Key Points
-
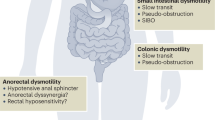
Gut involvement in systemic sclerosis occurs more commonly in the diffuse than the limited cutaneous variant
-
Oesophageal involvement is the most common gastrointestinal manifestation of systemic sclerosis, with severe reflux disease and dysphagia as the main presentations
-
When treating small intestinal bacterial overgrowth in patients with systemic sclerosis, effectiveness of treatment and decisions about cyclical courses of antibiotic are best assessed by symptoms rather than breath testing
-
Malnutrition in patients with systemic sclerosis is associated with more aggressive disease progression, and the risk of developing malnutrition is related to both gut and extraintestinal factors
Abstract
Systemic sclerosis is a multisystem autoimmune disorder that involves the gastrointestinal tract in more than 90% of patients. This involvement can extend from the mouth to the anus, with the oesophagus and anorectum most frequently affected. Gut complications result in a plethora of presentations that impair oral intake and faecal continence and, consequently, have an adverse effect on patient quality of life, resulting in referral to gastroenterologists. The cornerstones of gastrointestinal symptom management are to optimize symptom relief and monitor for complications, in particular anaemia and malabsorption. Early intervention in patients who develop these complications is critical to minimize disease progression and improve prognosis. In the future, enhanced therapeutic strategies should be developed, based on an ever-improving understanding of the intestinal pathophysiology of systemic sclerosis. This Review describes the most commonly occurring clinical scenarios of gastrointestinal involvement in patients with systemic sclerosis as they present to the gastroenterologist, with recommendations for the suggested assessment protocol and therapy in each situation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Allcock, R. J. et al. A study of the prevalence of systemic sclerosis in northeast england. Rheumatology (Oxford) 43, 596–602 (2004).
Chifflot, H. et al. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin. Arthritis Rheum. 37, 223–235 (2008).
Andréasson, K. et al. Prevalence and incidence of systemic sclerosis in southern Sweden: population-based data with case ascertainment using the 1980 ARA criteria and the proposed ACR-EULAR classification criteria. Ann. Rheum. Dis. 73, 1788–1792 (2014).
Mayes, M. D. et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 48, 2246–2255 (2003).
Steen, V. D. & Medsger, T. A. Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 43, 2437–2444 (2000).
Lock, G., Holstege, A., Lang, B. & Scholmerich, J. Gastrointestinal manifestations of progressive systemic sclerosis. Am. J. Gastroenterol. 92, 763–771 (1997).
Attar, A. Digestive manifestations in systemic sclerosis. Ann. Med. Interne (Paris). 153, 260–264 (in French) (2002).
Hunzelmann, N. et al. The registry of the German Network for Systemic Scleroderma: frequency of disease subsets and patterns of organ involvement. Rheumatology (Oxford) 47, 1185–1192 (2008).
Sjogren, R. W. Gastrointestinal features of scleroderma. Curr. Opin. Rheumatol. 8, 569–575 (1996).
Malandrini, A. et al. Autonomic nervous system and smooth muscle cell involvement in systemic sclerosis: ultrastructural study of 3 cases. J. Rheumatol. 27, 1203–1206 (2000).
Dessein, P. H. et al. Autonomic dysfunction in systemic sclerosis: sympathetic overactivity and instability. Am. J. Med. 93, 143–150 (1992).
Eaker, E. Y. et al. Myenteric neuronal antibodies in scleroderma — passive transfer evokes alterations in intestinal myoelectric activity in a rat model. J. Lab. Clin. Med. 133, 551–556 (1999).
Kawaguchi, Y. et al. Muscarinic-3 acetylcholine receptor autoantibody in patients with systemic sclerosis: contribution to severe gastrointestinal tract dysmotility. Ann. Rheum. Dis. 68, 710–714 (2009).
Singh, J. et al. Immunoglobulins from scleroderma patients inhibit the muscarinic receptor activation in internal anal sphincter smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol. 297, 206–213 (2009).
