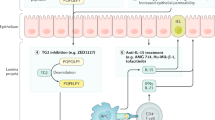
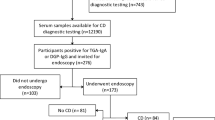
Key Points
-
The fundamental step in diagnosing coeliac disease is awareness of symptom diversity; anti-transglutaminase antibodies are very specific for the diagnosis and, in children, duodenal biopsies can sometimes be omitted
-
Prospective studies show that coeliac disease manifests at a young age, more often in girls, and is related to the HLA genotype, but not the timing of gluten introduction or breastfeeding
-
Wheat allergy is one of the most common food allergies in children beginning in early childhood; it is less common in adolescents and adults; most children outgrow wheat allergy by 12 years
-
Noncoeliac gluten sensitivity is a clinical condition in which symptoms are triggered by gluten ingestion in the absence of coeliac disease and wheat allergy
-
No biological markers exist for noncoeliac gluten sensitivity, exclusion of coeliac disease and of wheat allergy is the most important diagnostic step
-
Once a gluten-related disorder is diagnosed, children should be referred to a paediatric dietitian for in-depth guidance about the necessary dietary treatment
Abstract
Gluten-related disorders such as coeliac disease, wheat allergy and noncoeliac gluten sensitivity are increasingly being diagnosed in children. Coeliac disease occurs frequently, affecting 1–3% of the Western population. The condition manifests at a very young age, more so in girls, and is related to the HLA genotype. Coeliac disease might be considered a public health problem and, as primary prevention is not possible, the debate on mass screening should be reopened. Wheat proteins, including gluten, are responsible for one of the most common food allergies in children: wheat allergy. Unlike coeliac disease and wheat allergy, noncoeliac gluten sensitivity is an unclear and controversial entity. These three gluten-related disorders are treated with a gluten-free diet. In coeliac disease, the diet should be strictly followed, whereas wheat allergy only requires wheat elimination and in noncoeliac gluten sensitivity occasional trials of gluten reintroduction can be done. A good diagnostic work-up is important for gluten-related disorders in childhood to avoid unnecessary restrictive diets in children. In this Review, we provide an overview of the pathogenesis, diagnosis and management of the most common gluten-related disorders in children.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Meijer, C. R., Shamir, R. & Mearin, M. L. Coeliac disease and gluten sensitivity. J. Pediatr. Gastroenterol. Nutr. 60, 429–432 (2015).
Husby, S. et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 54, 136–160 (2012).
Myleus, A. et al. Celiac disease revealed in 3% of Swedish 12-year-olds born during an epidemic. J. Pediatr. Gastroenterol. Nutr. 49, 170–176 (2009).
Catassi, C., Gatti, S. & Fasano, A. The new epidemiology of celiac disease. J. Pediatr. Gastroenterol. Nutr. 59 (Suppl. 1), S7–S9 (2014).
Gandolfi, L. et al. Prevalence of celiac disease among blood donors in Brazil. Am. J. Gastroenterol. 95, 689–692 (2000).
Gomez, J. C. et al. Prevalence of celiac disease in Argentina: screening of an adult population in the La Plata area. Am. J. Gastroenterol. 96, 2700–2704 (2001).
Barada, K., Bitar, A., Mokadem, M. A., Hashash, J. G. & Green, P. Celiac disease in Middle Eastern and North African countries: a new burden? World J. Gastroenterol. 16, 1449–1457 (2010).
Masjedizadeh, R. et al. Celiac disease in South-West of Iran. World J. Gastroenterol. 12, 4416–4419 (2006).
Yuan, J. et al. The tip of the “celiac iceberg” in China: a systematic review and meta-analysis. PLoS ONE 8, e81151 (2013).
Byass, P., Kahn, K. & Ivarsson, A. The global burden of childhood coeliac disease: a neglected component of diarrhoeal mortality? PLoS ONE 6, e22774 (2011).
Fasano, A. et al. Federation of International Societies of Pediatric Gastroenterology, Hepatology, and Nutrition consensus report on celiac disease. J. Pediatr. Gastroenterol. Nutr. 47, 214–219 (2008).
Rubio-Tapia, A. et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology 137, 88–93 (2009).
Catassi, C. et al. Detection of Celiac disease in primary care: a multicenter case-finding study in North America. Am. J. Gastroenterol. 102, 1454–1460 (2007).
Csizmadia, C. G., Mearin, M. L., von Blomberg, B. M., Brand, R. & Verloove-Vanhorick, S. P. An iceberg of childhood coeliac disease in the Netherlands. Lancet 353, 813–814 (1999).
