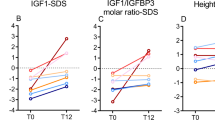
Abstract
Crohn's disease manifests during childhood or adolescence in up to 25% of patients. The potential for linear growth impairment as a complication of chronic intestinal inflammation is unique to pediatric patient populations. Insulin-like growth factor I (IGF-I), produced by the liver in response to growth hormone (GH) stimulation, is the key mediator of GH effects at the growth plate of bones. An association between impaired growth in children with Crohn's disease and low IGF-I levels is well recognized. Early studies emphasized the role of malnutrition in suppression of IGF-I production. However, a simple nutritional hypothesis fails to explain all the observations related to growth in children with Crohn's disease. The direct, growth-inhibitory effects of proinflammatory cytokines are increasingly recognized and explored. The potential role of noncytokine factors, such as lipopolysaccharides, and their potential to negatively influence the growth axis have recently been investigated with intriguing results. There is now reason for optimism that the modern anticytokine therapeutic agents available for treating children and adolescents with Crohn's disease will reduce the prevalence of this otherwise common complication. As our understanding of the mechanisms that underlie growth impairment advance, so too should the opportunity for developing further novel and targeted therapies.
Key Points
-
Chronic undernutrition and direct effects of proinflammatory cytokines are the two major and interrelated factors responsible for the impairment of linear growth in children with Crohn's disease
-
The mechanisms by which cytokines impede linear growth are complex and involve, but are not limited to, disruption of growth hormone–insulin-like growth factor I pathways
-
The potential effects that other noncytokine molecular factors may have on the growth axis are an evolving focus of research
-
Normal growth is a marker of the success of therapy in children with Crohn's disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kelsen, J. & Baldassano, R. Inflammatory bowel disease: the difference between children and adults. Inflamm. Bowel Dis. 14 (Suppl. 2), S9–S11 (2008).
Heuschkel, R. et al. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm. Bowel Dis. 14, 839–849 (2008).
Karlberg, J., Jalil, F., Lam, B., Low, L. & Yeung, C. Y. Linear growth retardation in relation to the three phases of growth. Eur. J. Clin. Nutr. 48 (Suppl. 1), S25–S44 (1994).
Rogol, A. D., Roemmich, J. N. & Clark, P. A. Growth at puberty. J. Adolesc. Health 31, 192–200 (2002).
Salmon, W. D. Jr & Daughaday, W. H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J. Lab. Clin. Med. 116, 408–419 (1990).
Daughaday, W. H. A personal history of the origin of the somatomedin hypothesis and recent challenges to its validity. Perspect. Biol. Med. 32, 194–211 (1989).
Rinderknecht, E. & Humbel, R. E. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J. Biol. Chem. 253, 2769–2776 (1978).
Isaksson, O. G., Jansson, J. O. & Gause, I. A. Growth hormone stimulates longitudinal bone growth directly. Science 216, 1237–1239 (1982).
Isaksson, O. G., Lindahl, A., Nilsson, A. & Isgaard, J. Mechanism of the stimulatory effect of growth hormone on longitudinal bone growth. Endocr. Rev. 8, 426–438 (1987).
Green, H., Morikawa, M. & Nixon, T. A dual effector theory of growth-hormone action. Differentiation 29, 195–198 (1985).
Frank, S. J., Messina, J. L., Baumann, G., Black, R. A. & Bertics, P. J. Insights into modulation of (and by) growth hormone signaling. J. Lab. Clin. Med. 136, 14–20 (2000).
Teglund, S. et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93, 841–850 (1998).
Bergad, P. L. et al. Inhibition of growth hormone action in models of inflammation. Am. J. Physiol. Cell Physiol. 279, C1906–C1917 (2000).
Denson, L. A. et al. Interleukin-6 inhibits hepatic growth hormone signaling via upregulation of Cis and Socs-3. Am. J. Physiol. Gastrointest. Liver Physiol. 284, G646–G654 (2003).
Ram, P. A. & Waxman, D. J. SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J. Biol. Chem. 274, 35553–35561 (1999).
