Abstract
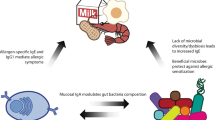
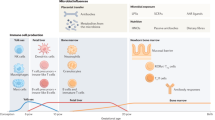
Numerous genes are involved in innate and adaptive immunity and these have been modified over millions of years. During this evolution, the mucosal immune system has developed two anti-inflammatory strategies: immune exclusion by the use of secretory antibodies to control epithelial colonization of microorganisms and to inhibit the penetration of potentially harmful agents; and immunosuppression to counteract local and peripheral hypersensitivity against innocuous antigens, such as food proteins. The latter strategy is called oral tolerance when induced via the gut. Homeostatic mechanisms also dampen immune responses to commensal bacteria. The mucosal epithelial barrier and immunoregulatory network are poorly developed in newborns. The perinatal period is, therefore, critical with regard to the induction of food allergy. The development of immune homeostasis depends on windows of opportunity during which innate and adaptive immunity are coordinated by antigen-presenting cells. The function of these cells is not only orchestrated by microbial products but also by dietary constituents, including vitamin A and lipids, such as polyunsaturated omega-3 fatty acids. These factors may in various ways exert beneficial effects on the immunophenotype of the infant. The same is true for breast milk, which provides immune-inducing factors and secretory immunoglobulin A, which reinforces the gut epithelial barrier. It is not easy to dissect the immunoregulatory network and identify variables that lead to food allergy. This Review discusses efforts to this end and outlines the scientific basis for future food allergy prevention.
Key Points
-
Homeostasis in the gut mucosa is normally preserved by secretory IgA-dependent immune exclusion of antigens and by the suppression of proinflammatory responses to innocuous antigens by mucosally induced tolerance (oral tolerance)
-
Food allergy is considered to be the consequence of abrogation of oral tolerance due to inappropriate interactions between genes and the environment
-
Any event causing epithelial barrier defects may underlie food allergen sensitization, not only in the gut but also elsewhere in the body, such as the skin and airways
-
The successful induction of oral tolerance depends on the dose and timing of enteric exposure to potential allergens, immune-modulating microbial components and dietary factors, such as vitamin A and lipids
-
Strict allergen avoidance during pregnancy, lactation, and early childhood to prevent food allergy in families that are genetically at high risk of allergy seems to be based on mythology rather than science
-
Exclusive breastfeeding for 4 months and mixed feeding thereafter will probably promote tolerance to food allergens in newborns
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Bischoff, S. & Crowe, S. E. Gastrointestinal food allergy: new insights into pathophysiology and clinical perspectives. Gastroenterology 128, 1089–1113 (2005).
Sicherer, S. H. & Sampson, H. A. Food allergy: recent advances in pathophysiology and treatment. Annu. Rev. Med. 60, 261–277 (2009).
Cianferoni, A. & Spergel, J. M. Food allergy: review, classification and diagnosis. Allergol. Int. 58, 457–466 (2009).
Eigenmann, P. A. et al. New visions for food allergy: an iPAC summary and future trends. Pediatr. Allergy Immunol. 19 (Suppl. 19), 26–39 (2008).
Devereux, G. The increase in the prevalence of asthma and allergy: food for thought. Nat. Rev. Immunol. 6, 869–874 (2006).
Rona, R. J. et al. The prevalence of food allergy: a meta-analysis. J. Allergy Clin. Immunol. 120, 638–646 (2007).
Venter, C. et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy 63, 354–359 (2008).
Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 121, 1331–1336 (2008).
Bruijnzeel-Koomen, C. et al. Adverse reactions to food. European Academy of Allergology and Clinical Immunology Subcommittee. Allergy 50, 623–635 (1995).
Johansson, S. G. et al. A revised nomenclature for allergy: an EAACI position statement from the EAACI nomenclature task force. Allergy 56, 813–824 (2001).
Zopf, Y., Baenkler, H. W., Silbermann, A., Hahn E. G. & Raithel, M. The differential diagnosis of food intolerance. Dtsch Arztebl. Int. 106, 359–369 (2009).
Gell, P. G. H. & Coombs, R. R. A. (Eds) Clinical Aspects of Immunology 1st edn (Blackwell Science Ltd, Oxford, 1963).
Brandtzaeg, P. The changing immunological paradigm in coeliac disease. Immunol. Lett. 105, 127–139 (2006).
Johansson, S. G. et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 113, 832–836 (2004).
Plaut, M., Sawyer, R. T. & Fenton, M. J. Summary of the 2008 National Institute of Allergy and Infectious Diseases—US Food and Drug Administration workshop on food allergy clinical trial design. J. Allergy Clin. Immunol. 124, 671–678e1 (2009).
Niggemann, B. et al. Controlled oral food challenges in children—when indicated, when superfluous? Allergy 60, 865–870 (2005).
Allen, K. J. et al. Management of cow's milk protein allergy in infants and young children: an expert panel perspective. J. Paediatr. Child. Health 45, 481–486 (2009).
Bischoff, S. C., Mayer, J. H. & Manns, M. P. Allergy and the gut. Int. Arch. Allergy Immunol. 121, 270–283 (2000).
Veres, G., Westerholm-Ormio, M., Kokkonen, J., Arato, A. & Savilahti, E. Cytokines and adhesion molecules in duodenal mucosa of children with delayed-type food allergy. J. Pediatr. Gastroenterol. Nutr. 37, 27–34 (2003).
Majamaa, H., Moisio, P., Holm, K., Kautiainen, H. & Turjanmaa, K. Cow's milk allergy: diagnostic accuracy of skin prick and patch tests and specific IgE. Allergy 54, 346–351 (1999).
