Abstract
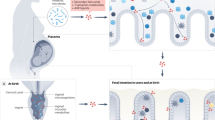
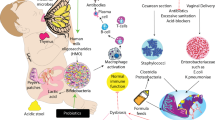
Interaction with colonizing intestinal bacteria is essential for healthy intestinal and immunological development in infancy. Advances in understanding early host–microbe interactions indicate that this early microbial programming begins in utero and is substantially modulated by mode of birth, perinatal antibiotics and breastfeeding. Furthermore, it has become evident that this stepwise microbial colonization process, as well as immune and metabolic programming by the microbiota, might have a long-lasting influence on the risk of not only gastrointestinal disease, but also allergic, autoimmune and metabolic disease, in later life. Modulating early host–microbe interaction by maternal probiotic intervention during pregnancy and breastfeeding offers a promising novel tool to reduce the risk of disease. In this Review, we describe the current body of knowledge regarding perinatal microbial contact, initial intestinal colonization and its association with human disease, as well as means of modulating early host–microbe interaction to reduce the risk of disease in the child.
Key Points
-
Contact with microbes begins in utero and proceeds in a stepwise manner during birth and early infancy
-
Early host–microbe interaction is a crucial component of healthy immune and metabolic programming
-
Mode of delivery, prematurity, perinatal antibiotic exposure and breastfeeding have a major influence on early microbial contact, intestinal colonization and subsequent risk of disease
-
Maternal probiotic supplementation during pregnancy and breastfeeding has shown potential in reducing the risk of immune-mediated and metabolic disease via modulation of early host–microbe interactions
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
DuPont, A. W. & DuPont, H. L. The intestinal microbiota and chronic disorders of the gut. Nat. Rev. Gastroenterol. Hepatol. 16, 523–531 (2011).
Roduit, C. et al. Prenatal animal contact and gene expression of innate immunity receptors at birth are associated with atopic dermatitis. J. Allergy Clin. Immunol. 127, 179–185 (2011).
Conrad, M. L. et al. Maternal TLR signalling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J. Exp. Med. 206, 2869–2877 (2009).
Kalliomäki, M. et al. Disticnt patterns of neonatal gut microflora in infants in whom allergy was and was not developing. J. Allergy Clin. Immunol. 107, 129–134 (2001).
Björkstén, B., Sepp, E., Julge K., Voor, T. & Mikelsaar, M. Allergy development and the intestinal microflora during the first year of life. J. Allergy Clin. Immunol. 108, 516–520 (2001).
Kalliomäki, M., Collado, M. C., Salminen, S. & Isolauri, E. Early differences in fecal microbiota composition in children may predict overweight. Am. J. Clin. Nutr. 87, 534–538 (2008).
Johansson, M. A., Sjögren, Y. M., Persson, J. O., Nilsson, C. & Sverremark-Ekström, E. Early colonization with a group of Lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PLoS ONE 6, e23031 (2011).
Ajslev, T., Andersen, C. S., Gamborg, M., Sørensen, T. I. & Jess, T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int. J. Obes. (Lond). 35, 522–529 (2011).
Harmsen, H. J. et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 30, 61–67 (2000).
Roger, L. C., Costabile, A., Holland, D. T., Hoyles, L. & McCartney, A. L. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology 156, 3329–3341 (2010).
Roger, L. C. & McCartney, A. L. Longitudinal investigation of the faecal microbiota of healthy full-term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiology 156, 3317–3328 (2010).
Matsumiya, Y., Kato, N., Watanabe, K. & Kato, H. Molecular epidemiological study of vertical transmission of vaginal Lactobacillus species from mothers to newborn infants in Japanese, by arbitrarily primed polymerase chain reaction. J. Infect. Chemother. 8, 43–49 (2002).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
Gajer, P. et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 4, 132ra52 (2012).
Penders, J. et al. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol. Lett. 243, 141–147 (2005).
Penders, J. et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118, 511–521 (2006).