Asplin, C. M. et al. Alterations in the pulsatile mode of growth hormone release in men and women with insulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 69, 239–245 (1989).
Herrington, J., Smit, L. S., Schwartz, J. & Carter-Su, C. The role of STAT proteins in growth hormone signaling. Oncogene 19, 2585–2597 (2000).
Liu, X., Robinson, G. W., Gouilleux, F., Groner, B. & Hennighausen, L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc. Natl Acad. Sci. USA 92, 8831–8835 (1995).
Jones, J. I. & Clemmons, D. R. Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 16, 3–34 (1995).
Govoni, K. E., Baylink, D. J. & Mohan, S. The multi-functional role of insulin-like growth factor binding proteins in bone. Pediatr. Nephrol. 20, 261–268 (2005).
Rechler, M. M. Insulin-like growth factor binding proteins. Vitam. Horm. 47, 1–114 (1993).
Miyakoshi, N., Richman, C., Qin, X., Baylink, D. J. & Mohan, S. Effects of recombinant insulin-like growth factor-binding protein-4 on bone formation parameters in mice. Endocrinology 140, 5719–5728 (1999).
Rajaram, S., Baylink, D. J. & Mohan, S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr. Rev. 18, 801–831 (1997).
Thissen, J. P., Davenport, M. L., Pucilowska, J. B., Miles, M. V. & Underwood, L. E. Increased serum clearance and degradation of 125I-labeled IGF-I in protein-restricted rats. Am. J. Physiol. 262, E406–E411 (1992).
Underwood, L. E., Thissen, J. P., Lemozy, S., Ketelslegers, J. M. & Clemmons, D. R. Hormonal and nutritional regulation of IGF-I and its binding proteins. Horm. Res. 42, 145–151 (1994).
Nilsson, O. & Baron, J. Impact of growth plate senescence on catch-up growth and epiphyseal fusion. Pediatr. Nephrol. 20, 319–322 (2005).
Walker, K. V. & Kember, N. F. Cell kinetics of growth cartilage in the rat tibia. II. Measurements during ageing. Cell Tissue Kinet. 5, 409–419 (1972).
Weise, M. et al. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc. Natl Acad. Sci. USA 98, 6871–6876 (2001).
Gafni, R. I. et al. Catch-up growth is associated with delayed senescence of the growth plate in rabbits. Pediatr. Res. 50, 618–623 (2001).
Baron, J. et al. Catch-up growth after glucocorticoid excess: a mechanism intrinsic to the growth plate. Endocrinology 135, 1367–1371 (1994).
Wei, W. & Sedivy, J. M. Differentiation between senescence (M1) and crisis (M2) in human fibroblast cultures. Exp. Cell Res. 253, 519–522 (1999).
Prader, A., Tanner, J. M. & von Harnack, H. G. Catch-up growth following illness or starvation: an example of developmental canalization in man. J. Pediatr. 62, 646–659 (1963).
Cutler, G. B. Jr. The role of estrogen in bone growth and maturation during childhood and adolescence. J. Steroid Biochem. Mol. Biol. 61, 141–144 (1997).
Veldhuis, J. D. & Bowers, C. Y. Three-peptide control of pulsatile and entropic feedback-sensitive modes of growth hormone secretion: modulation by estrogen and aromatizable androgen. J. Pediatr. Endocrinol. Metab. 16 (Suppl. 3), 587–605 (2003).
Keenan, B. S. et al. Androgen-stimulated pubertal growth: the effects of testosterone and dihydrotestosterone on growth hormone and insulin-like growth factor-I in the treatment of short stature and delayed puberty. J. Clin. Endocrinol. Metab. 76, 996–1001 (1993).
Nilsson, K. O. et al. Improved final height in girls with Turner's syndrome treated with growth hormone and oxandrolone. J. Clin. Endocrinol. Metab. 81, 635–640 (1996).
Stanhope, R. et al. Double blind placebo controlled trial of low dose oxandrolone in the treatment of boys with constitutional delay of growth and puberty. Arch. Dis. Child 63, 501–505 (1988).