Sandstrom, O. et al. Transglutaminase IgA antibodies in a celiac disease mass screening and the role of HLA-DQ genotyping and endomysial antibodies in sequential testing. J. Pediatr. Gastroenterol. Nutr. 57, 472–476 (2013).
Steens, R. F. et al. A national prospective study on childhood celiac disease in the Netherlands 1993–2000: an increasing recognition and a changing clinical picture. J. Pediatr. 147, 239–243 (2005).
Vriezinga, S. L. et al. Randomized feeding intervention in infants at high risk for celiac disease. N. Engl. J. Med. 371, 1304–1315 (2014).
Lundin, K. E. et al. Gliadin-specific, HLA-DQ(α 1*0501, β 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J. Exp. Med. 178, 187–196 (1993).
Anderson, R. P., Degano, P., Godkin, A. J., Jewell, D. P. & Hill, A. V. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat. Med. 6, 337–342 (2000).
Arentz-Hansen, H. et al. The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J. Exp. Med. 191, 603–612 (2000).
Shan, L. et al. Structural basis for gluten intolerance in celiac sprue. Science 297, 2275–2279 (2002).
Sjostrom, H. et al. Identification of a gliadin T-cell epitope in coeliac disease: general importance of gliadin deamidation for intestinal T-cell recognition. Scand. J. Immunol. 48, 111–115 (1998).
Tye-Din, J. A. et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci. Transl. Med. 2, 41ra51 (2010).
Vader, L. W. et al. Characterization of cereal toxicity for celiac disease patients based on protein homology in grains. Gastroenterology 125, 1105–1113 (2003).
Vader, W. et al. The gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology 122, 1729–1737 (2002).
van de Wal, Y. et al. Small intestinal T cells of celiac disease patients recognize a natural pepsin fragment of gliadin. Proc. Natl. Acad. Sci. USA 95, 10050–10054 (1998).
van de Wal, Y. et al. Glutenin is involved in the gluten-driven mucosal T cell response. Eur. J. Immunol. 29, 3133–3139 (1999).
Molberg, O. et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 4, 713–717 (1998).
Vader, L. W. et al. Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J. Exp. Med. 195, 643–649 (2002).
van de Wal, Y. et al. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. 161, 1585–1588 (1998).
Mearin, M. L. et al. HLA-DR phenotypes in Spanish coeliac children: their contribution to the understanding of the genetics of the disease. Gut 24, 532–537 (1983).
Vader, W. et al. The HLA-DQ2 gene dose effect in celiac disease is directly related to the magnitude and breadth of gluten-specific T cell responses. Proc. Natl. Acad. Sci. USA 100, 12390–12395 (2003).
Vermeulen, B. A. et al. Phenotypic variance in childhood coeliac disease and the HLA-DQ/DR dose effect. Scand. J. Gastroenterol. 44, 40–45 (2009).
Tjon, J. M., van, B. J. & Koning, F. Celiac disease: how complicated can it get? Immunogenetics 62, 641–651 (2010).
van, Bergen, J., Mulder, C. J., Mearin, M. L. & Koning, F. Local communication among mucosal immune cells in patients with celiac disease. Gastroenterology 148, 1187–1194 (2015).
Abadie, V., Sollid, L. M., Barreiro, L. B. & Jabri, B. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu. Rev. Immunol. 29, 493–525 (2011).
Jarvinen, T. T. et al. Villous tip intraepithelial lymphocytes as markers of early-stage coeliac disease. Scand. J. Gastroenterol. 39, 428–433 (2004).
Meresse, B. et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 21, 357–366 (2004).
Hue, S. et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21, 367–377 (2004).
Kutlu, T. et al. Numbers of T cell receptor (TCR) αβ+ but not TcR γδ+ intraepithelial lymphocytes correlate with the grade of villous atrophy in coeliac patients on a long term normal diet. Gut 34, 208–214 (1993).
Schmitz, F. et al. Identification of a potential physiological precursor of aberrant cells in refractory coeliac disease type II. Gut 62, 509–519 (2013).
Ludvigsson, J. F. & Green, P. H. The missing environmental factor in celiac disease. N. Engl. J. Med. 371, 1341–1343 (2014).
Olivares, M. et al. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut 64, 406–417 (2015).
Guandalini, S. & Assiri, A. Celiac disease: a review. JAMA Pediatr. 168, 272–278 (2014).
Giersiepen, K. et al. Accuracy of diagnostic antibody tests for coeliac disease in children: summary of an evidence report. J. Pediatr. Gastroenterol. Nutr. 54, 229–241 (2012).