Hendel, L., Kobayasi, T. & Petri, M. Ultrastructure of the small intestinal mucosa in progressive systemic sclerosis (PSS). Acta Pathol. Microbiol. Immunol. Scand. 95, 41–46 (1987).
Ponge, T. & Bruley des Varannes, S. Digestive involvement of scleroderma. Rev. Pract. 52, 1896–1900 (2002).
Poirier, T. J. & Rankin, G. B. Gastrointestinal manifestations of progressive systemic scleroderma based on a review of 364 cases. Am. J. Gastroenterol. 58, 30–44 (1972).
Young, M. A., Rose, S. & Reynolds, J. C. Gastrointestinal manifestations of scleroderma. Rheum. Dis. Clin. North Am. 22, 797–823 (1996).
Sallam, P. J. et al. Assessment of gastrointestinal involvement. Clin. Exp. Rheumatol. 21 (Suppl. 29), S15–S18 (2003).
Weber, P. et al. 24-h intraesophageal pH monitoring in children and adolescents with scleroderma and mixed connective tissue disease. J. Rheumatol. 27, 2692–2695 (2000).
Thoua, N. et al. Assessment of gastrointestinal symptoms in patients with systemic slcerosis in a UK tertiary referral centre. Rheumatology (Oxford) 49, 1770–1775 (2010).
Harrison, E., Herrick, A. L., McLaughlin, J. T. & Lal, S. Malnutrition in systemic sclerosis. Rheumatology 51, 1747–1756 (2012).
Hansi, N. et al. Consensus best practice pathway of the UK scleroderma study group: gastrointestinal manifestations of systemic sclerosis. Clin. Exp. Rheumatol. 32 (Suppl. 86), S-214–S-221 (2014).
Marshall, J. B. et al. Gastrointestinal manifestations of mixed connective tissue disease. Gastroenterology 98, 1232–1238 (1990).
Bestetti, A. et al. Esophageal scintigraphy with a semisolid meal to evaluate esophageal dysmotility in systemic sclerosis and Raynaud's phenomenon. J. Nucl. Med. 40, 77–84 (1999).
Akesson, A. & Wollheim, F. A. Organ manifestations in 100 patients with progressive systemic sclerosis: a comparison between the CREST syndrome and diffuse scleroderma. Br. J. Rheumatol. 28, 281–286 (1989).
Sallam, H., McNearney, T. A. & Chen, J. D. Z. Systematic review: pathophysiology and management of gastrointestinal dysmotility in systemic sclerosis (scleroderma). Aliment. Pharmacol. Ther. 23, 691–712 (2006).
Lepri, G. et al. Evidence for oesophageal and anorectal involvement in very early systemic sclerosis (VEDOSS): report from a single VEDOSS/EUSTAR centre. Ann. Rheum. Dis. 74, 124–128 (2015).
Weston, S., Thumshirn, M., Wiste, J. & Carnilleri, M. Clinical and upper gastrointestinal motility features in systemic sclerosis and related disorders. Am. J. Gastroenterol. 93, 1085–1089 (1998).
Roman, S. et al. Esophageal dysmotility associated with systemic sclerosis: a high-resolution manometry study. Dis. Esophagus 24, 299–304 (2011).
Dantas, R. O. & Aprile, L. Esophageal striated muscle contractions in patients with gastroesophageal reflux symptoms. Dig. Dis. Sci. 47, 2586–2590 (2002).
Emmanuel, A., Kamm, M. A., Roy, A. J. & Antonelli, K. Effect of a novel prokinetic drug, R093877, on gastrointestinal transit in healthy volunteers. Gut 42, 511–516 (1998).
Boeckxstaens, G. E., Bartelsman, J. F., Lauwers, L. & Tytgat, G. N. Treatment of GI dysmotility in scleroderma with the new enterokinetic agent prucalopride. Am. J. Gastroenterol. 97, 194–197 (2002).