Kirschner, B. S. & Sutton, M. M. Somatomedin-C levels in growth-impaired children and adolescents with chronic inflammatory bowel disease. Gastroenterology 91, 830–836 (1986).
Tenore, A., Berman, W. F., Parks, J. S. & Bongiovanni, A. M. Basal and stimulated serum growth hormone concentrations in inflammatory bowel disease. J. Clin. Endocrinol. Metab. 44, 622–628 (1977).
Straus, D. S. & Takemoto, C. D. Effect of fasting on insulin-like growth factor-I (IGF-I) and growth hormone receptor mRNA levels and IGF-I gene transcription in rat liver. Mol. Endocrinol. 4, 91–100 (1990).
Thissen, J. P. et al. Evidence that pretranslational and translational defects decrease serum insulin-like growth factor-I concentrations during dietary protein restriction. Endocrinology 129, 429–435 (1991).
Kelts, D. G. et al. Nutritional basis of growth failure in children and adolescents with Crohn's disease. Gastroenterology 76, 720–727 (1979).
Kirschner, B. S., Klich, J. R., Kalman, S. S., deFavaro, M. V. & Rosenberg, I. H. Reversal of growth retardation in Crohn's disease with therapy emphasizing oral nutritional restitution. Gastroenterology 80, 10–15 (1981).
Ballinger, A. et al. The role of medial hypothalamic serotonin in the suppression of feeding in a rat model of colitis. Gastroenterology 118, 544–553 (2000).
Filipsson, S., Hulten, L. & Lindstedt, G. Malabsorption of fat and vitamin B12 before and after intestinal resection for Crohn's disease. Scand. J. Gastroenterol. 13, 529–536 (1978).
Griffiths, A. M., Drobnies, A., Soldin, S. J. & Hamilton, J. R. Enteric protein loss measured by fecal α1-antitrypsin clearance in the assessment of Crohn's disease activity: a study of children and adolescents. J. Pediatr. Gastroenterol. Nutr. 5, 907–911 (1986).
Azcue, M., Rashid, M., Griffiths, A. & Pencharz, P. B. Energy expenditure and body composition in children with Crohn's disease: effect of enteral nutrition and treatment with prednisolone. Gut 41, 203–208 (1997).
Ballinger, A. B., Azooz, O., El-Haj, T., Poole, S. & Farthing, M. J. Growth failure occurs through a decrease in insulin-like growth factor 1 which is independent of undernutrition in a rat model of colitis. Gut 46, 694–700 (2000).
De Benedetti, F. et al. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammation. J. Clin. Invest. 99, 643–650 (1997).
Martensson, K., Chrysis, D. & Savendahl, L. Interleukin-1β and TNF-α act in synergy to inhibit longitudinal growth in fetal rat metatarsal bones. J. Bone Miner. Res. 19, 1805–1812 (2004).
Varghese, S., Wyzga, N., Griffiths, A. M. & Sylvester, F. A. Effects of serum from children with newly diagnosed Crohn disease on primary cultures of rat osteoblasts. J. Pediatr. Gastroenterol. Nutr. 35, 641–648 (2002).
Wang, X. et al. Endotoxin-induced proteolytic reduction in hepatic growth hormone (GH) receptor: a novel mechanism for GH insensitivity. Mol. Endocrinol. 22, 1427–1437 (2008).
Denson, L. A. et al. TNF-α downregulates murine hepatic growth hormone receptor expression by inhibiting Sp1 and Sp3 binding. J. Clin. Invest. 107, 1451–1458 (2001).
Dejkhamron, P. et al. Lipopolysaccharide (LPS) directly suppresses growth hormone receptor (GHR) expression through MyD88-dependent and -independent Toll-like receptor-4/MD2 complex signaling pathways. Mol. Cell Endocrinol. 274, 35–42 (2007).
Colson, A., Le Cam, A., Maiter, D., Edery, M. & Thissen, J. P. Potentiation of growth hormone-induced liver suppressors of cytokine signaling messenger ribonucleic acid by cytokines. Endocrinology 141, 3687–3695 (2000).