Bischoff, S. C. Physiological and pathophysiological functions of intestinal mast cells. Semin. Immunopathol. 31, 185–205 (2009).
Knutson, T. W. et al. Intestinal reactivity in allergic and nonallergic patients: an approach to determine the complexity of the mucosal reaction. J. Allergy Clin. Immunol. 91, 553–559 (1993).
Bengtsson, U. et al. Increased levels of hyaluronan and albumin after intestinal challenge in adult patients with cow's milk intolerance. Clin. Exp. Allergy 26, 96–103 (1996).
Bengtsson, U. et al. Eosinophil cationic protein and histamine after intestinal challenge in patients with cow's milk intolerance. J. Allergy Clin. Immunol. 100, 216–221 (1997).
Lin, X. P. et al. Local allergic reaction in food-hypersensitive adults despite a lack of systemic food-specific IgE. J. Allergy Clin. Immunol. 109, 879–887 (2002).
Brandtzaeg, P. Mechanisms of gastrointestinal reactions to food. Environ. Toxicol. Pharmacol. 4, 9–24 (1997).
Groschwitz, K. R. et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc. Natl Acad. Sci. USA 106, 22381–22386 (2009).
Maeda, N., Inomata, N., Morita, A., Kirino, M. & Ikezawa, Z. Correlation of oral allergy syndrome due to plant-derived foods with pollen sensitization in Japan. Ann. Allergy Asthma Immunol. 104, 205–210 (2010).
Silva, R. et al. Anaphylaxis to mango fruit and crossreactivity with Artemisia vulgaris pollen. J. Investig. Allergol. Clin. Immunol. 19, 420–422 (2009).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Brandtzaeg, P. “ABC” of mucosal immunology. In Microbial–Host Interaction: Tolerance versus Allergy Vol. 64 (eds Brandtzaeg, P., Isolauri, E. & Prescott, S. L.) 23–43 (Nestec Ltd, Vevey & Karger, A. G., Basel, Switzerland, 2009).
Brandtzaeg, P. Mucosal immunity: induction, dissemination, and effector functions. Scand. J. Immunol. 70, 505–515 (2009).
Guarner, F. et al. Mechanisms of disease: the hygiene hypothesis revisited. Nat. Clin. Pract. Gastroenterol. Hepatol. 3, 275–284 (2006).
Björksten, B. The hygiene hypothesis: do we still believe in it? In Microbial–Host Interaction: Tolerance versus Allergy Vol. 64 (eds Brandtzaeg, P., Isolauri, E. & Prescott, S. L.) 11–22 (Nestec Ltd, Vevey & Karger, A. G., Basel, Switzerland, 2009).
Vickery, B. P. & Burks, A. W. Immunotherapy in the treatment of food allergy: focus on oral tolerance. Curr. Opin. Allergy Clin. Immunol. 9, 364–370 (2009).
Scurlock, A. M., Burks, A. W. & Jones, S. M. Oral immunotherapy for food allergy. Curr. Allergy Asthma Rep. 9, 186–193 (2009).
von Hertzen, L. C. et al. Scientific rationale for the Finnish Allergy Programme 2008–2018: emphasis on prevention and endorsing tolerance. Allergy 64, 678–701 (2009).
Brandtzaeg, P. History of oral tolerance and mucosal immunity. Ann. NY Acad. Sci. 778, 1–27 (1996).
Duchmann, R., Neurath, M., Märker-Hermann, E. & Meyer Zum Büschenfelde, K. H. Immune responses towards intestinal bacteria—current concepts and future perspectives. Z. Gastroenterol. 35, 337–346 (1997).
Helgeland, L. & Brandtzaeg, P. Development and function of intestinal B and T cells. Microbiol. Ecol. Health Dis. 2 (Suppl. 2), 110–127 (2000).
Konrad, A., Cong, Y., Duck, W., Borlaza, R. & Elson, C. O. Tight mucosal compartmentation of the murine immune response to antigens of the enteric microbiota. Gastroenterology 130, 2050–2059 (2006).
Macpherson, A. J., Geuking, M. B. & McCoy, K. D. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology 115, 153–162 (2005).
Neish, A. S. Microbes in gastrointestinal health and disease. Gastroenterology 136, 65–80 (2009).
Duerkop, B. A., Vaishnava, S. & Hooper, L. V. Immune responses to the microbiota at the intestinal mucosal surface. Immunity 31, 368–376 (2009).
Brandtzaeg, P., Nilssen, D. E., Rognum, T. O. & Thrane, P. S. Ontogeny of the mucosal immune system and IgA deficiency. Gastroenterol. Clin. North Am. 20, 397–439 (1991).
Holt, P. G. & Jones, C. A. The development of the immune system during pregnancy and early life. Allergy 55, 688–697 (2000).
Brandtzaeg, P. Development and basic mechanisms of human gut immunity. Nutr. Rev. 56, S5–S18 (1998).
Yamanaka, T. et al. Microbial colonization drives lymphocyte accumulation and differentiation in the follicle-associated epithelium of Peyer's patches. J. Immunol. 170, 816–822 (2003).
Round, J. L. & Mazmanian, S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323 (2009).
Brandtzaeg, P. & Johansen, F. E. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol. Rev. 206, 32–63 (2005).
Brandtzaeg, P. Function of mucosa-associated lymphoid tissue in antibody formation. Immunol. Invest. 39, 303–355 (2010).
Neutra, M. R., Mantis, N. J. & Kraehenbuhl, J. P. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2, 1004–1009 (2001).
Brandtzaeg, P. & Pabst, R. Let's go mucosal: communication on slippery ground. Trends Immunol. 25, 570–577 (2004).