Maeda, M., Ichiki, Y., Sumi, A. & Mori, S. A trial of acupuncture for progressive systemic sclerosis. J. Dermatol. 15, 133–140 (1988).
Mearin, F. et al. Effect of transcutaneous nerve stimulation on esophageal motility in patients with achalasia and scleroderma. Scand. J. Gastroenterol. 25, 1018–1023 (1990).
Thonhofer, R., Siegel, C., Trummer, M. & Graninger, W. Early endoscopy in systemic sclerosis without gastrointestinal symptoms. Rheumatol. Int. 32, 165–168 (2012).
Zamost, B. J. et al. Esophagitis in scleroderma. Prevalence and risk factors. Gastroenterology 92, 421–428 (1987).
Marie, I. et al. Gastric involvement in systemic sclerosis — a prospective study. Am. J. Gastroenterol. 96, 77–83 (2001).
Katzka, D. A. et al. Barrett's metaplasia and adenocarcinoma of the esophagus in scleroderma. Am. J. Med. 82, 46–52 (1987).
Domsic, R., Fasanella, K. & Bielefeldt, K. Gastrointestinal manifestations of systemic sclerosis. Dig. Dis. Sci. 53, 1163–1174 (2008).
McMahan, Z. H. & Hummers, L. K. Systemic sclerosis — challenges for clinical practice. Nat. Rev. Rheumatol. 9, 90–100 (2003).
Nagaraja, V., McMahan, Z. H., Getzug, T. & Khanna, D. Management of gastrointestinal involvement in scleroderma. Curr. Treatm. Opt. Rheumatol. 1, 82–105 (2015).
Shoenut, J. P., Wieler, J. A. & Micflikier, A. B. The extent and pattern of gastro-oesophageal reflux in patients with scleroderma oesophagus: the effect of low-dose omeprazole. Aliment. Pharmacol. Ther. 7, 509–513 (1993).
Hendel, L., Hage, E., Hendel, J. & Stentoft, P. Omeprazole in the long-term treatment of severe gastro-oesophageal reflux disease in patients with systemic sclerosis. Aliment. Pharmacol. Ther. 6, 565–577 (1992).
Corleto, V. D., Festa, S., Di Giulio, E. & Annibale, B. Proton pump inhibitor therapy and potential long-term harm. Curr. Opin. Endocrinol. Diabetes Obes. 21, 3–8 (2014).
Abraham, N. S. Proton pump inhibitors: potential adverse effects. Curr. Opin. Gastroenterol. 28, 615–620 (2012).
Petrokubi, R. J. & Jeffries, G. H. Cimetidine versus antacid in scleroderma with reflux esophagitis. A randomized double-blind controlled study. Gastroenterology 77, 691–695 (1979).
Wang, Y., Pan, T., Wang, Q. & Guo, Z. Additional bedtime H2-receptor antagonist for the control of nocturnal management of gastric acid breakthrough. Cochrane Database Syst Rev. 4, CD004275 (2009).
Zuccaro, G. Esophagoscopy and endoscopic esophageal ultrasound in the assessment of esophageal function. Semin. Thorac. Cardiovasc. Surg. 13, 226–233 (2001).
Wipff, J. et al. Outcomes of Barrett's oesophagus related to systemic sclerosis: a 3-year EULAR Scleroderma Trials and Research prospective follow-up study. Rheumatology 50, 1440–1444 (2011).
Conio, M. et al. Long-term endoscopic surveillance of patients with Barrett's esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am. J. Gastroenterol. 98, 1931–1939 (2003).
Appel, J. Z. 3rd et al. Characterization of the innate immune response to chronic aspiration in a novel rodent model. Respir. Res. 8, 87–90 (2007).
Savarino, E. et al. Gastroesophageal reflux and pulmonary fibrosis in scleroderma. A study using pH-impedance monitoring. Am. J. Respir. Crit. Care Med. 179, 408–413 (2009).