Cohney, S. J. et al. SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation. Mol. Cell Biol. 19, 4980–4988 (1999).
Ram, P. A. & Waxman, D. J. Role of the cytokine-inducible SH2 protein CIS in desensitization of STAT5b signaling by continuous growth hormone. J. Biol. Chem. 275, 39487–39496 (2000).
Shumate, M. L., Yumet, G., Ahmed, T. A. & Cooney, R. N. Interleukin-1 inhibits the induction of insulin-like growth factor-I by growth hormone in CWSV-1 hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G227–G239 (2005).
De Benedetti, F. et al. Effect of IL-6 on IGF binding protein-3: a study in IL-6 transgenic mice and in patients with systemic juvenile idiopathic arthritis. Endocrinology 142, 4818–4826 (2001).
Mauras, N. Growth hormone therapy in the glucocorticosteroid-dependent child: metabolic and linear growth effects. Horm. Res. 56 (Suppl. 1), 13–18 (2001).
De Benedetti, F. et al. Impaired skeletal development in interleukin-6-transgenic mice: a model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum. 54, 3551–3563 (2006).
Kamimura, D., Ishihara, K. & Hirano, T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev. Physiol. Biochem. Pharmacol. 149, 1–38 (2003).
Tamura, T. et al. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc. Natl Acad. Sci. USA 90, 11924–11928 (1993).
Franchimont, N., Wertz, S. & Malaise, M. Interleukin-6: an osteotropic factor influencing bone formation? Bone 37, 601–606 (2005).
Bross, D. A., Leichtner, A. M., Zurakowski, D., Law, T. & Bousvaros, A. Elevation of serum interleukin-6 but not serum-soluble interleukin-2 receptor in children with Crohn's disease. J. Pediatr. Gastroenterol. Nutr. 23, 164–171 (1996).
Suzuki, A. et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J. Exp. Med. 193, 471–481 (2001).
Tebbutt, N. C. et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat. Med. 8, 1089–1097 (2002).
Nicholson, S. E. et al. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc. Natl Acad. Sci. USA 97, 6493–6498 (2000).
Carey, R. et al. Activation of an IL-6–STAT3-dependent transcriptome in pediatric-onset inflammatory bowel disease. Inflamm. Bowel Dis. 14, 446–457 (2008).
Mudter, J. et al. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am. J. Gastroenterol. 100, 64–72 (2005).
Cassidy, J. T. & Hillman, L. S. Abnormalities in skeletal growth in children with juvenile rheumatoid arthritis. Rheum. Dis. Clin. North Am. 23, 499–522 (1997).
MacRae, V. E., Farquharson, C. & Ahmed, S. F. The pathophysiology of the growth plate in juvenile idiopathic arthritis. Rheumatology (Oxford) 45, 11–19 (2006).
Sawczenko, A. et al. Intestinal inflammation-induced growth retardation acts through IL-6 in rats and depends on the −174 IL-6 G>C polymorphism in children. Proc. Natl Acad. Sci. USA 102, 13260–13265 (2005).
Cezard, J. P. et al. Growth in paediatric Crohn's disease. Horm. Res. 58 (Suppl. 1), 11–15 (2002).
Bernstein, C. N. & Leslie, W. D. The pathophysiology of bone disease in gastrointestinal disease. Eur. J. Gastroenterol. Hepatol. 15, 857–864 (2003).
Lien, G. et al. A two-year prospective controlled study of bone mass and bone turnover in children with early juvenile idiopathic arthritis. Arthritis Rheum. 52, 833–840 (2005).
Ito, H. et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology 126, 989–996 (2004).
Nishimoto, N. & Kishimoto, T. Inhibition of IL-6 for the treatment of inflammatory diseases. Curr. Opin. Pharmacol. 4, 386–391 (2004).
Nishimoto, N. et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 50, 1761–1769 (2004).
Yokota, S. et al. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 52, 818–825 (2005).
Wolk, K. et al. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn's disease. J. Immunol. 178, 5973–5981 (2007).