Kraus, T. A. et al. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J. Clin. Invest. 115, 2234–2243 (2005).
Worbs, T. et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 203, 519–527 (2006).
Brandtzaeg, P. Role of local immunity and breast-feeding in mucosal homeostasis and defence against infections. In Nutrition and Immune Function 1st edn Vol. 1 Ch. 14 (eds Calder, P. C., Field, C. J. & Gill, H. S.) 273–320 (CABI Publishing, Oxon, UK, 2002).
Brandtzaeg, P. The mucosal immune system and its integration with the mammary glands. J. Pediatr. 156 (Suppl. 2), S8–S15 (2010).
Nahmias, A. et al. IgA-secreting cells in the blood of premature and term infants: normal development and effect of intrauterine infections. Adv. Exp. Med. Biol. 310, 59–69 (1991).
Stoll, B. J. et al. Immunoglobulin secretion by the normal and the infected newborn infant. J. Pediatr. 122, 780–786 (1993).
Siegrist, C. A. & Aspinall, R. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 9, 185–194 (2009).
Crabbé, P. A., Nash, D. R., Bazin, H., Eyssen, H. & Heremans, J. F. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab. Invest. 22, 448–457 (1970).
Lodinová, R., Jouja, V. & Wagner, V. Serum immunoglobulins and coproantibody formation in infants after artificial intestinal colonization with Escherichia coli 083 and oral lysozyme administration. Pediatr. Res. 7, 659–669 (1973).
Moreau, M. C., Ducluzeau, R., Guy-Grand, D. & Muller, M. C. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Infect. Immun. 21, 532–539 (1978).
Adkins, B., Leclerc, C. & Marshall-Clarke, S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4, 553–564 (2004).
Upham, J. W. et al. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect. Immun. 74, 1106–1112 (2006).
Marzi, M. et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin. Exp. Immunol. 106, 127–133 (1996).
Brandtzaeg, P. & Prydz, H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature 311, 71–73 (1984).
Johansson, M. E. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008).
Bollinger, R. R. et al. Secretory IgA and mucin-mediated biofilm formation by environmental strains of Escherichia coli: role of type 1 pili. Mol. Immunol. 43, 378–387 (2006).
Mellander, L., Carlsson, B., Jalil, F., Söderström, T. & Hanson, L. A. Secretory IgA antibody response against Escherichia coli antigens in infants in relation to exposure. J. Pediatr. 107, 430–433 (1985).
Martino, D. J., Currie, H., Taylor, A., Conway, P. & Prescott, S. L. Relationship between early intestinal colonization, mucosal immunoglobulin A production and systemic immune development. Clin. Exp. Allergy 38, 69–78 (2008).
Kukkonen, K. et al. High intestinal IgA associates with reduced risk of IgE-associated allergic diseases. Pediatr. Allergy Immunol. 21, 67–73 (2010).
Ogra, S. S., Weintraub, D. & Ogra, P. L. Immunologic aspects of human colostrum and milk. III. Fate and absorption of cellular and soluble components in the gastrointestinal tract of the newborn. J. Immunol. 119, 245–248 (1977).
Klemola, T., Savilahti, E. & Leinikki, P. Mumps IgA antibodies are not absorbed from human milk. Acta Paediatr. Scand. 75, 230–232 (1986).
Weaver, L. T., Wadd, N., Taylor, C. E., Greenwell, J. & Toms, G. L. The ontogeny of serum IgA in the newborn. Pediatr. Allergy Immunol. 2, 2–75 (1991).
van Elburg, R. M., Uil, J. J., de Monchy, J. G. & Heymans, H. S. Intestinal permeability in pediatric gastroenterology. Scand. J. Gastroenterol. 194 (Suppl.), 19–24 (1992).
Johansen, F. E. et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J. Exp. Med. 190, 915–921 (1999).
Sait, L. C. et al. Secretory antibodies reduce systemic antibody responses against the gastrointestinal commensal flora. Int. Immunol. 19, 257–265 (2007).
Karlsson, M. R., Johansen, F. E., Kahu, H., Macpherson, A. & Brandtzaeg, P. Hypersensitivity and oral tolerance in the absence of a secretory immune system. Allergy 65, 561–570 (2010).
Smith, M. et al. Children with egg allergy have evidence of reduced neonatal CD4+CD25+CD127lo/- regulatory T cell function. J. Allergy Clin. Immunol. 121, 1460–1466, 1466.e1–e7 (2008).
Martino, D. J. & Prescott, S. L. Silent mysteries: epigenetic paradigms could hold the key to conquering the epidemic of allergy and immune disease. Allergy 65, 1–15 (2009).
Haddeland, U. et al. Putative regulatory T cells are impaired in cord blood from neonates with hereditary allergy risk. Pediatr. Allergy Immunol. 16, 104–112 (2005).
Janzi, M. et al. Selective IgA deficiency in early life: association to infections and allergic diseases during childhood. Clin. Immunol. 133, 78–85 (2009).
Brandtzaeg, P. Impact of immunodeficiency on immunological homeostasis in the gut. In Falk Symposium 135—Immunological Diseases of Liver and Gut (eds Lukáš, M., Manns, M. P., Špicák, J. & Stange, E. F.) 180–209 (Kluwer Academic Publishers, Dordrecht, 2004).
Hooper, L. V. et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–884 (2001).
Neish, A. S. et al. Prokaryotic regulation of epithelial responses by inhibition of IκBα ubiquitination. Science 289, 1560–1563 (2000).
Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 8, 411–420 (2008).
Lee, J. et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat. Cell Biol. 8, 1327–1336 (2006).
Nenci, A. et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 (2007).
