Key Points
-
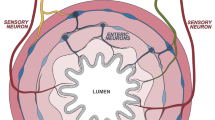
Neural reflex regulation of immune responses depends on the type and tissue compartment of the inflammatory response
-
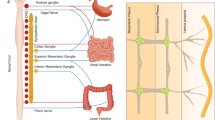
Although immune cells respond to cholinergic receptor activation, immune organs such as the spleen and lymphoid organs are innervated by adrenergic pathways rather than cholinergic pathways
-
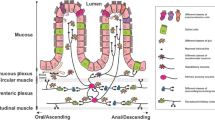
A variety of cholinergic, adrenergic and enteric neurotransmitters has strong immune-regulating potential, but only limited evidence exists for neural afferent–efferent reflex pathways in intestinal immune regulation
-
Clinical and preclinical studies using electrical (implanted devices) or nutritional (cholecystokinin-afferent activation) activation of vagal reflex are ongoing and suggest an anti-inflammatory potential of vagus nerve stimulation (afferent or efferent)
Abstract
Studies in neuroscience and immunology have clarified much of the anatomical and cellular basis for bidirectional interactions between the nervous and immune systems. As with other organs, intestinal immune responses and the development of immunity seems to be modulated by neural reflexes. Sympathetic immune modulation and reflexes are well described, and in the past decade the parasympathetic efferent vagus nerve has been added to this immune-regulation network. This system, designated 'the inflammatory reflex', comprises an afferent arm that senses inflammation and an efferent arm that inhibits innate immune responses. Intervention in this system as an innovative principle is currently being tested in pioneering trials of vagus nerve stimulation using implantable devices to treat IBD. Patients benefit from this treatment, but some of the working mechanisms remain to be established, for instance, treatment is effective despite the vagus nerve not always directly innervating the inflamed tissue. In this Review, we will focus on the direct neuronal regulatory mechanisms of immunity in the intestine, taking into account current advances regarding the innervation of the spleen and lymphoid organs, with a focus on the potential for treatment in IBD and other gastrointestinal pathologies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Costa, M., Brookes, S. J. & Hennig, G. W. Anatomy and physiology of the enteric nervous system. Gut 47 (Suppl. 4), 15–19 (2000).
Llewellyn-Smith, I. J., Furness, J. B., O'Brien, P. E. & Costa, M. Noradrenergic nerves in human small intestine. Distribution and ultrastructure. Gastroenterology 87, 513–529 (1984).
Sharkey, K. A. Emerging roles for enteric glia in gastrointestinal disorders. J. Clin. Invest. 125, 918–925 (2015).
Psichas, A., Reimann, F. & Gribble, F. M. Gut chemosensing mechanisms. J. Clin. Invest. 125, 908–917 (2015).
Mawe, G. M. Colitis-induced neuroplasticity disrupts motility in the inflamed and post-inflamed colon. J. Clin. Invest. 125, 949–955 (2015).
Avetisyan, M., Schill, E. M. & Heuckeroth, R. O. Building a second brain in the bowel. J. Clin. Invest. 125, 899–907 (2015).
Costes, L. M., Boeckxstaens, G. E., de Jonge, W. J. & Cailotto, C. Neural networks in intestinal immunoregulation. Organogenesis 9, 216–223 (2013).
Gonzalez-Rey, E., Chorny, A. & Delgado, M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat. Rev. Immunol. 7, 52–63 (2007).
Berthoud, H. R., Jedrzejewska, A. & Powley, T. L. Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with dil injected into the dorsal vagal complex in the rat. J. Comp. Neurol. 301, 65–79 (1990).
Pavlov, V. A. & Tracey, K. J. The cholinergic anti-inflammatory pathway. Brain Behav. Immun. 19, 493–499 (2005).
Pavlov, V. A. & Tracey, K. J. Controlling inflammation: the cholinergic anti-inflammatory pathway. Biochem. Soc. Trans. 34, 1037–1040 (2006).
Tracey, K. J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 117, 289–296 (2007).
Wieczorek, M. & Dunn, A. J. Effect of subdiaphragmatic vagotomy on the noradrenergic and HPA axis activation induced by intraperitoneal interleukin-1 administration in rats. Brain Res. 1101, 73–84 (2006).
