Key Points
-
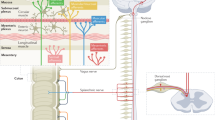
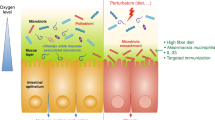
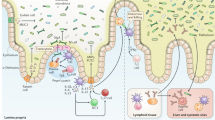
Altered gut microbiota composition, aberrant expression pattern and function of enterochromaffin cells, abnormal gut permeability and dysregulated immune activity have been found in at least subgroups of patients with IBS
-
The complex interaction between these systems has been demonstrated in different animal models of IBS
-
The association between these abnormalities and the symptom profile in patients with IBS has been demonstrated
-
Targeting these alterations in the development of new therapies for IBS seems promising
Abstract
The aetiology and pathology of IBS, a functional bowel disorder thought to lack an organic cause, is largely unknown. However, studies suggest that various features, such as altered composition of the gut microbiota, together with increased intestinal permeability, a changed balance in the enteroendocrine system and a dysregulated immune system in the gut, most likely have an important role in IBS. Exactly how these entities act together and give rise to symptoms is still unknown, but an altered gut microbiota composition could lead to dysregulation of the intestinal barrier as well as the enteroendocrine and the immune systems, which (through interactions with the nervous system) might generate symptoms. This Review highlights the crosstalk between the gut microbiota, the enteroendocrine system, the immune system and the role of intestinal permeability in patients with IBS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Longstreth, G. F. et al. Functional bowel disorders. Gastroenterology 130, 1480–1491 (2006).
Lovell, R. M. & Ford, A. C. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 10, 712–721.e4 (2012).
White, B. C. J. Mucous colitis: a delineation of the syndrome with certain observations on its mechanisms and on the role of emotional tension as a precipitating factor. Ann. Int. Med. 14, 854–872 (1940).
Whitehead, W. E., Palsson, O. & Jones, K. R. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 122, 1140–1156 (2002).
Chaudhary, N. A. & Truelove, S. C. The irritable colon syndrome. A study of the clinical features, predisposing causes, and prognosis in 130 cases. Q. J. Med. 31, 307–322 (1962).
Spiller, R. et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut 56, 1770–1798 (2007).
Törnblom, H. et al. Colonic transit time and IBS symptoms: what's the link? Am. J. Gastroenterol. 107, 754–760 (2012).
Ritchie, J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut 14, 125–132 (1973).
Whitehead, W. E. et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology 98, 1187–1192 (1990).
Posserud, I. et al. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology 133, 1113–1123 (2007).
Tillisch, K., Mayer, E. A. & Labus, J. S. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 140, 91–100 (2011).
Furness, J. B. et al. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 10, 729–740 (2013).
Mawe, G. M. & Hoffman, J. M. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 10, 473–486 (2013).
Simren, M. et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 62, 159–176 (2013).
Bercik, P. & Collins, S. M. The effects of inflammation, infection and antibiotics on the microbiota–gut–brain axis. Adv. Exp. Med. Biol. 817, 279–289 (2014).
De Palma, G., Collins, S. M. & Bercik, P. The microbiota–gut–brain axis in functional gastrointestinal disorders. Gut Microbes 5, 419–429 (2014).
Mayer, E. A. & Tillisch, K. The brain-gut axis in abdominal pain syndromes. Annu. Rev. Med. 62, 381–396 (2011).
Mayer, E. A., Savidge, T. & Shulman, R. J. Brain–gut microbiome interactions and functional bowel disorders. Gastroenterology 146, 1500–1512 (2014).
Tanaka, Y. et al. Biopsychosocial model of irritable bowel syndrome. J. Neurogastroenterol. Motil. 17, 131–139 (2011).
Sommer, F. & Backhed, F. The gut microbiota—masters of host development and physiology. Nat. Rev. Microbiol. 11, 227–238 (2013).
Peterson, L. W. & Artis, D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14, 141–153 (2014).
Gibson, M. K., Pesesky, M. W. & Dantas, G. The yin and yang of bacterial resilience in the human gut microbiota. J. Mol. Biol. http://dx.doi.org/10.1016/j.jmb.2014.05.029.