Rescigno, M., Lopatin, U. & Chieppa, M. Interactions among dendritic cells, macrophages, and epithelial cells in the gut: implications for immune tolerance. Curr. Opin. Immunol. 20, 669–675 (2008).
Shale, M. & Ghosh, S. How intestinal epithelial cells tolerise dendritic cells and its relevance to inflammatory bowel disease. Gut 58, 1291–1299 (2009).
Iliev, I. D. et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 58, 1481–1489 (2009).
Groschwitz, K. R. & Hogan, S. P. Intestinal barrier function: molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 124, 3–20 (2009).
Abreu, M. T., Palladino, A. A., Arnold, E. T., Kwon, R. S. & McRoberts, J. A. Modulation of barrier function during Fas-mediated apoptosis in human intestinal epithelial cells. Gastroenterology 119, 1524–1536 (2000).
Lotz, M. et al. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J. Exp. Med. 203, 973–984 (2006).
Eggesbø, M., Botten, G., Stigum, H., Nafstad, P. & Magnus, P. Is delivery by cesarean section a risk factor for food allergy? J. Allergy Clin. Immunol. 112, 420–426 (2003).
Latcham, F. et al. A consistent pattern of minor immunodeficiency and subtle enteropathy in children with multiple food allergy. J. Pediatr. 143, 39–47 (2003).
van Odijk, J. et al. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966–2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy 58, 833–843 (2003).
Ip, S. et al. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Agency for Healthcare Research and Quality [online], (2007).
Høst, A. et al. Dietary prevention of allergic diseases in infants and small children. Pediatr. Allergy Immunol. 19, 1–4 (2008).
Prescott, S. L. et al. The importance of early complementary feeding in the development of oral tolerance: concerns and controversies. Pediatr. Allergy Immunol. 19, 375–380 (2008).
Nwaru, B. I. et al. Age at the introduction of solid foods during the first year and allergic sensitization at age 5 years. Pediatrics 125, 50–59 (2010).
Hanson, L. A. Session 1: feeding and infant development breast-feeding and immune function. Proc. Nutr. Soc. 66, 384–396 (2007).
Corthésy, B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J. Immunol. 178, 27–32 (2007).
Savilahti E. et al. Low colostral IgA associated with cow's milk allergy. Acta Paediatr. Scand. 80, 1207–1213 (1991).
Järvinen, K. M., Laine, S. T., Järvenpää, A. L. & Suomalainen, H. K. Does low IgA in human milk predispose the infant to development of cow's milk allergy? Pediatr. Res. 48, 457–462 (2000).
Field, C. J. The immunological components of human milk and their effect on immune development in infants. J. Nutr. 135, 1–4 (2005).
Planchon, S. M., Martins, C. A., Guerrant, R. L. & Roche, J. K. Regulation of intestinal epithelial barrier function by TGF-β1. Evidence for its role in abrogating the effect of a T cell cytokine. J. Immunol. 153, 5730–5739 (1994).
Benn, C. S. et al. Breastfeeding and risk of atopic dermatitis, by parental history of allergy, during the first 18 months of life. Am. J. Epidemiol. 160, 217–223 (2004).
Verhasselt, V. et al. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat. Med. 14, 170–175 (2008).
Matson, A. P., Thrall, R. S., Rafti, E. & Puddington, L. Breastmilk from allergic mothers can protect offspring from allergic airway inflammation. Breastfeed Med. 4, 167–174 (2009).
López-Expósito, I., Song, Y., Järvinen, K. M., Srivastava, K. & Li, X. M. Maternal peanut exposure during pregnancy and lactation reduces peanut allergy risk in offspring. J. Allergy Clin. Immunol. 124, 1039–1046 (2009).
Simons, F. E. & Sampson, H. A. Anaphylaxis epidemic: fact or fiction? J. Allergy Clin. Immunol. 122, 1166–1168 (2008).
Saurer, L. & Mueller, C. T cell-mediated immunoregulation in the gastrointestinal tract. Allergy 64, 505–519 (2009).
Westendorf, A. M. et al. CD4+Foxp3+ regulatory T cell expansion induced by antigen-driven interaction with intestinal epithelial cells independent of local dendritic cells. Gut 58, 211–219 (2009).
Broere, F. et al. Cyclooxygenase-2 in mucosal DC mediates induction of regulatory T cells in the intestine through suppression of IL-4. Mucosal Immunol. 2, 254–264 (2009).
Rothberg, R. M. & Farr, R. S. Anti-bovine serum albumine and anti-alpha lactalbumin in the serum of children and adults. Pediatrics 35, 571–588 (1965).
Scott, H., Rognum, T. O., Midtvedt, T. & Brandtzaeg, P. Age-related changes of human serum antibodies to dietary and colonic bacterial antigens measured by an enzyme-linked immunosorbent assay. Acta Pathol. Microbiol. Immunol. Scand. C 93, 65–70 (1985).
Korenblat, P. E., Rothberg, R. M., Minden, P. & Farr, R. S. Immune responses of human adults after oral and parenteral exposure to bovine serum albumin. J. Allergy 41, 226–235 (1968).
Waldo, F. B., van den Wall Bake, A. W., Mestecky, J. & Husby, S. Suppression of the immune response by nasal immunization. Clin. Immunol. Immunopathol. 72, 30–34 (1994).
Husby, S., Mestecky, J., Moldoveanu, Z., Holland, S. & Elson, C. O. Oral tolerance in humans. T cell but not B cell tolerance after antigen feeding. J. Immunol. 152, 4663–4670 (1994).
Kraus, T. A., Toy, L., Chan, L., Childs, J. & Mayer, L. Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology 126, 1771–1778 (2004).