Berthoud, H. R., Kressel, M., Raybould, H. E. & Neuhuber, W. L. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat. Embryol. (Berl.) 191, 203–212 (1995).
Yamamoto, T. et al. Anti-allergic role of cholinergic neuronal pathway via alpha7 nicotinic ACh receptors on mucosal mast cells in a murine food allergy model. PLoS ONE 9, e85888 (2014).
Williams, R. M., Berthoud, H. R. & Stead, R. H. Vagal afferent nerve fibres contact mast cells in rat small intestinal mucosa. Neuroimmunomodulation 4, 266–270 (1997).
Ek, M., Kurosawa, M., Lundeberg, T. & Ericsson, A. Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. J. Neurosci. 18, 9471–9479 (1998).
Wan, W., Wetmore, L., Sorensen, C. M., Greenberg, A. H. & Nance, D. M. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res. Bull. 34, 7–14 (1994).
Maier, S. F., Goehler, L. E., Fleshner, M. & Watkins, L. R. The role of the vagus nerve in cytokine-to-brain communication. Ann. N. Y. Acad. Sci. 840, 289–300 (1998).
Saper, C. B., Romanovsky, A. A. & Scammell, T. E. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat. Neurosci. 15, 1088–1095 (2012).
Borovikova, L. V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000).
Wang, H. et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 10, 1216–1221 (2004).
Bernik, T. R. et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J. Exp. Med. 195, 781–788 (2002).
Tracey, K. J. The inflammatory reflex. Nature 420, 853–859 (2002).
Pavlov, V. A. et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl Acad. Sci. USA 103, 5219–5223 (2006).
Andersson, U. & Tracey, K. J. Neural reflexes in inflammation and immunity. J. Exp. Med. 209, 1057–1068 (2012).
Andersson, U. & Tracey, K. J. Reflex principles of immunological homeostasis. Annu. Rev. Immunol. 30, 313–335 (2012).
Guarini, S. et al. Efferent vagal fibre stimulation blunts nuclear factor-κB activation and protects against hypovolemic hemorrhagic shock. Circulation 107, 1189–1194 (2003).
Guarini, S. et al. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc. Res. 63, 357–365 (2004).
Martelli, D., McKinley, M. J. & McAllen, R. M. The cholinergic anti-inflammatory pathway: a critical review. Auton. Neurosci. 182, 65–69 (2014).
Bratton, B. O. et al. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp. Physiol. 97, 1180–1185 (2012).
Coupland, R. E., Parker, T. L., Kesse, W. K. & Mohamed, A. A. The innervation of the adrenal gland. III. Vagal innervation. J. Anat. 163, 173–181 (1989).
Torres-Rosas, R. et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat. Med. 20, 291–295 (2014).
Rhen, T. & Cidlowski, J. A. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N. Engl. J. Med. 353, 1711–1723 (2005).
Fuentes, J. M., Hanly, E. J., Aurora, A. R., De Maio, A. & Talamini, M. A. Anesthesia-specific protection from endotoxic shock is not mediated through the vagus nerve. Surgery 138, 766–771 (2005).
Bonaz, B., Picq, C., Sinniger, V., Mayol, J. F. & Clarencon, D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol. Motil. 25, 208–221 (2013).
Clarencon, D. et al. Long term effects of low frequency (10 Hz) vagus nerve stimulation on EEG and heart rate variability in Crohn's disease: a case report. Brain Stimul. 7, 914–916 (2014).
Birmingham, K. et al. Bioelectronic medicines: a research roadmap. Nat. Rev. Drug Discov. 13, 399–400 (2014).
Tobin, G., Giglio, D. & Lundgren, O. Muscarinic receptor subtypes in the alimentary tract. J. Physiol. Pharmacol. 60, 3–21 (2009).
Xu, Y. et al. Molecular dynamics of nicotinic acetylcholine receptor correlating biological functions. Curr. Protein Pept. Sci. 7, 195–200 (2006).
Kawashima, K., Fujii, T., Moriwaki, Y. & Misawa, H. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci. 91, 1027–1032 (2012).