Akiho, H. et al. Mechanisms underlying the maintenance of muscle hypercontractility in a model of postinfective gut dysfunction. Gastroenterology 129, 131–141 (2005).
Bercik, P. et al. Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology 127, 179–187 (2004).
Gwee, K. A. et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut 44, 400–406 (1999).
Kanazawa, M. et al. Motility response to colonic distention is increased in postinfectious irritable bowel syndrome (PI-IBS). Neurogastroenterol. Motil. 26, 696–704 (2014).
Kimball, E. S. et al. Acute colitis induction by oil of mustard results in later development of an IBS-like accelerated upper GI transit in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1266–G1273 (2005).
Rao, S. S. et al. Studies on the mechanism of bowel disturbance in ulcerative colitis. Gastroenterology 93, 934–940 (1987).
Rao, S. S. et al. Anorectal sensitivity and responses to rectal distention in patients with ulcerative colitis. Gastroenterology 93, 1270–1275 (1987).
Verdu, E. F. et al. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology 127, 826–837 (2004).
Verdu, E. F. et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 55, 182–90 (2006).
Verma-Gandhu, M. et al. Visceral pain perception is determined by the duration of colitis and associated neuropeptide expression in the mouse. Gut 56, 358–364 (2007).
Wu, T. et al. Gut motility and enteroendocrine secretion. Curr. Opin. Pharmacol. 13, 928–934 (2013).
Khan, W. I. & Ghia, J. E. Gut hormones: emerging role in immune activation and inflammation. Clin. Exp. Immunol. 161, 19–27 (2010).
Sekirov, I. et al. Gut microbiota in health and disease. Physiol. Rev. 90, 859–904 (2010).
Young, V. B. & Schmidt, T. M. Overview of the gastrointestinal microbiota. Adv. Exp. Med. Biol. 635, 29–40 (2008).
O'Hara, A. M. & Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 7, 688–693 (2006).
Frank, D. N. et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl Acad. Sci. USA 104, 13780–13785 (2007).
Dethlefsen, L., McFall-Ngai M & Relman, D. A. An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature 449, 811–818 (2007).
Zoetendal, E. G., Rajilic-Stojanovic, M. & de Vos, W. M. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut 57, 1605–1615 (2008).
Swidsinski, A., Loening-Baucke, V., Lochs, H. & Hale, L. P. Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J. Gastroenterol. 11, 113–1140 (2005).
Wu, G. D. & Lewis, J. D. Analysis of the human gut microbiome and association with disease. Clin. Gastroenterol. Hepatol. 11, 774–777 (2013).
Hyland, N. P., Quigley, E. M. & Brint, E. Microbiota–host interactions in irritable bowel syndrome: epithelial barrier, immune regulation and brain-gut interactions. World J. Gastroenterol. 20, 8859–8866 (2014).
Lee, K. N. & Lee, O. Y. Intestinal microbiota in pathophysiology and management of irritable bowel syndrome. World J. Gastroenterol. 20, 8886–8897 (2014).
Spiller, R. & Garsed, K. Postinfectious irritable bowel syndrome. Gastroenterology 136, 1979–1988 (2009).
Thabane, M., Kottachchi, D. T. & Marshall, J. K. Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment. Pharmacol. Ther. 26, 535–544 (2007).
Halvorson, H. A., Schlett, C. D. & Riddle, M. S. Postinfectious irritable bowel syndrome—a meta-analysis. Am. J. Gastroenterol. 101, 1894–1899 (2006).
Cremon, C. et al. Salmonella gastroenteritis during childhood is a risk factor for irritable bowel syndrome in adulthood. Gastroenterology 147, 69–77 (2014).
Pimentel, M., Chow, E. J. & Lin, H. C. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am. J. Gastroenterol. 95, 3503–3506 (2000).
Posserud, I. et al. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 56, 802–808 (2007).
Walters, B. & Vanner, S. J. Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: comparison with 14C-D-xylose and healthy controls. Am. J. Gastroenterol. 100, 1566–1570 (2005).
Vanner, S. The small intestinal bacterial overgrowth. Irritable bowel syndrome hypothesis: implications for treatment. Gut 57, 1315–1321 (2008).
Halmos, E. P. et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 146, 67–75.e5 (2014).
Moayyedi, P. et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut 59, 325–32 (2010).