Kraus, T. A., Cheifetz, A., Toy, L., Meddings, J. B. & Mayer, L. Evidence for a genetic defect in oral tolerance induction in inflammatory bowel disease. Inflamm. Bowel Dis. 12, 82–88 (2006).
Qiao, L. et al. Differential regulation of human T cell responsiveness by mucosal versus blood monocytes. Eur. J. Immunol. 26, 922–927 (1996).
Smith, P. D. et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J. Immunol. 167, 2651–2656 (2001).
Rugtveit, J. et al. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. Gastroenterology 112, 1493–1505 (1997).
Weber, B., Saurer, L. & Mueller, C. Intestinal macrophages: differentiation and involvement in intestinal immunopathologies. Semin. Immunopathol. 31, 171–184 (2009).
Denning, T. L., Wang, Y. C., Patel, S. R., Williams, I. R. & Pulendran, B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8, 1086–1094 (2007).
Rescigno, M. Before they were gut dendritic cells. Immunity 31, 454–456 (2009).
Milling, S. W., Cousins, L. & MacPherson, G. G. How do DCs interact with intestinal antigens? Trends Immunol. 26, 349–352 (2005).
Mowat, A. M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 3, 331–341 (2003).
Smythies, L. E. et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 115, 66–75 (2005).
Grindebacke, H. et al. Dynamic development of homing receptor expression and memory cell differentiation of infant CD4+CD25high regulatory T cells. J. Immunol. 183, 4360–4370 (2009).
Mora, J. R., Iwata, M. & von Andrian, U. H. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 8, 685–698 (2008).
Hammerschmidt, S. I. et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J. Exp. Med. 205, 2483–2490 (2008).
Sakaguchi, S., Wing, K. & Yamaguchi, T. Dynamics of peripheral tolerance and immune regulation mediated by Treg. Eur. J. Immunol. 39, 2331–2336 (2009).
Belkaid, Y. & Oldenhove, G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity 29, 362–371 (2008).
Barnes, M. J. & Powrie, F. Regulatory T cells reinforce intestinal homeostasis. Immunity 31, 401–411 (2009).
Horwitz, D. A., Zheng, S. G. & Gray, J. D. Natural and TGF-β-induced Foxp3+CD4+ CD25+ regulatory T cells are not mirror images of each other. Trends Immunol. 29, 429–435 (2008).
Akbar, A. N., Vukmanovic-Stejic, M., Taams, L. S. & Macallan, D. C. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat. Rev. Immunol. 7, 231–237 (2007).
Schnoeller, C. et al. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J. Immunol. 180, 4265–4272 (2008).
Honda, K. & Takeda, K. Regulatory mechanisms of immune responses to intestinal bacteria. Mucosal Immunol. 2, 187–196 (2009).
Weinstock, J. V. & Elliott, D. E. Helminths and the IBD hygiene hypothesis. Inflamm. Bowel Dis. 15, 128–133 (2009).
Blümer, N., Herz, U., Wegmann, M. & Renz, H. Prenatal lipopolysaccharide-exposure prevents allergic sensitization and airway inflammation, but not airway responsiveness in a murine model of experimental asthma. Clin. Exp. Allergy 35, 397–402 (2005).
Gerhold, K. et al. Prenatal initiation of endotoxin airway exposure prevents subsequent allergen-induced sensitization and airway inflammation in mice. J. Allergy Clin. Immunol. 118, 666–673 (2006).
Eder, W. & von Mutius, E. Genetics in asthma: the solution to a lasting conundrum? Allergy 60, 1482–1484 (2005).
Hong, X., Tsai, H. J. & Wang, X. Genetics of food allergy. Curr. Opin. Pediatr. 21, 770–776 (2009).
Rook, G. A. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology 126, 3–11 (2009).
Fallon, P. G. & Mangan, N. E. Suppression of TH2-type allergic reactions by helminth infection. Nat. Rev. Immunol. 7, 220–230 (2007).
Tang, M. L. Probiotics and prebiotics: immunological and clinical effects in allergic disease. In Microbial–Host Interaction: Tolerance versus Allergy Vol. 64 (eds Brandtzaeg, P., Isolauri, E. & Prescott, S. L.) 219–238 (Nestec Ltd, Vevey & Karger, A. G., Basel, Switzerland, 2009).
Salminen, S., Collado, M. C., Isolauri, E. & Gueimonde, M. Microbial-host interactions: selecting the right probiotics and prebiotics for infants. In Microbial–Host Interaction: Tolerance versus Allergy Vol. 64 (eds Brandtzaeg, P., Isolauri, E. & Prescott, S. L.) 201–217 (Nestec Ltd, Vevey & Karger, A. G., Basel, Switzerland, 2009).
Ghadimi, D., Vrese, M., Heller, K. J. & Schrezenmeir, J. Effect of natural commensal-origin DNA on toll-like receptor 9 (TLR9) signaling cascade, chemokine IL-8 expression, and barrier integritiy of polarized intestinal epithelial cells. Inflamm. Bowel Dis. 16, 410–427 (2010).
Broide, D. H. Immunomodulation of allergic disease. Annu. Rev. Med. 60, 279–291 (2009).
Rancé, F., Boguniewicz, M. & Lau, S. New visions for atopic eczema: an iPAC summary and future trends. Pediatr. Allergy Immunol. 19 (Suppl. 19), 17–25 (2008).
Vercelli, D. Discovering susceptibility genes for asthma and allergy. Nat. Rev. Immunol. 8, 169–182 (2008).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Herz, U., Lacy, P., Renz, H. & Erb, K. The influence of infections on the development and severity of allergic disorders. Curr. Opin. Immunol. 12, 632–640 (2000).