Palmer, C., Bik, E. M., DiGiulio, D. B., Relman, D. A. & Brown, P. O. Development of the human infant intestinal microbiota. PLoS Biol. 5, e177 (2007).
Koenig, J. E. et al. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4578–4585 (2011).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature 473, 174–180 (2011).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Goldenberg, R. L., Culhane, J. F., Iams, J. D. & Romero, R. Epidemiology and causes of preterm birth. Lancet 371, 75–84 (2008).
Stark, P. L. & Lee, A. The bacterial colonization of the large bowel of pre-term low birth weight neonates. J. Hyg. (Lond). 89, 59–67 (1982).
Björkström, M. V. et al. Intestinal flora in very low-birth weight infants. Acta Paediatr. 98, 1762–1767 (2009).
Butel, M. J. et al. Conditions of bifidobacterial colonization in preterm infants: a prospective analysis. J. Pediatr. Gastroenterol. Nutr. 44, 577–582 (2007).
Chang, J. Y., Shin, S. M., Chun, J., Lee, J. H. & Seo, J. K. Pyrosequencing-based molecular monitoring of the intestinal bacterial colonization in preterm infants. J. Pediatr. Gastroenterol. Nutr. 53, 512–519 (2011).
Wang, Y. et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 3, 944–954 (2009).
Rougé, C. et al. Investigation of the intestinal microbiota in preterm infants using different methods. Anaerobe 16, 362–370 (2010).
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
de la Cochetiere, M. F. et al. Early intestinal bacterial colonization and necrotizing enterocolitis in premature infants: the putative role of Clostridium. Pediatr. Res. 56, 366–370 (2004).
Mai, V. et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE 6, e20647 (2011).
Sudo, N. et al. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J. Immunol. 159, 739–745 (1997).
Olszak, T. et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 (2012).
Round, J. L. et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977 (2011).
Mazmanian, S. K., Liu, C. H., Tzianabos, A. O. & Kasper, D. L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118 (2005).
Ruiz, P. A., Hoffmann, M., Szcesny, S., Blaut, M. & Haller, D. Innate mechanisms for Bifidobacterium lactis to activate transient pro-inflammatory host responses in intestinal epithelial cells after the colonization of germ-free rats. Immunology 115, 441–450 (2005).
Wang, Q. et al. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J. Exp. Med. 203, 2853–2863 (2006).
Otte, J. M., Cario, E. & Podolsky, D. K. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 126, 1054–1070 (2004).
Bashir, M. E., Louie, S., Shi, H. N. & Nagler-Anderson, C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J. Immunol. 172, 6978–6987 (2004).
Hooper, L. V. et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–884 (2001).
Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl Acad. Sci. USA 101, 15718–15723 (2004).
Bäckhed, F., Manchester, J. K., Semenkovich, C. F. & Gordon, J. I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl Acad. Sci. USA 104, 979–984 (2007).
Ley, R. E. et al. Obesity alters gut microbial ecology. Proc. Natl Acad. Sci. USA 102, 11070–11075 (2005).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Rautava, S., Kalliomäki, M. & Isolauri, E. New therapeutic strategy for combating the increasing burden of allergic disease: Probiotics-A Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota (NAMI) Research Group report. J. Allergy Clin. Immunol. 116, 31–37 (2005).
Isolauri, E., Kalliomäki, M., Rautava, S., Salminen, S. & Laitinen, K. Obesity—extending the hygiene hypothesis. Nestle Nutr. Workshop Ser. Pediatr. Program. 64, 75–85 (2009).
Abraham, C. & Medzhitov, R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology 140, 1729–1737 (2011).
Decker, E. et al. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics 125, 1433–1440 (2010).
Mårild, K., Stephansson, O., Montgomery, S., Murray, J. A. & Ludvigsson, J. F. Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology 142, 39–45 (2012).
Algert, C. S., McElduff, A., Morris, J. M. & Roberts, C. L. Perinatal risk factors for early onset of Type 1 diabetes in a 2000–2005 birth cohort. Diabet. Med. 26, 1193–1197 (2009).