Gilson, M. et al. Prognostic factors for lung function in systemic sclerosis: prospective study of 105 cases. Eur. Respir. J. 35, 112–117 (2010).
Christmann, R. B. et al. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: clinical, radiologic, histopathologic, and treatment evidence. Semin. Arthritis Rheum. 40, 241–249 (2010).
de Souza, R. B. C. et al. Centrilobular fibrosis: an underrecognized pattern in systemic sclerosis. Respiration 77, 389–397 (2009).
Madsen, J. L. & Hendel, L. Gastrointestinal transit times of radiolabeled meal in progressive systemic sclerosis. Dig. Dis. Sci. 37, 1404–1408 (1992).
Cozzi, F. et al. Gastric dysmotility after liquid bolus ingestion in systemic sclerosis: an ultrasonographic study. Rheumatol. Int. 32, 1219–1223 (2012).
Franck-Larsson, K., Hedenstrom, H., Dahl, R. & Ronnblom, A. Delayed gastric emptying in patients with diffuse versus limited systemic sclerosis, unrelated to gastrointestinal symptoms and myoelectric gastric activity. Scand. J. Rheumatol. 32, 348–355 (2003).
Tack, J., Carbone, F. & Rotondo, A. Gastroparesis. Curr. Opin. Gastroenterol. 31, 499–505 (2015).
Fiorucci, S., Distrufti, V., Gerli, R. & Morelli, A. Effect of erythromycin on gastric and gallbladder emptying and gastrointestinal symptoms in scleroderma patients is maintained medium term. Am. J. Gastroenterol. 89, 550–555 (1994).
Verne, G. N., Eaker, E. Y., Hardy, E. & Sninsky, C. A. Effect of octreotide and erythromycin on idiopathic and scleroderma-associated intestinal pseudoobstruction. Dig. Dis. Sci. 40, 1892–1901 (1995).
Edmunds, M. C. et al. Effect of octreotide on gastric and small bowel motility in patients with gastroparesis. Aliment. Pharmacol. Ther. 12, 167–174 (1998).
Murray, C., Kamm, M., Bloom, S. & Emmanuel, A. Ghrelin for the gastroenterologist: history and potential. Gastroenterology 125, 1492–1502 (2003).
Ariyasu, H. et al. Clinical effects of ghrelin on gastrointestinal involvement in patients with systemic sclerosis. Endocr. J. 61, 735–742 (2014).
McCallum, R. et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin. Gastroenterol. Hepatol. 3, 49–54 (2005).
Sallam, H., Doshi, D., McNearney, T. & Chen, J. D. Z. Transcutaneous electrical nerve stimulation improves gastric motility, dyspeptic symptoms and physical functioning in patients with systemic sclerosis by balancing the sympathovagal activity. Gastroenterology 128, A-468 (2005).
Oiwa, H. et al. A case of systemic sclerosis sine scleroderma associated with perforation of an afferent loop after subtotal gastrectomy with Bilroth 2 anastomosis for its severe gastrointestinal involvement. Mod. Rheumatol. 15, 371–373 (2005).
You, C. H. et al. Gastric and small intestinal myoelectrical dysrhythmia associated with chronic intractable nausea and vomiting. Ann. Int. Med. 95, 449–451 (1981).
Ghoshal, U. C., Srivastava, D., Ghoshal, U. & Misra, A. Breath tests in the diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome in comparison with quantitative upper gut aspirate culture. Eur. J. Gastroenterol. Hepatol. 26, 753–760 (2014).
Simrén, M. & Stotzer, P. O. Use and abuse of hydrogen breath tests. Gut 55, 297–303 (2006).
Quigley, E. M. Small intestinal bacterial overgrowth: what it is and what it is not. Curr. Opin. Gastroenterol. 30, 141–146 (2014).
Quigley, E. M. & Quera, R. Small intestinal bacterial overgrowth: roles of antibiotics, prebiotics, and probiotics. Gastroenterology 130 (Suppl. 1), S78–S90 (2006).