Isolauri, E., Grönlund, M. M., Salminen, S. & Arvilommi, H. Why don't we bud? J. Pediatr. Gastroenterol. Nutr. 30, 214–216 (2000).
Prescott, S. L. et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the TH2 cytokine profile. J. Immunol. 160, 4730–4737 (1998).
Michaëlsson, J., Mold, J. E., McCune, J. M. & Nixon, D. F. Regulation of T cell responses in the developing human fetus. J. Immunol. 176, 5741–5748 (2006).
Cupedo, T., Nagasawa, M., Weijer, K., Blom, B. & Spits, H. Development and activation of regulatory T cells in the human fetus. Eur. J. Immunol. 35, 383–390 (2005).
Darrasse-Jèze, G., Marodon, G., Salomon, B. L., Catala, M. & Klatzmann, D. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood 105, 4715–4721 (2005).
Renz, H., Pfefferle, P. I., Teich, R. & Garn, H. Development and regulation of immune responses to food antigens in pre- and postnatal life. In Microbial–Host Interaction: Tolerance versus Allergy Vol. 64 (eds Brandtzaeg, P., Isolauri, E. & Prescott, S. L.) 139–157 (Nestec Ltd, Vevey & Karger, A. G., Basel, Switzerland, 2009).
Huehn, J., Polansky, J. K. & Hamann, A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat. Rev. Immunol. 9, 83–89 (2009).
Wilson, C. B., Rowell, E. & Sekimata, M. Epigenetic control of T-helper-cell differentiation. Nat. Rev. Immunol. 9, 91–105 (2009).
Conrad, M. L. et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J. Exp. Med. 206, 2869–2877 (2009).
Takahashi, K., Sugi, Y., Hosono, A. & Kaminogawa, S. Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J. Immunol. 183, 6522–6529 (2009).
Alford, S. H. et al. Parental history of atopic disease: disease pattern and risk of pediatric atopy in offspring. J. Allergy Clin. Immunol. 114, 1046–1050 (2004).
Renz, H., Blümer, N., Virna, S., Sel, S. & Garn, H. in Allergy and Asthma in Modern Society: A Scientific Approach Vol. 91 (ed. Crameri, R.) 30–48 (Chem. Immunol. Allergy, Karger, Basel, 2006).
van der Kleij, D. et al. A novel host–parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates Toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 277, 48122–48129 (2002).
Rautava, S., Ruuskanen, O., Ouwehand, A., Salminen, S. & Isolauri, E. The hygiene hypothesis of atopic disease—an extended version. J. Pediatr. Gastroenterol. Nutr. 38, 378–388 (2004).
Rowe, J. et al. Prenatal versus postnatal sensitization to environmental allergens in a high-risk birth cohort. J. Allergy Clin. Immunol. 119, 1164–1173 (2007).
Holt, P. G. & Thomas, W. R. Sensitization to airborne environmental allergens: unresolved issues. Nat. Immunol. 6, 957–960 (2005).
Skripak, J. M., Matsui, E. C., Mudd, K. & Wood, R. A. The natural history of IgE-mediated cow's milk allergy. J. Allergy Clin. Immunol. 120, 1172–1177 (2007).
Bird, J. A. & Burks, A. W. Food allergy and asthma. Prim. Care Respir. J. 18, 258–265 (2009).
Sepp, E. et al. Intestinal microflora of Estonian and Swedish infants. Acta Paediatr. 86, 956–961 (1997).
Björkstén, B., Naaber, P., Sepp, E. & Mikelsaar, M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin. Exp. Allergy 29, 342–346 (1999).
Kalliomäki, M. et al. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 107, 129–134 (2001).
Eggesbø, M. et al. Cesarean delivery and cow milk allergy/intolerance. Allergy 60, 1172–1173 (2005).
Koplin, J. et al. Is caesarean delivery associated with sensitization to food allergens and IgE-mediated food allergy: a systematic review. Pediatr. Allergy Immunol. 19, 682–687 (2008).
Kuitunen, M. et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J. Allergy Clin. Immunol. 123, 335–341 (2009).
Zeiger, R. S. Dietary aspects of food allergy prevention in infants and children. J. Pediatr. Gastroenterol. Nutr. 30 (Suppl.), S77–S86 (2000).
Prescott, S. L. Role of dietary immunomodulatory factors in the development of immune tolerance. In Microbial–Host Interaction: Tolerance versus Allergy Vol. 64 (eds Brandtzaeg, P., Isolauri, E. & Prescott, S. L.). 185–200 (Nestec Ltd, Vevey & Karger, A. G., Basel, Switzerland, 2009).
Perez, P. F. et al. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 119, e724–e732 (2007).
Miettinen, M. et al. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect. Immun. 66, 6058–6062 (1998).
Hessle, C., Hanson, L. A. & Wold, A. E. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin. Exp. Immunol. 116, 276–282 (1999).
Hessle, C., Andersson, B. & Wold, A. E. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infect. Immun. 68, 3581–3586 (2000).
Hessle, C., Hanson, L. A. & Wold, A. E. Interleukin-10 produced by the innate immune system masks in vitro evidence of acquired T-cell immunity to E. coli. Scand. J. Immunol. 52, 13–20 (2000).
Steidler, L. et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289, 1352–1355 (2000).
Caramalho, I. et al. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 197, 403–411 (2003).
Murai, M. et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 10, 1178–1184 (2009).
Li, B. et al. FOXP3 is a homo-oligomer and a component of a supramolecular regulatory complex disabled in the human XLAAD/IPEX autoimmune disease. Int. Immunol. 19, 825–835 (2007).
Holt, P. G. Parasites, atopy, and the hygiene hypothesis: resolution of a paradox? Lancet 356, 1699–1701 (2000).
