease and in gluten intolerance. Clin. Rev. Allergy Immunol. http://dx.doi.org/10.1007/s12016-010-8223-1.
Verdu, E. F. Can gluten contribute to irritable bowel syndrome? Am. J. Gastroenterol. 106, 516–518 (2011).
Cooper, B. T. et al. Gluten-sensitive diarrhea without evidence of celiac disease. Gastroenterology 79, 801–806 (1980).
Kaukinen, K. et al. Intolerance to cereals is not specific for coeliac disease. Scand. J. Gastroenterol. 35, 942–946 (2000).
Sapone, A. et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 9, 23 (2011).
Granzotto, M. et al. Regulatory T-cell function is impaired in celiac disease. Dig. Dis. Sci. 54, 1513–1519 (2009).
Vorobjova, T. et al. Increased FOXP3 expression in small-bowel mucosa of children with coeliac disease and type I diabetes mellitus. Scand. J. Gastroenterol. 44, 422–430 (2009).
Hansson, T. et al. Transforming growth factor-β (TGF-β) and tissue transglutaminase expression in the small intestine in children with coeliac disease. Scand. J. Immunol. 56, 530–537 (2002).
Schuppan, D., Junker, Y. & Barisani, D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology 137, 1912–1933 (2009).
Biesiekierski, J. R. et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am. J. Gastroenterol. 106, 508–514 (2011).
Verdu, E. F. et al. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G217–G225 (2008).
Natividad, J. M. et al. Host responses to intestinal microbial antigens in gluten sensitive mice. PLoS ONE 4, e6472 (2009).
Volta, U. et al. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J. Clin. Gastroenterol. http://dx.doi.org/10.1097/MCG.0b013e3182372541.
Troncone, R. et al. In siblings of celiac children, rectal gluten challenge reveals gluten sensitization not restricted to celiac HLA. Gastroenterology 111, 318–324 (1996).
Dezi, R. et al. Gluten sensitivity in the rectal mucosa of first-degree relatives of celiac disease patients. Am. J. Gastroenterol. 92, 1326–1330 (1997).
Wahnschaffe, U., Schulzke, J., Zeitz, M. & Ullrich, R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 5, 844–850 (2007).
Volta, U. et al. Usefulness of antibodies to deamidated gliadin peptides in celiac disease diagnosis and follow-up. Dig. Dis. Sci. 53, 1582–1588 (2008).
Volta, U. et al. High prevalence of celiac disease in the general population. Dig. Dis. Sci. 46, 1500–1505 (2001).
Hadjivassiliou, M. et al. Does cryptic gluten sensitivity play a part in neurological illness? Lancet 347, 369–371 (1996).
Volta, U. et al. IgA antigliadin antibodies and persistence of jejunal lesions in adult coeliac disease. Digestion 47, 111–114 (1990).
Oberhüber, G., Granditsch, G. & Vogelsang, H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 11, 1185–1194 (1999).
Brown, I., Mino-Kenudson, M., Deshpande, V. & Lauwers, G. Y. Intraepithelial lymphocytosis in architecturally preserved proximal small intestinal mucosa: an increasing diagnostic problem with a wide differential diagnosis. Arch. Pathol. Lab. Med. 130, 1020–1025 (2006).
Salmi, T. T. et al. Immunoglobulin A autoantibodies against transglutaminase 2 in the small intestinal mucosa predict forthcoming coeliac disease. Aliment. Pharmacol. Ther. 24, 541–552 (2006).
Maglio, M. et al. Intestinal deposits of anti-tissue transglutaminase IgA in childhood celiac disease. Dig. Liver Dis. 43, 604–608 (2011).
Acknowledgements
The authors would like to acknowledge the support of grants PRIN/COFIN from the Italian Ministry of University, Research and Education 2008 and 2010, RFO funds from the University of Bologna and research grants from Fondazione Del Monte di Bologna e Ravenna.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Volta, U., De Giorgio, R. New understanding of gluten sensitivity. Nat Rev Gastroenterol Hepatol 9, 295–299 (2012). https://doi.org/10.1038/nrgastro.2012.15
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2012.15
This article is cited by
-
An updated overview of spectrum of gluten-related disorders: clinical and diagnostic aspects
BMC Gastroenterology (2020)
-
Disturbi dell’umore e patologie glutine correlate
La Rivista Italiana della Medicina di Laboratorio - Italian Journal of Laboratory Medicine (2017)
-
The relation between celiac disease, nonceliac gluten sensitivity and irritable bowel syndrome
Nutrition Journal (2015)
-
Diet in irritable bowel syndrome
Nutrition Journal (2015)
-
The spectrum of noncoeliac gluten sensitivity
Nature Reviews Gastroenterology & Hepatology (2015)