Bonifacio, E., Warncke, K., Winkler, C., Wallner, M. & Ziegler, A. G. Cesarean section and interferon-induced helicase gene polymorphisms combine to increase childhood type 1 diabetes risk. Diabetes 60, 3300–3306 (2011).
Kero, J. et al. Mode of delivery and asthma—is there a connection? Pediatr. Res. 52, 6–11 (2002).
Roduit, C. et al. Asthma at 8 years of age in children born by caesarean section. Thorax 64, 107–113 (2009).
Nistal, E. et al. Differences of small intestinal bacteria populations in adults and children with/without celiac disease: effect of age, gluten diet, and disease. Inflamm. Bowel Dis. 18, 649–656 (2012).
Giongo, A. et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 5, 82–91 (2011).
Russell, S. L. et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 13, 440–447 (2012).
Turnbaugh, P. J. et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484 (2009).
Jumpertz, R. et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 94, 58–65 (2011).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Membrez, M. et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 22, 2416–2426 (2008).
Mold, J. E. et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322, 1562–1565 (2008).
Roberts, K. A. et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta 32, 247–254 (2011).
Collado, M. C., Isolauri, E., Laitinen, K. & Salminen, S. Effect of mother's weight on infant's microbiota acquisition, composition, and activity during early infancy: a prospective follow-up study initiated in early pregnancy. Am. J. Clin. Nutr. 92, 1023–1030 (2010).
Andrews, W. W. et al. Endometrial microbial colonization and plasma cell endometritis after spontaneous or indicated preterm versus term delivery. Am. J. Obstet. Gynecol. 193, 739–745 (2005).
Onderdonk, A. B., Delaney, M. L., DuBois, A. M., Allred, E. N. & Leviton, A. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am. J. Obstet. Gynecol. 198, 1–7 (2008).
Bengtson, M. B. et al. Relationships between inflammatory bowel disease and perinatal factors: both maternal and paternal disease are related to preterm birth of offspring. Inflamm. Bowel Dis. 16, 847–855 (2010).
Goepfert, A. R. et al. Periodontal disease and upper genital tract inflammation in early spontaneous preterm birth. Obstet. Gynecol. 104, 777–783 (2004).
Bearfield, C., Davenport, E. S., Sivapathasundaram, V. & Allaker, R. P. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG 109, 527–533 (2002).
León, R. et al. Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J. Periodontol. 78, 1249–1255 (2007).
DiGiulio, D. B. Diversity of microbes in amniotic fluid. Semin. Fetal Neonatal Med. 17, 2–11 (2012).
Sánchez, P. J. & Regan, J. A. Vertical transmission of Ureaplasma urealyticum from mothers to preterm infants. Pediatr. Infect. Dis. J. 9, 398–401 (1990).
Sánchez, P. J. & Regan, J. A. Ureaplasma urealyticum colonization and chronic lung disease in low birth weight infants. Pediatr. Infect. Dis. J. 7, 542–546 (1988).
Okogbule-Wonodi, A. C. et al. Necrotizing enterocolitis is associated with Ureaplasma colonization in preterm infants. Pediatr. Res. 69, 442–447 (2011).
Leviton, A. et al. Microbiologic and histologic characteristics of the extremely preterm infant's placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatr. Res. 67, 95–101 (2010).
Goldenberg, R. L., Hauth, J. C. & Andrews, W. W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 342, 1500–1507 (2000).
Satokari, R., Grönroos, T., Laitinen, K., Salminen, S. & Isolauri, E. Bifidobacterium and Lactobacillus DNA in the human placenta. Lett. Appl. Microbiol. 48, 8–12 (2009).
Steel, J. H. et al. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr. Res. 57, 404–411 (2005).
Rautava, S., Collado, M. C., Salminen, S. & Isolauri, E. Probiotics modulate host–microbe interaction in the placenta and fetal gut—a randomized, double-blind, placebo-controlled trial. Neonatology 102, 178–184 (2005).