Schoenfeld, P. et al. Safety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: a pooled analysis of randomised, double-blind, placebo-controlled trials. Aliment. Pharmacol. Ther. 39, 1161–1168 (2014).
Carroll, I. M. et al. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G799–G807 (2011).
Matto, J. et al. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome—a longitudinal study in IBS and control subjects. FEMS Immunol. Med. Microbiol. 43, 213–222 (2005).
Maukonen, J. et al. Prevalence and temporal stability of selected clostridial groups in irritable bowel syndrome in relation to predominant faecal bacteria. J. Med. Microbiol. 55, 625–633 (2006).
Malinen, E. et al. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J. Gastroenterol. 16, 4532–4540 (2010).
Malinen, E. et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am. J. Gastroenterol. 100, 373–382 (2005).
Rajilic-Stojanovic, M. et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 141, 1792–1801 (2011).
Jeffery, I. B. et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61, 997–1006 (2012).
Carroll, I. M. et al. Luminal and mucosal-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Gut Pathog. 2, 19 (2010).
Carroll, I. M. et al. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 24, 521–530.e248 (2012).
Codling, C. et al. A molecular analysis of fecal and mucosal bacterial communities in irritable bowel syndrome. Dig. Dis. Sci. 55, 392–397 (2010).
Kassinen, A. et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 133, 24–33 (2007).
Kerckhoffs, A. P. et al. Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J. Gastroenterol. 15, 2887–2892 (2009).
Krogius-Kurikka, L. et al. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 9, 95 (2009).
Lyra, A. et al. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J. Gastroenterol. 15, 5936–5945 (2009).
Noor, S. O. et al. Ulcerative colitis and irritable bowel patients exhibit distinct abnormalities of the gut microbiota. BMC Gastroenterol. 10, 134 (2010).
Saulnier, D. M. et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 141, 1782–1791 (2011).
Tana, C. et al. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. 22, 512–519.e114–115 (2010).
Jalanka-Tuovinen, J. et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 63, 1737–1745 (2014).
Rigsbee, L. et al. Quantitative profiling of gut microbiota of children with diarrhea-predominant irritable bowel syndrome. Am. J. Gastroenterol. 107, 1740–1751 (2012).
Ponnusamy, K. et al. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J. Med. Microbiol. 60, 817–827 (2011).
Carroll, I. M. et al. Fecal protease activity is associated with compositional alterations in the intestinal microbiota. PLoS ONE 8, e78017 (2013).
Durban, A. et al. Instability of the faecal microbiota in diarrhoea-predominant irritable bowel syndrome. FEMS Microbiol. Ecol. 86, 581–589 (2013).
Balsari, A. et al. The fecal microbial population in the irritable bowel syndrome. Microbiologica 5, 185–194 (1982).
Durban, A. et al. Structural alterations of faecal and mucosa-associated bacterial communities in irritable bowel syndrome. Environ. Microbiol. Rep. 4, 242–247 (2012).
Rinttila, T. et al. Real-time PCR analysis of enteric pathogens from fecal samples of irritable bowel syndrome subjects. Gut Pathog. 3, 6 (2011).
Si, J. M. et al. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J. Gastroenterol. 10, 1802–1805 (2004).
Parkes, G. C. et al. Distinct microbial populations exist in the mucosa-associated microbiota of sub-groups of irritable bowel syndrome. Neurogastroenterol. Motil. 24, 31–39 (2012).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Kashyap, P. C. et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 144, 967–977 (2013).
Halmos, E. P. et al. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut http://dx.doi.org/10.1136/gutjnl-2014-307264.
Lewis, S. & Cochrane, S. Alteration of sulfate and hydrogen metabolism in the human colon by changing intestinal transit rate. Am. J. Gastroenterol. 102, 624–633 (2007).
Oufir, L. E. et al. Relationships between transit time in man and in vitro fermentation of dietary fiber by fecal bacteria. Eur. J. Clin. Nutr. 54, 603–609 (2000).
Jeffery, I. B. et al. The microbiota link to irritable bowel syndrome: an emerging story. Gut Microbes 3, 572–576 (2012).
Bercik, P. et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609.e1–3 (2011).
Park, A. J. et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol. Motil. 25, 733–e575 (2013).