Wang, H. et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388 (2003).
Kawashima, K., Yoshikawa, K., Fujii, Y. X., Moriwaki, Y. & Misawa, H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 80, 2314–2319 (2007).
Qian, J., Galitovskiy, V., Chernyavsky, A. I., Marchenko, S. & Grando, S. A. Plasticity of the murine spleen T-cell cholinergic receptors and their role in in vitro differentiation of naive CD4 T cells toward the Th1, Th2 and Th17 lineages. Genes Immun. 12, 222–230 (2011).
Skok, M., Grailhe, R. & Changeux, J. P. Nicotinic receptors regulate B lymphocyte activation and immune response. Eur. J. Pharmacol. 517, 246–251 (2005).
Skok, M., Grailhe, R., Agenes, F. & Changeux, J. P. The role of nicotinic acetylcholine receptors in lymphocyte development. J. Neuroimmunol. 171, 86–98 (2006).
Olofsson, P. S., Rosas-Ballina, M., Levine, Y. A. & Tracey, K. J. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol. Rev. 248, 188–204 (2012).
Matteoli, G. et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63, 938–948 (2014).
Cailotto, C. et al. Neuroanatomical evidence demonstrating the existence of the vagal anti-inflammatory reflex in the intestine. Neurogastroenterol. Motil. 24, 191–200 (2012).
Kirchgessner, A. L. & Gershon, M. D. Identification of vagal efferent fibers and putative target neurons in the enteric nervous system of the rat. J. Comp. Neurol. 285, 38–53 (1989).
Holst, M. C., Kelly, J. B. & Powley, T. L. Vagal preganglionic projections to the enteric nervous system characterized with Phaseolus vulgaris-leucoagglutinin. J. Comp. Neurol. 381, 81–100 (1997).
Walter, G. C., Phillips, R. J., Baronowsky, E. A. & Powley, T. L. Versatile, high-resolution anterograde labeling of vagal efferent projections with dextran amines. J. Neurosci. Methods 178, 1–9 (2009).
de Jonge, W. J. et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 6, 844–851 (2005).
Nemethova, A., Michel, K., Gomez-Pinilla, P. J., Boeckxstaens, G. E. & Schemann, M. Nicotine attenuates activation of tissue resident macrophages in the mouse stomach through the beta2 nicotinic acetylcholine receptor. PLoS ONE 8, e79264 (2013).
Matteoli, G. & Boeckxstaens, G. E. The vagal innervation of the gut and immune homeostasis. Gut 62, 1214–1222 (2013).
Lubbers, T. et al. Lipid-enriched enteral nutrition controls the inflammatory response in murine Gram-negative sepsis. Crit. Care Med. 38, 1996–2002 (2010).
de Haan, J. J. et al. Lipid-rich enteral nutrition regulates mucosal mast cell activation via the vagal anti-inflammatory reflex. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G383–G391 (2013).
Felten, D. L., Felten, S. Y., Carlson, S. L., Olschowka, J. A. & Livnat, S. Noradrenergic and peptidergic innervation of lymphoid tissue. J. Immunol. 135, 755s–765s (1985).
Nance, D. M. & Burns, J. Innervation of the spleen in the rat: evidence for absence of afferent innervation. Brain Behav. Immun. 3, 281–290 (1989).
Bellinger, D. L., Lorton, D., Felten, S. Y. & Felten, D. L. Innervation of lymphoid organs and implications in development, aging, and autoimmunity. Int. J. Immunopharmacol. 14, 329–344 (1992).
Rosas-Ballina, M. et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl Acad. Sci. USA 105, 11008–11013 (2008).
Berthoud, H. R. & Powley, T. L. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J. Auton. Nerv. Syst. 42, 153–169 (1993).
Schafer, M. K., Eiden, L. E. & Weihe, E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system. Neuroscience 84, 361–376 (1998).
Vida, G., Pena, G., Deitch, E. A. & Ulloa, L. α7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J. Immunol. 186, 4340–4346 (2011).
Vida, G. et al. beta2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J. 25, 4476–4485 (2011).