Green, P. H. & Jabri, B. Coeliac disease. Lancet 362, 383–391 (2003).
Hadithi, M. et al. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann. Intern. Med. 147, 294–302 (2007).
Wessels, M. M. et al. Impact on parents of HLA-DQ2/DQ8 genotyping in healthy children from coeliac families. Eur. J. Hum. Genet. 23, 405–408 (2014).
Marsh, M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology 102, 330–354 (1992).
Oberhuber, G., Granditsch, G. & Vogelsang, H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 11, 1185–1194 (1999).
Aronsson, C. A. et al. Age at gluten introduction and risk of celiac disease. Pediatrics 135, 239–245 (2015).
Werkstetter, K. ProCeDE: Prospective Celiac Disease Diagnostic Evaluation [online], (2015).
Kolsteren, M. M., Koopman, H. M., Schalekamp, G. & Mearin, M. L. Health-related quality of life in children with celiac disease. J. Pediatr. 138, 593–595 (2001).
van Doorn, R. K., Winkler, L. M., Zwinderman, K. H., Mearin, M. L. & Koopman, H. M. CDDUX: a disease-specific health-related quality-of-life questionnaire for children with celiac disease. J. Pediatr. Gastroenterol. Nutr. 47, 147–152 (2008).
Mariani, P. et al. The gluten-free diet: a nutritional risk factor for adolescents with celiac disease? J. Pediatr. Gastroenterol. Nutr. 27, 519–523 (1998).
Hopman, E. G., le Cessie, S., von Blomberg, B. M. & Mearin, M. L. Nutritional management of the gluten-free diet in young people with celiac disease in The Netherlands. J. Pediatr. Gastroenterol. Nutr. 43, 102–108 (2006).
Ohlund, K., Olsson, C., Hernell, O. & Ohlund, I. Dietary shortcomings in children on a gluten-free diet. J. Hum. Nutr. Diet. 23, 294–300 (2010).
Alvarez-Jubete, L., Arendt, E. K. & Gallagher, E. Nutritive value and chemical composition of pseudocereals as gluten-free ingredients. Int. J. Food Sci. Nutr. 60 (Suppl. 4), 240–257 (2009).
do Nascimento, A. B., Fiates, G. M., Dos, A. A. & Teixeira, E. Analysis of ingredient lists of commercially available gluten-free and gluten-containing food products using the text mining technique. Int. J. Food Sci. Nutr. 64, 217–222 (2013).
Sjoberg, V. et al. Noncontaminated dietary oats may hamper normalization of the intestinal immune status in childhood celiac disease. Clin. Transl. Gastroenterol. 5, e58 (2014).
Hogen Esch, C. E. et al. Specific celiac disease antibodies in children on a gluten-free diet. Pediatrics 128, 547–552 (2011).
James, S. P. National Institutes of Health Consensus Development Conference Statement on Celiac Disease, June 28–30, 2004. Gastroenterology 128 (Suppl. 1), S1–S9 (2005).
Nederlandse Vereniging van Maag-Darm-Leverartsen. Richtlijn Coeliakie en Dermatitis Herpetiformis Richtlijn Coeliakie en Dermatitis Herpetiformis. Haarlem: Nederlandse Vereniging voor Maag-Darm-Leverartsen [online], (2008).
Hill, I. D. et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 40, 1–19 (2005).
Murch, S. et al. Joint BSPGHAN and Coeliac UK guidelines for the diagnosis and management of coeliac disease in children. Arch. Dis. Child 98, 806–811 (2013).
Freeman, H. J. Non-dietary forms of treatment for adult celiac disease. World J. Gastrointest. Pharmacol. Ther. 4, 108–112 (2013).
Mitea, C. et al. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for coeliac disease. Gut 57, 25–32 (2008).
Siegel, M. et al. Safety, tolerability, and activity of ALV003: results from two phase 1 single, escalating-dose clinical trials. Dig. Dis. Sci. 57, 440–450 (2012).
Kapoerchan, V. V. et al. Design, synthesis and evaluation of high-affinity binders for the celiac disease associated HLA-DQ2 molecule. Mol. Immunol. 47, 1091–1097 (2010).
Xia, J. et al. Cyclic and dimeric gluten peptide analogues inhibiting DQ2-mediated antigen presentation in celiac disease. Bioorg. Med. Chem. 15, 6565–6573 (2007).
Klock, C., Herrera, Z., Albertelli, M. & Khosla, C. Discovery of potent and specific dihydroisoxazole inhibitors of human transglutaminase 2. J. Med. Chem. 57, 9042–9064 (2014).