Naseribafrouei, A. et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 26, 1155–1162 (2014).
Simrén, M. IBS with intestinal microbial dysbiosis: a new and clinically relevant subgroup? Gut 63, 1685–1686 (2014).
Parkes, G. C. et al. Gastrointestinal microbiota in irritable bowel syndrome: their role in its pathogenesis and treatment. Am. J. Gastroenterol. 103, 1557–1567 (2008).
May, C. L. & Kaestner, K. H. Gut endocrine cell development. Mol. Cell Endocrinol. 323, 70–75 (2010).
Gunawardene, A. R., Corfe, B. M. & Staton, C. A. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int. J. Exp. Pathol. 92, 219–231 (2011).
Kellum, J. M. et al. Stroking human jejunal mucosa induces 5-HT release and Cl- secretion via afferent neurons and 5-HT4 receptors. Am. J. Physiol. 277, G515–G520 (1999).
Wang, H. et al. CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut 56, 949–957 (2007).
Fukumoto, S. et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R1269–R1276 (2003).
Gershon, M. D. & Tack, J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 (2007).
Fujita, T. et al. Effect of MKC-733, a 5-HT receptor partial agonist, on bowel motility and symptoms in subjects with constipation: an exploratory study. J. Clin. Pharm. Ther. 30, 611–622 (2005).
Camilleri, M. et al. A placebo-controlled trial of prucalopride for severe chronic constipation. N. Engl. J. Med. 358, 2344–2354 (2008).
Talley, N. J. et al. GR 38032F (ondansetron), a selective 5HT3 receptor antagonist, slows colonic transit in healthy man. Dig. Dis. Sci. 35, 477–480 (1990).
Houghton, L. A., Foster, J. M. & Whorwell, P. J. Alosetron, a 5-HT3 receptor antagonist, delays colonic transit in patients with irritable bowel syndrome and healthy volunteers. Aliment. Pharmacol. Ther. 14, 775–782 (2000).
Gregory, R. E. & Ettinger, D. S. 5-HT3 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting. A comparison of their pharmacology and clinical efficacy. Drugs 55, 173–189 (1998).
Fuller, R. W. & Wong, D. T. Serotonin uptake and serotonin uptake inhibition. Ann. N. Y. Acad. Sci. 600, 68–78 (1990).
Erspamer, V. & Testini, A. Observations on the release and turnover rate of 5 hydroxytryptamine in the gastrointestinal tract. J. Pharm. Pharmacol. 11, 618–623 (1959).
Bearcroft, C. P., Perrett D & Farthing, M. J. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut 42, 42–46 (1998).
Dunlop, S. P. et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 3, 349–357 (2005).
Atkinson, W. et al. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology 130, 34–43 (2006).
Shekhar, C. et al. Rome III functional constipation and irritable bowel syndrome with constipation are similar disorders within a spectrum of sensitization, regulated by serotonin. Gastroenterology 145, 749–757 (2013).
Miwa, J. et al. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion 63, 188–194 (2001).
Kerckhoffs, A. P. et al. Trypsinogen IV, serotonin transporter transcript levels and serotonin content are increased in small intestine of irritable bowel syndrome patients. Neurogastroenterol. Motil. 20, 900–907 (2008).
Costedio, M. M. et al. Mucosal serotonin signaling is altered in chronic constipation but not in opiate-induced constipation. Am. J. Gastroenterol. 105, 1173–1180 (2010).
Dunlop, S. P. et al. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology 125, 1651–1659 (2003).
Coates, M. D. et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126, 1657–1664 (2004).
Faure, C. et al. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology 139, 249–258 (2010).
El-Salhy, M. et al. Chromogranin A cell density in the rectum of patients with irritable bowel syndrome. Mol. Med. Report 6, 1223–1225 (2012).
El-Salhy, M., Lomholt-Beck, B. & Hausken, T. Chromogranin A as a possible tool in the diagnosis of irritable bowel syndrome. Scand. J. Gastroenterol. 45, 1435–1439 (2010).
El-Salhy, M., Wendelbo, I. H. & Gundersen, D. Reduced chromogranin A cell density in the ileum of patients with irritable bowel syndrome. Mol. Med. Report 7, 1241–1244 (2013).