Mshvildadze, M. et al. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J. Pediatr. 156, 20–25 (2010).
Markenson, G. R., Adams, L. A., Hoffman, D. E. & Reece, M. T. Prevalence of Mycoplasma bacteria in amniotic fluid at the time of genetic amniocentesis using the polymerase chain reaction. J. Reprod. Med. 48, 775–779 (2003).
Fichorovna, R. N. et al. Maternal microbe-specific modulation of inflammatory response in extremely low-gestational-age newborns. MBio 2, e00280-10 (2011).
Zhu, M. J., Du, M., Nathanielsz, P. W. & Ford, S. P. Maternal obesity up-regulates inflammatory signalling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta 31, 387–391 (2010).
Kenyon, S. L., Taylor, D. J., Tarnow-Mordi, W. & ORACLE Collaborative Group. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet 357, 979–988 (2001).
Weintraub, A. S. et al. Antenatal antibiotic exposure in preterm infants with necrotizing enterocolitis. J. Perinatol. http://dx.doi.org/10.1038/jp.2011.180.
Kenyon, S. et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet 372, 1319–1327 (2008).
Cotten, C. M. et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 123, 58–66 (2009).
Kuppala, V. S., Meinzen-Derr, J., Morrow, A. L. & Schibler, K. R. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J. Pediatr. 159, 720–725 (2011).
Shaw, S. Y., Blanchard, J. F. & Bernstein, C. N. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am. J. Gastroenterol. 105, 2687–2692 (2010).
Rautava, S. & Walker, W. A. Academy of Breastfeeding Medicine founder's lecture 2008: breastfeeding—an extrauterine link between mother and child. Breastfeed. Med. 4, 3–10 (2010).
Gueimonde, M., Laitinen, K., Salminen, S. & Isolauri, E. Breast milk: a source of bifidobacteria for infant gut development and maturation? Neonatology 92, 64–66 (2007).
Grönlund, M. M. et al. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clin. Exp. Allergy 37, 1764–1772 (2007).
Yektaei-Karin, E. et al. The stress of birth enhances in vitro spontaneous and IL-8-induced neutrophil chemotaxis in the human newborn. Pediatr. Allergy Immunol. 18, 643–651 (2007).
Grönlund, M. M. et al. Mode of delivery directs the phagocyte functions of infants for the first 6 months of life. Clin. Exp. Immunol. 116, 521–526 (1999).
Huurre, A. et al. Mode of delivery—effects on gut microbiota and humoral immunity. Neonatology 93, 236–240 (2008).
Cabrera-Rubio, R. et al. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. http://dx.doi.org/10.3945/ajcn.112.037382.
Perez, P. F. et al. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 119, 724–732 (2007).
Grönlund, M. M., Grzes´kowiak, Ł., Isolauri, E. & Salminen, S. Influence of mother's intestinal microbiota on gut colonization in the infant. Gut Microbes 2, 227–233 (2011).
Martín, V. et al. Sharing of bacterial strains between breast milk and infant feces. J. Hum. Lact. 28, 36–44 (2012).
Abrahamsson, T. R., Sinkiewicz, G., Jakobsson, T., Fredrikson, M. & Björkstén, B. Probiotic lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life. J. Pediatr. Gastroenterol. Nutr. 49, 349–354 (2009).
Verhasselt, V. et al. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat. Med. 14, 170–175 (2008).
Rautava, S. et al. Breast milk-transforming growth factor-β2 specifically attenuates IL-1β-induced inflammatory responses in the immature human intestine via an SMAD6- and ERK-dependent mechanism. Neonatology 99, 192–201 (2011).
Rautava, S., Lu, L., Nanthakumar, N. N., Dubert-Ferrandon, A. & Walker, W. A. TGF-β2 induces maturation of immature human intestinal epithelial cells and inhibits inflammatory cytokine responses induced via the NF-κB pathway. J. Pediatr. Gastroenterol. Nutr. 54, 630–638 (2012).