Parodi, A. et al. Small intestinal bacterial overgrowth in patients suffering from scleroderma: clinical effectiveness of its eradication. Am. J. Gastroenterol. 103, 1257–1262 (2008).
Shah, S. C., Day, L. W., Somsouk, M. & Sewell, J. L. Metaanalysis: antibiotic therapy for small intestinal bacterial overgrowth. Aliment. Pharmacol. Ther. 38, 925–934 (2013).
Gyger, G. & Baron, M. Gastrointestinal manifestations of scleroderma: recent progress in evaluation, pathogenesis and management. Curr. Rheumatol. Rep. 14, 22–29 (2012).
Baron, M. et al. Screening and management for malnutrition and related gastro-intestinal disorders in systemic sclerosis: recommendations of a North American expert panel. Clin. Exp. Rheumatol. 28 (Suppl. 58), 42–46 (2010).
Soifer, L. O., Peralta, D., Dima, G. & Besasso, H. Comparative clinical efficacy of a probiotic versus an antibiotic in the treatment of patients with intestinal bacterial overgrowth and chronic abdominal functional distension: a pilot study. Acta Gastroenterol. Latinoam. 40, 323–327 (in Spanish) (2010).
Frech, T. M. et al. Probiotics for the treatment of systemic sclerosis-associated gastrointestinal bloating/distention. Clin. Exp. Rheumatol. 29, S22–S25 (2011).
Forbes, A. & Marie, I. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatology 48, 36–39 (2009).
da Silva Fink, J., Daniel de Mello, P. & Daniel de Mello, E. Subjective global assessment of nutritional status — a systematic review of the literature. Clin. Nutr. 34, 785–792 (2015).
Baron, M. et al. Malnutrition is common in systemic sclerosis: results from the Canadian scleroderma research group database. J. Rheumatol. 36, 2737–2743 (2009).
Khanna, D. et al. Reliability and validity of the University of California, Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument. Arthritis Rheum. 61, 1257–1263 (2009).
Krause, L. et al. Nutritional status as marker for disease activity and severity predicting mortality in patients with systemic sclerosis. Ann. Rheum. Dis. 69, 1951–1957 (2010).
Brown, M. et al. Home parenteral nutrition — an effective and safe long-term therapy for systemic sclerosisrelated intestinal failure. Rheumatology (Oxford) 47, 176–179 (2008).
Mann, S. D., Debinski, H. S. & Kamm, M. A. Clinical characteristics of chronic idiopathic intestinal pseudo-obstruction in adults. Gut 41, 675–681 (1997).
Stanghellini, V. et al. Natural history of intestinal failure induced by chronic idiopathic intestinal pseudo-obstruction. Transplant. Proc. 42, 15–18 (2010).
Lindberg, G., Iwarzon, M. & Tornblom, H. Clinical features and long-term survival in chronic intestinal pseudo-obstruction and enteric dysmotility. Scand. J. Gastroenterol. 44, 692–699 (2009).
Mecoli, C., Purohit, S., Sandorfi, N. & Derk, C. T. Mortality, recurrence, and hospital course of patients with systemic sclerosis-related acute intestinal pseudoobstruction. J. Rheumatol. 41, 2049–2054 (2014).
Lauro, A., De Giorgio, R. & Pinna, A. D. Advancement in the clinical management of intestinal pseudo-obstruction. Expert. Rev. Gastroenterol. Hepatol. 9, 197–208 (2015).
Emmanuel, A. V., Shand, A. & Kamm, M. A. Erythromycin for the treatment of chronic intestinal pseudo-obstruction: description of six cases with positive response. Aliment. Pharmacol. Ther. 19, 687–694 (2004).
Emmanuel, A. V. et al. Randomised clinical trial: the efficacy of prucalopride in patients with chronic intestinal pseudo-obstruction — a double-blind, placebo-controlled, cross-over, multiple n = 1 study. Aliment. Pharmacol. Ther. 35, 48–55 (2012).