Yumet, G., Shumate, M. L., Bryant, D. P., Lang, C. H. & Cooney, R. N. Hepatic growth hormone resistance during sepsis is associated with increased suppressors of cytokine signaling expression and impaired growth hormone signaling. Crit. Care Med. 34, 1420–1427 (2006).
Allen, D. B. Influence of inhaled corticosteroids on growth: a pediatric endocrinologist's perspective. Acta Paediatr. 87, 123–129 (1998).
Ballinger, A. B., Savage, M. O. & Sanderson, I. R. Delayed puberty associated with inflammatory bowel disease. Pediatr. Res. 53, 205–210 (2003).
Brain, C. E. & Savage, M. O. Growth and puberty in chronic inflammatory bowel disease. Baillieres Clin. Gastroenterol. 8, 83–100 (1994).
Mizokami, A., Gotoh, A., Yamada, H., Keller, E. T. & Matsumoto, T. Tumor necrosis factor-α represses androgen sensitivity in the LNCaP prostate cancer cell line. J. Urol. 164, 800–805 (2000).
Zadik, Z., Cooper, M., Chen, M. & Stern, N. Cushing's disease presenting as pubertal arrest. J. Pediatr. Endocrinol. 6, 201–204 (1993).
Russell, R. K. et al. Genotype–phenotype analysis in childhood-onset Crohn's disease: NOD2/CARD15 variants consistently predict phenotypic characteristics of severe disease. Inflamm. Bowel Dis. 11, 955–964 (2005).
Tomer, G., Ceballos, C., Concepcion, E. & Benkov, K. J. NOD2/CARD15 variants are associated with lower weight at diagnosis in children with Crohn's disease. Am. J. Gastroenterol. 98, 2479–2484 (2003).
Wine, E. et al. Pediatric Crohn's disease and growth retardation: the role of genotype, phenotype, and disease severity. Pediatrics 114, 1281–1286 (2004).
Russell, R. K. et al. Analysis of the influence of OCTN1/2 variants within the IBD5 locus on disease susceptibility and growth indices in early-onset inflammatory bowel disease. Gut 55, 1114–1123 (2006).
Levine, A. et al. TNF promoter polymorphisms and modulation of growth retardation and disease severity in pediatric Crohn's disease. Am. J. Gastroenterol. 100, 1598–1604 (2005).
Aiges, H., Markowitz, J., Rosa, J. & Daum, F. Home nocturnal supplemental nasogastric feedings in growth-retarded adolescents with Crohn's disease. Gastroenterology 97, 905–910 (1989).
Belli, D. C. et al. Chronic intermittent elemental diet improves growth failure in children with Crohn's disease. Gastroenterology 94, 603–610 (1988).
Wilschanski, M. et al. Supplementary enteral nutrition maintains remission in paediatric Crohn's disease. Gut 38, 543–548 (1996).
Walker-Smith, J. A. Management of growth failure in Crohn's disease. Arch. Dis. Child. 75, 351–354 (1996).
Fell, J. M. et al. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn's disease. Aliment. Pharmacol. Ther. 14, 281–289 (2000).
Borrelli, O. et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn's disease: a randomized controlled open-label trial. Clin. Gastroenterol. Hepatol. 4, 744–753 (2006).
Zachos, M., Tondeur, M. & Griffiths, A. M. Enteral nutritional therapy for inducing remission of Crohn's disease. Cochrane Database of Systematic Reviews CD000542 (2001).
Rigaud, D. et al. Controlled trial comparing two types of enteral nutrition in treatment of active Crohn's disease: elemental versus polymeric diet. Gut 32, 1492–1497 (1991).
Seidman, E., Jones, A. & Issenman, R. Cyclical exclusive enteral nutrition versus alternate day prednisone in maintaining remission of pediatric Crohn's disease. J. Pediatr. Gastroenterol. Nutr. 23, A344 (1996).
Rutgeerts, P. J. Review article: the limitations of corticosteroid therapy in Crohn's disease. Aliment. Pharmacol. Ther. 15, 1515–1525 (2001).
Markowitz, J., Grancher, K., Kohn, N., Lesser, M. & Daum, F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn's disease. Gastroenterology 119, 895–902 (2000).