Öhman, L. et al. Altered levels of fecal chromogranins and secretogranins in IBS: relevance for pathophysiology and symptoms? Am. J. Gastroenterol. 107, 440–447 (2012).
El-Salhy, M. et al. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front. Biosci. (Elite Ed.) 4, 2783–2800 (2012).
Lyte, M. Microbial endocrinology and infectious disease in the 21st century. Trends Microbiol. 12, 14–20 (2004).
Freestone, P. Communication between bacteria and their hosts. Scientifica (Cairo) 2013, 361073 (2013).
Coleman, N. S. et al. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin. Gastroenterol. Hepatol. 4, 874–881 (2006).
Foley, S. et al. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology 140, 1434–1443.e1 (2011).
Linden, D. R. et al. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G207–G216 (2003).
Linden, D. R. et al. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol. Motil. 17, 565–574 (2005).
Ghia, J. E. et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137, 1649–1660 (2009).
Haub, S. et al. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol. Motil. 22, 826–834.e229 (2010).
Feistritzer, C. et al. Effects of the neuropeptide secretoneurin on natural killer cell migration and cytokine release. Regul. Pept. 126, 195–201 (2005).
Shooshtarizadeh, P. et al. The antimicrobial peptides derived from chromogranin/secretogranin family, new actors of innate immunity. Regul. Pept. 165, 102–110 (2010).
Zhang, D. et al. Two chromogranin a-derived peptides induce calcium entry in human neutrophils by calmodulin-regulated calcium independent phospholipase A2. PLoS ONE 4, e4501 (2009).
Rhee, S. H., Pothoulakis, C. & Mayer, E. A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 6, 306–214 (2009).
Johansson, M. E., Sjovall, H. & Hansson, G. C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 352–361 (2013).
Scaldaferri, F. et al. The gut barrier: new acquisitions and therapeutic approaches. J. Clin. Gastroenterol. 46 (Suppl.), S12–S17 (2012).
Camilleri, M. et al. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 24, 503–512 (2012).
Rao, A. S. et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G919–G928 (2011).
Camilleri, M. et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol. Motil. 22, e15–e26 (2010).
Zuckerman, M. J. et al. Intestinal permeability to [51Cr]EDTA in infectious diarrhea. Dig. Dis. Sci. 38, 1651–1657 (1993).
Spiller, R. C. et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47, 804–811 (2000).
Marshall, J. K. et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment. Pharmacol. Ther. 20, 1317–1322 (2004).
Piche, T. et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 58, 196–201 (2009).
Dunlop, S. P. et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am. J. Gastroenterol. 101, 1288–1294 (2006).
Bertiaux-Vandaele, N. et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am. J. Gastroenterol. 106, 2165–2173 (2011).
Martinez, C. et al. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut 62, 1160–1168 (2013).
Wilcz-Villega, E., McClean S. & O'Sullivan, M. Reduced E-cadherin expression is associated with abdominal pain and symptom duration in a study of alternating and diarrhea predominant IBS. Neurogastroenterol. Motil. 26, 316–325 (2014).
Gustafsson, J. K., Hansson, G. C. & Sjovall, H. Ulcerative colitis patients in remission have an altered secretory capacity in the proximal colon despite macroscopically normal mucosa. Neurogastroenterol. Motil. 24, e381–e391 (2012).
Vivinus-Nebot, M. et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut 63, 744–752 (2014).
Zhou, Q., Zhang, B. & Verne, G. N. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 146, 41–46 (2009).
Annahazi, A. et al. Luminal cysteine-proteases degrade colonic tight junction structure and are responsible for abdominal pain in constipation-predominant IBS. Am. J. Gastroenterol. 108, 1322–1331 (2013).
Gecse, K. et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut 57, 591–599 (2008).
Vivinus-Nebot, M. et al. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: role of barrier defects and mast cells. Am. J. Gastroenterol. 107, 75–81 (2012).
Chandrasekharan, B. et al. Tumor necrosis factor-neuropeptide Y cross talk regulates inflammation, epithelial barrier functions, and colonic motility. Inflamm. Bowel Dis. 19, 2535–2546 (2013).