Keech, C. L., Dromey, J. A., Chen, Z., Anderson, R. P. & McCluskey, J. Immune tolerance induced by peptide immunotherapy in an HLA Dq2-dependent mouse model of gluten immunity [abstract 355]. Gastroenterology 136, A57 (2009).
Kelly, C. P. et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment. Pharmacol. Ther. 37, 252–262 (2013).
Ivarsson, A. et al. Epidemic of coeliac disease in Swedish children. Acta Paediatr. 89, 165–171 (2000).
Norris, J. M. et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA 293, 2343–2351 (2005).
Agostoni, C. et al. Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 46, 99–110 (2008).
Akobeng, A. K., Ramanan, A. V., Buchan, I. & Heller, R. F. Effect of breast feeding on risk of coeliac disease: a systematic review and meta-analysis of observational studies. Arch. Dis. Child 91, 39–43 (2006).
Jansen, M. A. et al. Infant feeding and anti-tissue transglutaminase antibody concentrations in the Generation R Study. Am. J. Clin. Nutr. 100, 1095–1101 (2014).
Lionetti, E. et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N. Engl. J. Med. 371, 1295–1303 (2014).
Szajewska, H. et al. Systematic review with meta-analysis: early infant feeding and coeliac disease—update 2015. Aliment. Pharmacol. Ther. 41, 1038–1054 (2015).
Ivarsson, A. et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics 131, e687–e694 (2013).
Rosen, A. et al. Usefulness of symptoms to screen for celiac disease. Pediatrics 133, 211–218 (2014).
Mearin, M. L., Ivarsson, A. & Dickey, W. Coeliac disease: is it time for mass screening? Best. Pract. Res. Clin. Gastroenterol. 19, 441–452 (2005).
van Koppen, E. J. et al. Long-term health and quality-of-life consequences of mass screening for childhood celiac disease: a 10-year follow-up study. Pediatrics 123, e582–e588 (2009).
Kiefte-de Jong, J. C. et al. Levels of antibodies against tissue transglutaminase during pregnancy are associated with reduced fetal weight and birth weight. Gastroenterology 144, 726–735 (2013).
Kurppa, K. et al. Benefits of a gluten-free diet for asymptomatic patients with serologic markers of celiac disease. Gastroenterology 147, 610–617 (2014).
Catassi, C. & Fasano, A. Coeliac disease. The debate on coeliac disease screening—are we there yet? Nat. Rev. Gastroenterol. Hepatol. 11, 457–458 (2014).
Inomata, N. Wheat allergy. Curr. Opin. Allergy Clin. Immunol. 9, 238–243 (2009).
Johansson, S. G. et al. Revised nomenclature for allergy for global use: report of the nomenclature review committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 113, 832–836 (2004).
Benhamou, A. H., Vanini, G., Lantin, J. P. & Eigenmann, P. A. Antihistamine and sodium cromoglycate medication for food cold water exercise-induced anaphylaxis. Allergy 62, 1471–1472 (2007).
Mulder, C. J., van Wanrooij, R. L., Bakker, S. F., Wierdsma, N. & Bouma, G. Gluten-free diet in gluten-related disorders. Dig. Dis. 31, 57–62 (2013).
Nwaru, B. I. et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy 69, 992–1007 (2014).
Zuidmeer, L. et al. The prevalence of plant food allergies: a systematic review. J. Allergy Clin. Immunol. 121, 1210–1218 (2008).
Zeiger, R. S. & Heller, S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J. Allergy Clin. Immunol. 95, 1179–1190 (1995).
Poole, J. A. et al. Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics 117, 2175–2182 (2006).
Droste, J. H. et al. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin. Exp. Allergy 30, 1547–1553 (2000).
Husain, Z. & Schwartz, R. A. Food allergy update: more than a peanut of a problem. Int. J. Dermatol. 52, 286–294 (2013).
Makela, M. J. et al. Wheat allergy in children—new tools for diagnostics. Clin. Exp. Allergy 44, 1420–1430 (2014).
Leonard, M. M. & Vasagar, B. US perspective on gluten-related diseases. Clin. Exp. Gastroenterol. 7, 25–37 (2014).
Jones, S. M., Magnolfi, C. F., Cooke, S. K. & Sampson, H. A. Immunologic cross-reactivity among cereal grains and grasses in children with food hypersensitivity. J. Allergy Clin. Immunol. 96, 341–351 (1995).