Haass, M. & Kubler, W. Nicotine and sympathetic neurotransmission. Cardiovasc. Drugs Ther. 10, 657–665 (1997).
Huston, J. M. et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 203, 1623–1628 (2006).
Nakai, A., Hayano, Y., Furuta, F., Noda, M. & Suzuki, K. Control of lymphocyte egress from lymph nodes through beta2-adrenergic receptors. J. Exp. Med. 211, 2583–2598 (2014).
Gautron, L. et al. Neuronal and nonneuronal cholinergic structures in the mouse gastrointestinal tract and spleen. J. Comp. Neurol. 521, 3741–3767 (2013).
Buijs, R. M., van der Vliet, J., Garidou, M. L., Huitinga, I. & Escobar, C. Spleen vagal denervation inhibits the production of antibodies to circulating antigens. PLoS ONE 3, e3152 (2008).
Bellinger, D. L., Lorton, D., Hamill, R. W., Felten, S. Y. & Felten, D. L. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav. Immun. 7, 191–204 (1993).
Snoek, S. A., Borensztajn, K. S., van den Wijngaard, R. M. & de Jonge, W. J. Neuropeptide receptors in intestinal disease: physiology and therapeutic potential. Curr. Pharm. Des. 16, 1091–1105 (2010).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011).
Nijhuis, L. E. et al. Adrenergic β2 receptor activation stimulates anti-inflammatory properties of dendritic cells in vitro. PLoS ONE 9, e85086 (2014).
van der Zanden, E. P. et al. Vagus nerve activity augments intestinal macrophage phagocytosis via nicotinic acetylcholine receptor alpha4beta2. Gastroenterology 137, 1029–1039 (2009).
Ghia, J. E., Blennerhassett, P., Kumar-Ondiveeran, H., Verdu, E. F. & Collins, S. M. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 131, 1122–1130 (2006).
Ji, H. et al. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 7, 335–347 (2014).
Eliakim, R., Fan, Q. X. & Babyatsky, M. W. Chronic nicotine administration differentially alters jejunal and colonic inflammation in interleukin-10 deficient mice. Eur. J. Gastroenterol. Hepatol. 14, 607–614 (2002).
Eliakim, R., Karmeli, F., Rachmilewitz, D., Cohen, P. & Fich, A. Effect of chronic nicotine administration on trinitrobenzene sulphonic acid-induced colitis. Eur. J. Gastroenterol. Hepatol. 10, 1013–1019 (1998).
Snoek, S. A. et al. Selective alpha7 nicotinic acetylcholine receptor agonists worsen disease in experimental colitis. Br. J. Pharmacol. 160, 322–333 (2010).
Meregnani, J. et al. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton. Neurosci. 160, 82–89 (2011).
Fogel, R., Zhang, X. & Renehan, W. E. Relationships between the morphology and function of gastric and intestinal distention-sensitive neurons in the dorsal motor nucleus of the vagus. J. Comp. Neurol. 364, 78–91 (1996).
Bonaz, B. The cholinergic anti-inflammatory pathway and the gastrointestinal tract. Gastroenterology 133, 1370–1373 (2007).
Pellissier, S. et al. Relationship between vagal tone, cortisol, TNF-α, epinephrine and negative affects in Crohn's disease and irritable bowel syndrome. PLoS ONE 9, e105328 (2014).
Rubio, A., Pellissier, S., Picot, A., Dantzer, C. & Bonaz, B. The link between negative affect, vagal tone, and visceral sensitivity in quiescent Crohn's disease. Neurogastroenterol. Motil. 26, 1200–1203 (2014).
Jacobson, K., McHugh, K. & Collins, S. M. Experimental colitis alters myenteric nerve function at inflamed and noninflamed sites in the rat. Gastroenterology 109, 718–722 (1995).
Jacobson, K., McHugh, K. & Collins, S. M. The mechanism of altered neural function in a rat model of acute colitis. Gastroenterology 112, 156–162 (1997).
Straub, R. H. et al. Key role of the sympathetic microenvironment for the interplay of tumour necrosis factor and interleukin 6 in normal but not in inflamed mouse colon mucosa. Gut 54, 1098–1106 (2005).