Mellor, A. L. & Munn, D. Policing pregnancy: Tregs help keep the peace. Trends Immunol. 25, 563–565 (2004).
Vassallo, R., Tamada, K., Lau, J. S., Kroening, P. R. & Chen, L. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J. Immunol. 175, 2684–2691 (2005).
Traidl-Hoffmann, C. et al. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J. Exp. Med. 201, 627–636 (2005).
Lin, J. & Sampson, H. A. The role of immunoglobulin E-binding epitopes in the characterization of food allergy. Curr. Opin. Allergy Clin. Immunol. 9, 357–363 (2009).
O'Gorman, W. E. et al. The initial phase of an immune response functions to activate regulatory T cells. J. Immunol. 183, 332–339 (2009).
Du Toit, G. et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J. Allergy Clin. Immunol. 122, 984–991 (2008).
Fox, A. T., Sasieni, P., du Toit, G., Syed, H. & Lack, G. Household peanut consumption as a risk factor for the development of peanut allergy. J. Allergy Clin. Immunol. 123, 417–423 (2009).
Wennergren, G. What if it is the other way around? Early introduction of peanut and fish seems to be better than avoidance. Acta Paediatr. 98, 1085–1087 (2009).
Greer, F. R., Sicherer, S. H. & Burks, A. W. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 121, 183–191 (2008).
Sicherer, S. H. & Burks, A. W. Maternal and infant diets for prevention of allergic diseases: understanding menu changes in 2008. J. Allergy Clin. Immunol. 122, 29–33 (2008).
Mazer, B. Specific oral tolerance induction increased tolerance to milk in children with severe cow's milk allergy. Evid. Based Med. 14, 50 (2009).
Staden, U. et al. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy 62, 1261–1269 (2007).
Skripak, J. M. et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J. Allergy Clin. Immunol. 122, 1154–1160 (2008).
Rancé, F. Novel approaches in treating food allergy using allergens. In Microbial–Host Interaction: Tolerance versus Allergy Vol. 64 (eds Brandtzaeg, P., Isolauri, E. & Prescott, S. L.) 157–167 (Nestec Ltd, Vevey & Karger, A. G., Basel, Switzerland, 2009).
Nowak-Wegrzyn, A. et al. Tolerance to extensively heated milk in children with cow's milk allergy. J. Allergy Clin. Immunol. 122, 342–347.e2 (2008).
Bannon, G. A. et al. Engineering, characterization and in vitro efficacy of the major peanut allergens for use in immunotherapy. Int. Arch. Allergy Immunol. 124, 70–72 (2001).
Calder, P. C. et al. Early nutrition and immunity—progress and perspectives. Br. J. Nutr. 96, 774–790 (2006).
Sampson, H. A. Role of complementary and alternative medicine in the field of allergy and clinical immunology. J. Allergy Clin. Immunol. 123, 317–318 (2009).
Srivastava, K. D. et al. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J. Allergy Clin. Immunol. 123, 443–451 (2009).
Furuhjelm, C. et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr. 98, 1461–1467 (2009).
Sandin, A., Bråbäck, L., Norin, E. & Björkstén, B. Faecal short chain fatty acid pattern and allergy in early childhood. Acta Paediatr. 98, 823–827 (2009).
Korotkova, M., Telemo, E., Yamashiro, Y., Hanson, L. A. & Strandvik, B. The ratio of n-6 to n-3 fatty acids in maternal diet influences the induction of neonatal immunological tolerance to ovalbumin. Clin. Exp. Immunol. 137, 237–244 (2004).
Oddy, W. H. et al. Atopy, eczema and breast milk fatty acids in a high-risk cohort of children followed from birth to 5 yr. Pediatr. Allergy Immunol. 17, 4–10 (2006).
Wijga, A. H. et al. Breast milk fatty acids and allergic disease in preschool children: the prevention and incidence of asthma and mite allergy birth cohort study. J. Allergy Clin. Immunol. 117, 440–447 (2006).
Arita, M. et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 201, 713–722 (2005).
Iwata, M. et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Maloy, K. J. et al. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 197, 111–119 (2003).
Zorn, E. et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 108, 1571–1579 (2006).
Kretschmer, K. et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6, 1219–1227 (2005).
Sutmuller, R. P. et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Invest. 116, 485–494 (2006).
Hrncir, T., Stepankova, R., Kozakova, H., Hudcovic, T. & Tlaskalova-Hogenova, H. Gut microbiota and lipopolysaccharide content of the diet influence development of regulatory T cells: studies in germ-free mice. BMC Immunol. 9, 65 (2008).
Corthay, A. How do regulatory T cells work? Scand. J. Immunol. 70, 326–336 (2009).
Fontenot, J. D., Rasmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005).
Miyara, M. et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30, 899–911 (2009).
Thorstenson, K. M. & Khoruts, A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167, 188–195 (2001).
Zhang, X., Izikson, L., Liu, L. & Weiner, H. L. Activation of CD25+CD4+ regulatory T cells by oral antigen administration. J. Immunol. 167, 4245–4253 (2001).
Karlsson, M. R., Kahu, H., Hanson, L. A., Telemo, E. & Dahlgren, U. I. Tolerance and bystander suppression, with involvement of CD25-positive cells, is induced in rats receiving serum from ovalbumin-fed donors. Immunology 100, 326–333 (2000).
Karlsson, M. R., Kahu, H., Hanson, L. A., Telemo, E. & Dahlgren, U. I. An established immune response against ovalbumin is suppressed by a transferable serum factor produced after ovalbumin feeding: a role of CD25+ regulatory cells. Scand. J. Immunol. 55, 470–477 (2002).