Turner, D. et al. Methotrexate following unsuccessful thiopurine therapy in pediatric Crohn's disease. Am. J. Gastroenterol. 102, 2804–2812 (2007).
Hyams, J. et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology 132, 863–873 (2007).
Walters, T. D., Gilman, A. R. & Griffiths, A. M. Linear growth improves during infliximab therapy in children with chronically active severe Crohn's disease. Inflamm. Bowel Dis. 13, 424–430 (2007).
de Ridder, L. et al. Infliximab therapy in 30 patients with refractory pediatric crohn disease with and without fistulas in The Netherlands. J. Pediatr. Gastroenterol. Nutr. 39, 46–52 (2004).
Borrelli, O. et al. Infliximab heals intestinal inflammatory lesions and restores growth in children with Crohn's disease. Dig. Liver Dis. 36, 342–347 (2004).
Cezard, J. P. et al. A prospective study of the efficacy and tolerance of a chimeric antibody to tumor necrosis factors (Remicade) in severe pediatric Crohn disease. J. Pediatr. Gastroenterol. Nutr. 36, 632–636 (2003).
Griffiths, A. M., Hyams, J. S. & Crandall, W. Height of children with active Crohn's disease improves during treatment with infliximab. Gastroenterology 130 (Suppl. 2), A59 (2006).
Griffiths, A. M., Wesson, D. E., Shandling, B., Corey, M. & Sherman, P. M. Factors influencing postoperative recurrence of Crohn's disease in childhood. Gut 32, 491–495 (1991).
Davies, G., Evans, C. M., Shand, W. S. & Walker-Smith, J. A. Surgery for Crohn's disease in childhood: influence of site of disease and operative procedure on outcome. Br. J. Surg. 77, 891–894 (1990).
Baldassano, R. N. et al. Pediatric Crohn's disease: risk factors for postoperative recurrence. Am. J. Gastroenterol. 96, 2169–2176 (2001).
Mauras, N. et al. Growth hormone has anabolic effects in glucocorticosteroid-dependent children with inflammatory bowel disease: a pilot study. Metabolism 51, 127–135 (2002).
Han, X., Sosnowska, D., Bonkowski, E. L. & Denson, L. A. Growth hormone inhibits signal transducer and activator of transcription 3 activation and reduces disease activity in murine colitis. Gastroenterology 129, 185–203 (2005).
Slonim, A. E. et al. A preliminary study of growth hormone therapy for Crohn's disease. N. Engl. J. Med. 342, 1633–1637 (2000).
Leung, D. W. et al. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature 330, 537–543 (1987).
Acknowledgements
T. D. Walters receives joint fellowship funding from The Crohn's and Colitis Foundation of Canada, AstraZeneca Canada, The Canadian Association of Gastroenterology, and the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A. M. Griffiths has been a Consultant for Abbott, Centocor, Schering-Plough and UCB, and has received research support from Schering Plough Canada.
T. D. Walters has been a Consultant for Schering-Plough Canada and UCB.
Rights and permissions
About this article
Cite this article
Walters, T., Griffiths, A. Mechanisms of growth impairment in pediatric Crohn's disease. Nat Rev Gastroenterol Hepatol 6, 513–523 (2009). https://doi.org/10.1038/nrgastro.2009.124
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2009.124
This article is cited by
-
Exclusive enteral nutrition effect on the clinical course of pediatric Crohn’s disease: a single center experience
European Journal of Pediatrics (2020)
-
Assessing the feasibility of injectable growth-promoting therapy in Crohn’s disease
Pilot and Feasibility Studies (2016)
-
Mesalazine in the initial management of severely acutely malnourished children with environmental enteric dysfunction: a pilot randomized controlled trial
BMC Medicine (2014)
-
Fat body, fat pad and adipose tissues in invertebrates and vertebrates: the nexus
Lipids in Health and Disease (2014)
-
Endocrine Therapy for Growth Retardation in Paediatric Inflammatory Bowel Disease
Pediatric Drugs (2014)