Camilleri, M. et al. American College of Gastroenterology Clinical guideline: management of gastroparesis. Am. J. Gastroenterol. 108, 18–37 (2013).
Ponec, R. J., Saunders, M. D. & Kimmey, M. B. Neostigmine for the treatment of acute colonic pseudo-obstruction. N. Engl. J. Med. 341, 137–141 (1999).
Nikou, G. C. et al. Effect of octreotide on intestinal motility and bacterial overgrowth in scleroderma. N. Engl. J. Med. 325, 1461–1467 (1991).
Perlemuter, G. et al. Octreotide treatment of chronic intestinal pseudoobstruction secondary to connective tissue diseases. Arthritis Rheum. 42, 1545–1549 (1999).
Charalambopoulos, D. & Sfikakis, P. P. Treatment of small intestinal disease in systemic sclerosis with octreotide: a prospective study in seven patients. J. Clin. Rheumatol. 13, 119–123 (2007).
Klein-Weigel, P., Opitz, C. & Riemekasten, G. Systemic sclerosis — a systematic overview: part 1 — disease characteristics and classification, pathophysiologic concepts, and recommendations for diagnosis and surveillance. Vasa 40, 6–19 (2011).
Ruiter, G. et al. Iron deficiency in systemic sclerosis patients with and without pulmonary hypertension. Rheumatology (Oxford) 53, 285–292 (2014).
Ghrénassia, E. et al. Prevalence, correlates and outcomes of gastric antral vascular ectasia in systemic sclerosis: a EUSTAR case–control study. J. Rheumatol. 41, 99–105 (2014).
Marie, I. et al. Watermelon stomach in systemic sclerosis: its incidence and management. Aliment. Pharmacol. Ther. 28, 412–421 (2008).
Ingraham, K. M. et al. Gastric antral vascular ectasia in systemic sclerosis: demographics and disease predictors. J. Rheumatol. 37, 603–607 (2010).
Sellinger, C. P. & Ang, Y. S. Gastric antral vascular ectasia (GAVE): an update on clinical presentation, pathophysiology and treatment. Digestion 77, 131–137 (2008).
Gostout, C. J. et al. Endoscopic laser therapy for watermelon stomach. Gastroenterology 96, 1462–1465 (1989).
Shibukawa, G. et al. Gastric antral vascular ectasia (GAVE) associated with systemic sclerosis: relapse after endoscopic treatment by argon plasma coagulation. Intern. Med. 46, 279–283 (2007).
Swanson, E., Mahgoub, A., MacDonald, R. & Shaukat, A. Medical and endoscopic therapies for angiodysplasia and gastric antral vascular ectasia: a systematic review. Clin. Gastroenterol. Hepatol. 12, 571–582 (2014).
Wells, C. D. et al. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest. Endosc. 68, 231–236 (2008).
Watson, M. et al. Gastric antral vascular ectasia (watermelon stomach) in patients with systemic sclerosis. Arthritis Rheum. 39, 341–346 (1996).
Cho, S. et al. Endoscopic cryotherapy for the management of gastric antral vascular ectasia. Gastrointest. Endosc. 68, 895–902 (2008).
Schulz, S. W. et al. Improvement of severe systemic sclerosis-associated gastric antral vascular ectasia following immunosuppressive treatment with intravenous cyclophosphamide. J. Rheumatol. 36, 1653–1656 (2009).
Sebastian, S., O'Morain, C. A. & Buckley, M. J. Review article: current therapeutic options for gastric antral vascular ectasia. Aliment. Pharmacol. Ther. 18, 157–165 (2003).
van Laar, J. M. et al. Autologous hematopoietic stem cell transplantation versus intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 311, 2490–2498 (2014).
Bhattacharyya, A. et al. Autologous hematopoietic stem cell transplant for systemic sclerosis improves anemia from gastric antral vascular ectasia. J. Rheumatol. 42, 554–555 (2015).