Rautava, S., Kalliomäki, M. & Isolauri, E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J. Allergy Clin. Immunol. 109, 1191–1121 (2002).
Fujii, T. et al. Bifidobacterium breve enhances transforming growth factor β1 signaling by regulating Smad7 expression in preterm infants. J. Pediatr. Gastroenterol. Nutr. 43, 83–88 (2006).
Moro, G. E. et al. Effects of a new mixture of prebiotics on faecal flora and stools in term infants. Acta Paediatr. Suppl. 91, 77–91 (2003).
Veereman-Wauters, G. et al. Physiological and bifidogenic effects of prebiotic supplements in infant formulae. J. Pediatr. Gastroenterol. Nutr. 52, 763–771 (2011).
Salvini, F. et al. A specific prebiotic mixture added to starting infant formula has long-lasting bifidogenic effects. J. Nutr. 141, 1335–1339 (2011).
Holscher, H. D. et al. Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial. JPEN J. Parenter. Enteral Nutr. 36, 95S–105S (2012).
van Hoffen, E. et al. A specific mixture of short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides induces a beneficial immunoglobulin profile in infants at high risk for allergy. Allergy 64, 484–487 (2009).
Arslanoglu, S. et al. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J. Nutr. 138, 1091–1095 (2008).
Grüber, C. et al. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J. Allergy Clin. Immunol. 126, 791–797 (2010).
Duggan, C. et al. Oligofructose-supplemented infant cereal: 2 randomized, blinded community-based trials in Peruvian infants. Am. J. Clin. Nutr. 77, 937–942 (2003).
Szajewska, H. et al. Inulin and fructo-oligosaccharides for the prevention of antibiotic-associated diarrhea in children: report by the ESPGHAN working group on probiotics and prebiotics. J. Pediatr. Gastroenterol. Nutr. 54, 828–829 (2012).
Vandenplas, Y., De Hert, S. G. & PROBIOTICAL-study group. Randomised clinical trial: the symbiotic food supplement Probiotical vs. placebo for acute gastroenteritis in children. Aliment. Pharmacol. Ther. 34, 862–867 (2011).
Passariello, A. et al. Randomised clinical trial: efficacy of a new symbiotic formulation containing Lactobacillus paracasei B21060 plus arabinogalactan and xilooligosaccharides in children with acute diarrhea. Aliment. Pharmacol. Ther. 35, 782–788 (2012).
Kukkonen, K. et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 119, 192–198 (2007).
Van der Aa, L. B. et al. Effect of a new symbiotic mixture on atopic dermatitis in infants: a randomized-controlled trial. Clin. Exp. Allergy 40, 795–804 (2010).
Schultz, M., Göttl, C., Young, R. J., Iwen, P. & Vanderhoof, J. A. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J. Pediatr. Gastroenterol. Nutr. 38, 293–297 (2004).
Rinne, M., Kalliomäki, M., Salminen, S. & Isolauri, E. Probiotic intervention in the first months of life: short-term effects on gastrointestinal symptoms and long-term effects on gut microbiota. J. Pediatr. Gastroenterol. Nutr. 43, 200–205 (2006).
Gueimonde, M., Kalliomäki, M., Isolauri, E. & Salminen, S. Probiotic intervention in neonates—will permanent colonization ensue? J. Pediatr. Gastroenterol. Nutr. 42, 604–606 (2006).
Grzes´kowiak, Ł. et al. The impact of perinatal probiotic intervention on gut microbiota: double-blind placebo-controlled trials in Finland and Germany. Anaerobe 18, 7–13 (2012).
Kalliomäki, M. et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357, 1076–1079 (2001).
Kalliomäki, M., Salminen, S., Poussa, T., Arvilommi, H. & Isolauri, E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 361, 1869–1871 (2003).
Kalliomäki, M., Salminen, S., Poussa, T. & Isolauri, E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 119, 1019–1021 (2007).
Abrahamsson, T. R. et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 119, 1174–1180 (2007).
Wickens, K. et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 122, 788–794 (2008).