Hischenhuber, C. et al. Review article: safe amounts of gluten for patients with wheat allergy or coeliac disease. Aliment. Pharmacol. Ther. 23, 559–575 (2006).
Yang, H., Xiao, Y. Z., Luo, X. Y., Tan, Q. & Wang, H. Diagnostic accuracy of atopy patch tests for food allergy in children with atopic dermatitis aged less than two years. Allergol. Immunopathol. (Madr.) 42, 22–28 (2014).
Soares-Weiser, K. et al. The diagnosis of food allergy: a systematic review and meta-analysis. Allergy 69, 76–86 (2014).
Matsukura, S. et al. Two cases of wheat-dependent anaphylaxis induced by aspirin administration but not by exercise. Clin. Exp. Dermatol. 35, 233–237 (2010).
Keet, C. A. et al. The natural history of wheat allergy. Ann. Allergy Asthma Immunol. 102, 410–415 (2009).
Sapone, A. et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 10, 13 (2012).
Biesiekierski, J. R. et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am. J. Gastroenterol. 106, 508–514 (2011).
Catassi, C. et al. Non-Celiac Gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 5, 3839–3853 (2013).
Francavilla, R. et al. Clinical, serologic, and histologic features of gluten sensitivity in children. J. Pediatr. 164, 463–467 (2014).
Batista, I. C. et al. Autism spectrum disorder and celiac disease: no evidence for a link. Arq Neuropsiquiatr. 70, 28–33 (2012).
Whiteley, P. et al. The ScanBrit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutr. Neurosci. 13, 87–100 (2010).
Sapone, A. et al. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int. Arch. Allergy Immunol. 152, 75–80 (2010).
Volta, U., Bardella, M. T., Calabro, A., Troncone, R. & Corazza, G. R. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 12, 85 (2014).
Sapone, A. et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 9, 23 (2011).
Vazquez-Roque, M. I. et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 144, 903–911 (2013).
Junker, Y. et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 209, 2395–2408 (2012).
Volta, U. & De, G. R. New understanding of gluten sensitivity. Nat. Rev. Gastroenterol. Hepatol. 9, 295–299 (2012).
Lundin, K. E. & Alaedini, A. Non-celiac gluten sensitivity. Gastrointest. Endosc. Clin. N. Am. 22, 723–734 (2012).
Ahrens, B., Niggemann, B., Wahn, U. & Beyer, K. Positive reactions to placebo in children undergoing double-blind, placebo-controlled food challenge. Clin. Exp. Allergy 44, 572–578 (2014).
Biesiekierski, J. R., Newnham, E. D., Shepherd, S. J., Muir, J. G. & Gibson, P. R. Characterization of adults with a self-diagnosis of nonceliac gluten sensitivity. Nutr. Clin. Pract. 29, 504–509 (2014).
Peters, S. L., Biesiekierski, J. R., Yelland, G. W., Muir, J. G. & Gibson, P. R. Randomised clinical trial: gluten may cause depression in subjects with non-coeliac gluten sensitivity—an exploratory clinical study. Aliment. Pharmacol. Ther. 39, 1104–1112 (2014).
Biesiekierski, J. R. et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 145, 320–328 (2013).
Millward, C., Ferriter, M., Calver, S. & Connell-Jones, G. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD003498 http://dx.doi.org/10.1002/14651858.CD003498.pub3 (2008).
Acknowledgements
We thank Dr D. Amado, visiting paediatrician at Leiden University Medical Centre, for editing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Vriezinga, S., Schweizer, J., Koning, F. et al. Coeliac disease and gluten-related disorders in childhood. Nat Rev Gastroenterol Hepatol 12, 527–536 (2015). https://doi.org/10.1038/nrgastro.2015.98
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2015.98
This article is cited by
-
Reduced frequency of circulating regulatory T cells and their related immunosuppressive mediators in treated celiac patients
Molecular Biology Reports (2022)
-
Efficient implementation of the ‘non-biopsy approach’ for the diagnosis of childhood celiac disease in the Netherlands: a national prospective evaluation 2010–2013
European Journal of Pediatrics (2021)
-
Outlook for coeliac disease patients: towards bread wheat with hypoimmunogenic gluten by gene editing of α- and γ-gliadin gene families
BMC Plant Biology (2019)
-
The multiple roles of sucrase-isomaltase in the intestinal physiology
Molecular and Cellular Pediatrics (2016)
-
Fecal Gluten Peptides Reveal Limitations of Serological Tests and Food Questionnaires for Monitoring Gluten-Free Diet in Celiac Disease Patients
American Journal of Gastroenterology (2016)