Curotto de Lafaille, M. A. et al. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity 29, 114–126 (2008).
Boirivant, M. et al. A transient breach in the epithelial barrier leads to regulatory T-cell generation and resistance to experimental colitis. Gastroenterology 135, 1612–1623.e5 (2008).
Karlsson, M. R., Rugtveit, J. & Brandtzaeg, P. Allergen-responsive CD+CD25+ regulatory T cells in children who have outgrown cow's milk allergy. J. Exp. Med. 199, 1679–1688 (2004).
Sletten, G. B., Halvorsen, R., Egaas, E. & Halstensen, T. S. Memory T cell proliferation in cow's milk allergy after CD25+ regulatory T cell removal suggests a role for casein-specific cellular immunity in IgE-mediated but not in non-IgE-mediated cow's milk allergy. Int. Arch. Allergy Immunol. 142, 190–198 (2007).
Shreffler, W. G., Wanich, N., Moloney, M., Nowak-Wegrzyn, A. & Sampson, H. A. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J. Allergy Clin. Immunol. 123, 43–52.e7 (2009).
Tiemessen, M. M. et al. Cow's milk-specific T-cell reactivity of children with and without persistent cow's milk allergy: key role for IL-10. J. Allergy Clin. Immunol. 113, 932–939 (2004).
Frossard, C. P., Hauser, C. & Eigenmann, P. A. Antigen-specific secretory IgA antibodies in the gut are decreased in a mouse model of food allergy. J. Allergy Clin. Immunol. 114, 377–382 (2004).
Peterson, D. A., McNulty, N. P., Guruge, J. L. & Gordon. J. I. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell. Host Microbe 2, 328–339 (2007).
Atherton, J. C. & Blaser, M. J. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J. Clin. Invest. 119, 2475–2487 (2009).
Untersmayr, E. & Jensen-Jarolim, E. The role of protein digestibility and antacids on food allergy outcomes. J. Allergy Clin. Immunol. 121, 1301–1308 (2008).
Mullins, R. J. Paediatric food allergy trends in a community-based specialist allergy practice, 1995–2006. Med. J. Aust. 186, 618–621 (2007).
Rickard, C. Response to “Health risks of genetically modified foods”. Crit. Rev. Food Sci. Nutr. 50, 85–91 (2010).
Guimarães, V. D. et al. Comparative study of the adjuvanticity of Bacillus thuringiensis Cry1Ab protein and cholera toxin on allergic sensitisation and elicitation to peanut. Food Agric. Immunol. 19, 325–337 (2008).
Prescott, V. E. et al. Transgenic expression of bean α-amylase inhibitor in peas results in altered structure and immunogenicity. J. Agric. Food Chem. 53, 9023–9030 (2005).
Finamore, A. et al. Intestinal and peripheral immune response to MON810 maize ingestion in weaning and old mice. J. Agric. Food Chem. 56, 11533–11539 (2008).
Brandtzaeg, P. & Tolo, K. Mucosal penetrability enhanced by serum-derived antibodies. Nature 266, 262–263 (1977).
Lim, P. L. & Rowley, D. The effect of antibody on the intestinal absorption of macromolecules and on intestinal permeability in adult mice. Int. Arch. Allergy Appl. Immunol. 68, 41–46 (1982).
Werfel, T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J. Invest. Dermatol. 129, 1878–1891 (2009).
Arima, K. et al. Distinct signal codes generate dendritic cell functional plasticity. Sci. Signal. 3, ra4 (2010).
Michelson, P. H., Williams, L. W., Benjamin, D. K. & Barnato, A. E. Obesity, inflammation, and asthma severity in childhood: data from the National Health and Nutrition Examination Survey 2001–2004. Ann. Allergy Asthma Immunol. 103, 381–385 (2009).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
von Berg, A. Modified proteins in allergy prevention. In Microbial–Host Interaction: Tolerance versus Allergy Vol. 64 (eds Brandtzaeg, P., Isolauri, E. & Prescott, S. L.) 239–250 (Nestec Ltd, Vevey & Karger, A. G., Basel, Switzerland, 2009).
Sopo, S. M., Onesimo, R., Giorgio, V. & Fundarò, C. Specific oral tolerance induction (SOTI) in pediatric age: Clinical research or just routine practice? Pediatr. Allergy Immunol. 21, e446–e449 (2010).
Jones, S. M. et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J. Allergy Clin. Immunol. 124, 292–300.e97 (2009).
Goubier, A. et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity 29, 464–475 (2008).
Bamboat, Z. M. et al. Human liver dendritic cells promote T cell hyporesponsiveness. J. Immunol. 182, 1901–1911 (2009).
Acknowledgements
The author is grateful to H. Eliassen for excellent secretarial assistance. Studies discussed here conducted at LIIPAT were supported by the Research Council of Norway, the Norwegian Cancer Society, the University of Oslo, and Oslo University Hospital.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Brandtzaeg, P. Food allergy: separating the science from the mythology. Nat Rev Gastroenterol Hepatol 7, 380–400 (2010). https://doi.org/10.1038/nrgastro.2010.80
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2010.80
This article is cited by
-
Perinatal antibiotic exposure alters composition of murine gut microbiota and may influence later responses to peanut antigen
Allergy, Asthma & Clinical Immunology (2018)
-
Adverse reactions to food: the female dominance – A secondary publication and update
World Allergy Organization Journal (2017)
-
Immune suppression of IgG response against dairy proteins in major depression
BMC Psychiatry (2017)
-
Prostaglandin D2 metabolite in urine is an index of food allergy
Scientific Reports (2017)
-
Food, health, and complexity: towards a conceptual understanding to guide collaborative public health action
BMC Public Health (2016)