Niers, L. et al. The effects of selected probiotic strains on the development of eczema (the PandA study). Allergy 64, 1349–1358 (2009).
Dotterud, C. K., Storrø, O., Johnsen, R. & Oien, T. Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br. J. Dermatol. 163, 616–623 (2010).
Kim, J. Y. et al. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: a double-blind, randomized, placebo-controlled trial. Pediatr. Allergy Immunol. 21, 386–393 (2010).
Taylor, A. L., Dunstan, J. A. & Prescott, S. L. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J. Allergy Clin. Immunol. 119, 184–191 (2007).
Kopp, M. V., Hennemuth, I., Heinzmann, A. & Urbanek, R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics 121, 850–856 (2008).
Soh, S. E. et al. Probiotic supplementation in the first 6 months of life in at risk Asian infants—effects on eczema and atopic sensitization at the age of 1 year. Clin. Exp. Allergy 39, 571–578 (2009).
Huurre, A., Laitinen, K., Rautava, S., Korkeamäki, M. & Isolauri, E. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: a double-blind placebo-controlled study. Clin. Exp. Allergy 38, 1342–1348 (2008).
Luoto, R., Laitinen, K., Nermes, M. & Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br. J. Nutr. 103, 1792–1799 (2010).
Laitinen, K., Poussa, T. & Isolauri, E. Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Group. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br. J. Nutr. 101, 1679–1687 (2009).
Ilmonen, J., Isolauri, E., Poussa, T. & Laitinen, K. Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo-controlled trial. Clin. Nutr. 30, 156–164 (2011).
Luoto, R., Kalliomäki, M., Laitinen, K. & Isolauri, E. The impact of perinatal probiotic intervention on the development of overweight and obesity: follow-up study from birth to 10 years. Int. J. Obes. (Lond). 34, 1531–1537 (2010).
Björkstén, B. et al. Collecting and banking human milk: to heat or not to heat? Br. Med. J. 281, 765–769 (1980).
Martín, R. et al. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediatr. 143, 754–758 (2003).
Heikkilä, M. P. & Saris, P. E. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J. Appl. Microbiol. 95, 471–478 (2003).
Beasley, S. S. & Saris, P. E. Nisin-producing Lactococcus lactis strains isolated from human milk. Appl. Environ. Microbiol. 70, 5051–5053 (2004).
Martín, R. et al. Cultivation-independent assessment of the bacterial diversity of breast milk among healthy women. Res. Microbiol. 158, 31–37 (2007).
Delgado, S., Arroyo, R., Martín, R. & Rodríguez, J. M. PCR-DGGE assessment of the bacterial diversity of breast milk in women with lactational infectious mastitis. BMC Infect. Dis. 8, 51 (2008).
Jiménez, E. et al. Assessment of the bacterial diversity of human colostrum and screening of staphylococcal and enterococcal populations for potential virulence factors. Res. Microbiol. 159, 595–601 (2008).
Martín, R. et al. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl. Environ. Microbiol. 75, 965–969 (2009).
Hunt, K. M. et al. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 6, e21313 (2011).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Rautava, S., Luoto, R., Salminen, S. et al. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol 9, 565–576 (2012). https://doi.org/10.1038/nrgastro.2012.144
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2012.144
This article is cited by
-
Fewer culturable Lactobacillaceae species identified in faecal samples of pigs performing manipulative behaviour
Scientific Reports (2024)
-
The preterm gut microbiota and administration routes of different probiotics: a randomized controlled trial
Pediatric Research (2023)
-
The metagenomic and metabolomic profile of the gut microbes in Chinese full-term and late preterm infants treated with Clostridium butyricum
Scientific Reports (2023)
-
Lactobacillus rhamnosus Used in the Perinatal Period for the Prevention of Atopic Dermatitis in Infants: A Systematic Review and Meta-Analysis of Randomized Trials
American Journal of Clinical Dermatology (2022)
-
Gold standard for nutrition: a review of human milk oligosaccharide and its effects on infant gut microbiota
Microbial Cell Factories (2021)