Battle, W. M. et al. Abnormal colonic motility in progressive systemic sclerosis. Ann. Intern. Med. 94, 749–752 (1981).
Sacher, P., Buchmann, P. & Burger, H. Stenosis of the large intestine complicating scleroderma and mimicking a sigmoid carcinoma. Dis. Colon Rectum 26, 347–348 (1983).
Wang, S. J. et al. Colonic transit disorders in systemic sclerosis. Clin. Rheumatol. 20, 251–254 (2001).
Whitehead, W. E., Taitelbaum, G., Wigley, F. M. & Schuster, M. M. Rectosigmoid motility and myoelectric activity in progressive systemic sclerosis. Gastroenterology 96, 428–432 (1989).
Emmanuel, A., Tack, J., Quigley, E. & Talley, N. Pharmacological management of constipation. Neurogastroenterol. Motility 21 (Suppl. 2), 41–54 (2009).
Butt, S. & Emmanuel, A. Systemic sclerosis and the gut. Expert Rev. Gastroenterol. Hepatol. 7, 331–339 (2013).
Folwaczny, C. et al. Effects of various prokinetic drugs on gastrointestinal transit times in patients with progressive systemic scleroderma. Z. Gastroenterol. 35, 905–912 (in German) (1997).
Lindsey, I., Farmer, C. R. & Cunningham, I. G. Subtotal colectomy and cecosigmoid anastomosis for colonic systemic sclerosis: report of a case and review of the literature. Dis. Colon Rectum 46, 1706–1711 (2003).
Davis, R. P., Hines, J. R. & Flinn, W. R. Scleroderma of the colon with obstruction: report of a case. Dis. Colon Rectum 19, 256–259 (1976).
Shafik, A., Shafik, A. A., El-Sibai, O. & Ahmed, I. Colonic pacing: a therapeutic option for the treatment of constipation due to total colonic inertia. Arch. Surg. 139, 775–779 (2004).
Thaha, M. A. et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst. Rev. 8, CD004464 (2015).
Balbir-Gurman, A., Brook, O. R., Chermesh, I. & Braun-Moscovici, Y. Pneumatosis cystoides intestinalis in scleroderma-related conditions. Intern. Med. J. 42, 323–329 (2012).
Wu, L. L., Yang, Y. S., Dou, Y. & Liu, Q. S. A systematic analysis of pneumatosis cystoids intestinalis. World J. Gastroenterol. 19, 4973–4978 (2013).
Vischio, J., Matlyuk-Urman, Z. & Lakshminarayanan, S. Benign spontaneous pneumoperitoneum in systemic sclerosis. J. Clin. Rheumatol. 16, 379–381 (2010).
Andréasson, K. et al. Faecal levels of calprotectin in systemic sclerosis are stable over time and are higher compared to primary Sjögren's syndrome and rheumatoid arthritis. Arthritis Res. Ther. 16, R46 (2014).
Pazzi, P. et al. Bile acid malabsorption in progressive systemic sclerosis. Gut 29, 552–553 (1988).
Staudacher, H. M., Irving, P. M., Lomer, M. C. & Whelan, K. Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat. Rev. Gastroenterol. Hepatol. 11, 256–266 (2014).
Muir, J. G. & Gibson, P. R. The low FODMAP diet for treatment of irritable bowel syndrome and other gastrointestinal disorders. Gastroenterol. Hepatol. 9, 450–445 (2013).
Franck-Larsson, K., Graf, W. & Ronnblom, A. Lower gastrointestinal symptoms and quality of life in patients with systemic sclerosis: a population-based study. Eur. J. Gastroenterol. Hepatol. 21, 176–182 (2009).
Clements, P. J., Becvar, R., Drosos, A. A., Ghattas, L. & Gabrielli, A. Assessment of gastrointestinal involvement. Clin. Exp. Rheumatol. 21, S15–S18 (2003).