Hu, Y. J. et al. Regulation of paracellular permeability: factors and mechanisms. Mol. Biol. Rep. 40, 6123–6142 (2013).
Lee, H. et al. Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. J. Neurogastroenterol. Motil. 19, 244–250 (2013).
Wilcz-Villega, E. M., McClean, S. & O'Sullivan, M. A. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am. J. Gastroenterol. 108, 1140–1151 (2013).
Martinez, C. et al. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am. J. Gastroenterol. 107, 736–746 (2012).
Overman, E. L., Rivier, J. E. & Moeser, A. J. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS ONE 7, e39935 (2012).
Wallon, C. et al. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut 57, 50–58 (2008).
Vanuytsel, T. et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 63, 1293–1299 (2014).
Keita, A. V. et al. Vasoactive intestinal polypeptide regulates barrier function via mast cells in human intestinal follicle-associated epithelium and during stress in rats. Neurogastroenterol. Motil. 25, e406–e417 (2013).
Villani, A. C. et al. Genetic risk factors for post-infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology 138, 1502–1513 (2010).
Vazquez-Roque, M. I. et al. Association of HLA-DQ gene with bowel transit, barrier function, and inflammation in irritable bowel syndrome with diarrhea. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1262–G1269 (2012).
Barbara, G. et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126, 693–702 (2004).
Park, C. H. et al. Activated mast cells infiltrate in close proximity to enteric nerves in diarrhea-predominant irritable bowel syndrome. J. Korean Med. Sci. 18, 204–210 (2003).
Öhman, L. & Simren, M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat. Rev. Gastroenterol. Hepatol. 7, 163–173 (2010).
Ohman, L. et al. T-cell activation in patients with irritable bowel syndrome. Am. J. Gastroenterol. 104, 1205–1212 (2009).
Öhman, L. et al. A controlled study of colonic immune activity and β7+ blood T lymphocytes in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 3, 980–986 (2005).
Brint, E. K. et al. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am. J. Gastroenterol. 106, 329–336 (2011).
Belmonte, L. et al. Role of toll like receptors in irritable bowel syndrome: differential mucosal immune activation according to the disease subtype. PLoS ONE 7, e42777 (2012).
Öhman, L. et al. Increased TLR2 expression on blood monocytes in irritable bowel syndrome patients. Eur. J. Gastroenterol. Hepatol. 24, 398–405 (2012).
Scully, P. et al. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am. J. Gastroenterol. 105, 2235–2243 (2010).
Chang, L. et al. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am. J. Gastroenterol. 107, 262–272 (2012).
Dinan, T. G. et al. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am. J. Gastroenterol. 103, 2570–2576 (2008).
Dinan, T. G. et al. Hypothalamic–pituitary–gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology 130, 304–311 (2006).
Kindt, S. et al. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol. Motil. 21, 389–398 (2009).
Bashashati, M. et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol. Motil. 26, 1036–1048 (2014).
Liebregts, T. et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 132, 913–920 (2007).
Gwee, K. A. et al. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut 52, 523–526 (2003).
O'Malley, D., Dinan, T. G. & Cryan, J. F. Interleukin-6 modulates colonic transepithelial ion transport in the stress-sensitive wistar kyoto rat. Front. Pharmacol. 3, 190 (2012).
Olofsson, P. S. et al. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol. Rev. 248, 188–204 (2012).
Anitha, M. et al. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology 143, 1006–1016.e4 (2012).
Muller, P. A. et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313 (2014).
Robinette, M. L. & Colonna, M. GI motility: microbiota and macrophages join forces. Cell 158, 239–240 (2014).
Tornblom, H. et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology 123, 1972–1979 (2002).
Chadwick, V. S. et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 122, 1778–1783 (2002).
Cremon, C. et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am. J. Gastroenterol. 104, 392–400 (2009).
Sundin, J. et al. Aberrant mucosal lymphocyte number and subsets in the colon of post-infectious irritable bowel syndrome patients. Scand. J. Gastroenterol. 49, 1068–1075 (2014).
Chen, J., Zhang, Y. & Deng, Z. Imbalanced shift of cytokine expression between T helper 1 and T helper 2 (Th1/Th2) in intestinal mucosa of patients with post-infectious irritable bowel syndrome. BMC Gastroenterol. 12, 91 (2012).