Zitnik, R. J. Treatment of chronic inflammatory diseases with implantable medical devices. Cleve. Clin. J. Med. 78 (Suppl. 1), S30–S34 (2011).
US National Library of Medicine. ClinicalTrial.gov [online], (2014).
US National Library of Medicine. ClinicalTrial.gov [online], (2015).
US National Library of Medicine. ClinicalTrial.gov [online], (2014).
Lubbers, T. et al. Cholecystokinin/cholecystokinin-1 receptor-mediated peripheral activation of the afferent vagus by enteral nutrients attenuates inflammation in rats. Ann. Surg. 252, 376–382 (2010).
Lubbers, T. et al. Chylomicron formation and glucagon-like peptide 1 receptor are involved in activation of the nutritional anti-inflammatory pathway. J. Nutr. Biochem. 22, 1105–1111 (2011).
de Haan, J. J. et al. Lipid-rich enteral nutrition improves the defense against an opportunistic infection during polymicrobial sepsis. Shock 41, 109–114 (2014).
Luyer, M. D. et al. Pretreatment with high-fat enteral nutrition reduces endotoxin and tumor necrosis factor-alpha and preserves gut barrier function early after hemorrhagic shock. Shock 21, 65–71 (2004).
Lubbers, T. et al. Continuous administration of enteral lipid- and protein-rich nutrition limits inflammation in a human endotoxemia model. Crit. Care Med. 41, 1258–1265 (2013).
Lubbers, T. et al. Lipid-rich enteral nutrition reduces postoperative ileus in rats via activation of cholecystokinin-receptors. Ann. Surg. 249, 481–487 (2009).
Andersen, H. K., Lewis, S. J. & Thomas, S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database of Systematic Reviews, Issue 4. Art. No.: CD004080 http://dx.doi.org/10.1002/14651858.CD004080.pub2.
Boelens, P. G. et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled trial. Ann. Surg. 259, 649–655 (2014).
Han-Geurts, I. J. et al. Randomized clinical trial of the impact of early enteral feeding on postoperative ileus and recovery. Br. J. Surg. 94, 555–561 (2007).
van den Heijkant, T. C. et al. Randomized clinical trial of the effect of gum chewing on postoperative ileus and inflammation in colorectal surgery. Br. J. Surg. 102, 202–211 (2014).
US National Library of Medicine. ClinicalTrial.gov [online], (2014).
Peters, E. G. et al. The effects of stimulation of the autonomic nervous system via perioperative nutrition on postoperative ileus and anastomotic leakage following colorectal surgery (SANICS II trial): a study protocol for a double-blind randomized controlled trial. Trials 16, 20 (2015).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
R.A.W. is funded by a research grant from GlaxoSmithKline. M.D.L. receives research funding and consultancy fees from Covidien, Fonds NutsOhra, Nutricia and ZonMw. W.J.J. has received research and consultancy grants from GlaxoSmithKline, Mead Johnson Nutrition Paediatric Institute, Schwabe Corporation and Setpoint Medical. W.A.B. declares no competing interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Willemze, R., Luyer, M., Buurman, W. et al. Neural reflex pathways in intestinal inflammation: hypotheses to viable therapy. Nat Rev Gastroenterol Hepatol 12, 353–362 (2015). https://doi.org/10.1038/nrgastro.2015.56
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2015.56
This article is cited by
-
Different modulation effects of 1 Hz and 20 Hz transcutaneous auricular vagus nerve stimulation on the functional connectivity of the periaqueductal gray in patients with migraine
Journal of Translational Medicine (2021)
-
Dexmedetomidine post-conditioning attenuates cerebral ischemia following asphyxia cardiac arrest through down-regulation of apoptosis and neuroinflammation in rats
BMC Anesthesiology (2021)
-
Autonomic nervous system and inflammation interaction in endometriosis-associated pain
Journal of Neuroinflammation (2020)
-
Bioelectric neuromodulation for gastrointestinal disorders: effectiveness and mechanisms
Nature Reviews Gastroenterology & Hepatology (2019)
-
Tissue macrophages: heterogeneity and functions
BMC Biology (2017)