Mawdsley, A. H. Patient perception of UK scleroderma services — results of an anonymous questionnaire. Rheumatology 45, 1573 (2006).
Thoua, N. et al. Internal anal sphincter atrophy in patients with systemic sclerosis. Rheumatology 50, 1596–1602 (2011).
Fynne, L. et al. Distensibility of the anal canal in patients with systemic sclerosis: a study with the functional lumen imaging probe. Colorectal Dis. 15, e40–e47 (2013).
Thoua, N. M., Abdel-Halim, M., Forbes, A., Denton, C. P. & Emmanuel, A. V. Fecal incontinence in systemic sclerosis is secondary to neuropathy. Am. J. Gastroenterol. 107, 597–603 (2011).
Chiou, A. W., Lin, J. K. & Wang, F. M. Anorectal abnormalities in progressive systemic sclerosis. Dis. Colon Rectum 32, 417–421 (1989).
Leighton, J. A. et al. Anorectal dysfunction and rectal prolapse in progressive systemic sclerosis. Dis. Colon Rectum 36, 182–185 (1993).
Jaffin, B. W., Chang, P. & Spiera, H. Fecal incontinence in scleroderma. Clinical features, anorectal manometric findings, and their therapeutic implications. J. Clin. Gastroenterol. 25, 513–517 (1997).
Denton, C. & Black, C. Scleroderma — clinical and pathological advances. Best Pract. Res. Clin. Rheumatol. 18, 271–290 (2004).
Sporbeck, B. et al. Effect of biofeedback and deep oscillation on Raynaud's phenomenon secondary to systemic sclerosis: results of a controlled prospective randomized clinical trial. Rheumatol. Int. 32, 1469–1473 (2012).
Kenefick, N. J. et al. Sacral nerve stimulation for faecal incontinence due to systemic sclerosis. Gut 51, 881–883 (2002).
Butt, S. et al. Lack of effect of sacral nerve stimulation for incontinence in patients with systemic sclerosis. Colorectal Dis. 17, 903–907 (2015).
Butt, S. K. et al. Preliminary significant findings from a randomised controlled trial of posterior tibial nerve stimulation in systemic sclerosis associated faecal incontinence. UEG J. 2 (1 Suppl. 1), A407 (2014).
Bae, S. et al. Associations between a scleroderma specific gastrointestinal instrument and objective tests of upper gastrointestinal involvements in systemic sclerosis. Clin. Exp. Rheumatol. 31 (Suppl. 76), 57–63 (2013).
Alrubaiy, L. et al. Systematic review of health-related quality of life measures for inflammatory bowel disease. J. Crohns Colitis 9, 284–292 (2015).
Spiegel, B. M. et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am. J. Gastroenterol. 109, 1804–1814 (2014).
Nagaraja, V. et al. Construct validity of the Patient-Reported Outcomes Measurement Information System gastrointestinal symptom scales in systemic sclerosis. Arthritis Care Res. (Hoboken) 66, 1725–1730 (2014).
Acknowledgements
The author is supported by the National Institute of Health Research, University College London Hospitals Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Emmanuel, A. Current management of the gastrointestinal complications of systemic sclerosis. Nat Rev Gastroenterol Hepatol 13, 461–472 (2016). https://doi.org/10.1038/nrgastro.2016.99
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.99
This article is cited by
-
Gastric antral vascular ectasia in systemic sclerosis: a study of its epidemiology, disease characteristics and impact on survival
Arthritis Research & Therapy (2022)
-
Faecal incontinence in adults
Nature Reviews Disease Primers (2022)
-
Systemerkrankungen und Gastroenterologie
Die Gastroenterologie (2022)
-
Sklerodermie
Die Gastroenterologie (2022)
-
Soluble guanylate cyclase stimulator reduced the gastrointestinal fibrosis in bleomycin-induced mouse model of systemic sclerosis
Arthritis Research & Therapy (2021)