Holmen, N. et al. CD4+CD25+ regulatory T cells in irritable bowel syndrome patients. Neurogastroenterol. Motil. 19, 119–125 (2007).
Ohman, L. et al. B-cell activation in patients with irritable bowel syndrome (IBS). Neurogastroenterol. Motil. 21, 644–650.e27 (2009).
Vicario, M. et al. Increased humoral immunity in the jejunum of diarrhoea-predominant irritable bowel syndrome associated with clinical manifestations. Gut http://dx.doi.org/10.1136/gutjnl-2013-306236.
Schoepfer, A. M. et al. Antibodies to flagellin indicate reactivity to bacterial antigens in IBS patients. Neurogastroenterol. Motil. 20, 1110–1118 (2008).
Akiho, H. et al. Involvement of interleukin-17A-induced hypercontractility of intestinal smooth muscle cells in persistent gut motor dysfunction. PLoS ONE 9, e92960 (2014).
Akiho, H., Ihara, E., Motomura, Y. & Nakamura, K. Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders. World J. Gastrointest. Pathophysiol. 2, 72–81 (2011).
Bashashati, M. et al. Cytokine gene polymorphisms are associated with irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol. Motil. 24, 1102–e566 (2012).
van der Veek, P. P. et al. Role of tumor necrosis factor-alpha and interleukin-10 gene polymorphisms in irritable bowel syndrome. Am. J. Gastroenterol. 100, 2510–2516 (2005).
Romero-Valdovinos, M. et al. Interleukin-8 and -10 gene polymorphisms in irritable bowel syndrome. Mol. Biol. Rep. 39, 8837–8843 (2012).
Swan, C. et al. Identifying and testing candidate genetic polymorphisms in the irritable bowel syndrome (IBS): association with TNFSF15 and TNFα. Gut 63, 985–994 (2013).
Zucchelli, M. et al. Association of TNFSF15 polymorphism with irritable bowel syndrome. Gut 60, 1671–1677 (2011).
Camilleri, M. et al. RNA sequencing shows transcriptomic changes in rectosigmoid mucosa in patients with irritable bowel syndrome-diarrhea: a pilot case–control study. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G1089–G1098 (2014).
Akbar, A. et al. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 57, 923–929 (2008).
Wang, L. H., Fang, X. C. & Pan, G. Z. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut 53, 1096–1101 (2004).
Di Nardo, G. et al. Neuroimmune interactions at different intestinal sites are related to abdominal pain symptoms in children with IBS. Neurogastroenterol. Motil. 26, 196–204 (2014).
Nasser, Y. et al. Using human intestinal biopsies to study the pathogenesis of irritable bowel syndrome. Neurogastroenterol. Motil. 26, 455–469 (2014).
Barbara, G. et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132, 26–37 (2007).
Buhner, S. et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 137, 1425–1434 (2009).
Balestra, B. et al. Colonic mucosal mediators from patients with irritable bowel syndrome excite enteric cholinergic motor neurons. Neurogastroenterol. Motil. 24, 1118–e570 (2012).
Cenac, N. et al. Role for protease activity in visceral pain in irritable bowel syndrome. J. Clin. Invest. 117, 636–647 (2007).
Cremon, C. et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am. J. Gastroenterol. 106, 1290–1298 (2011).
Buhner, S. et al. Submucous rather than myenteric neurons are activated by mucosal biopsy supernatants from irritable bowel syndrome patients. Neurogastroenterol. Motil. 24, 1134–e572 (2012).
Valdez-Morales, E. E. et al. Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel syndrome patients: a role for PAR2. Am. J. Gastroenterol. 108, 1634–1643 (2013).
Hughes, P. A. et al. Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut 62, 1456–1465 (2013).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
L.O. has received an unrestricted research grant from AstraZeneca, served as an Advisory Board member for Genetic Analysis AS, and has received speaker honoraria from Abbvie and Takeda. H.T. has received unrestricted research support from Tillotts Pharma, and served as a Consultant and/or Advisory Board member for Almirall, Danone and Shire. M.S. has received unrestricted research grants from AstraZeneca and Danone, and served as a consultant and/or Advisory Board member for Albireo, Almirall, Danone, Chr Hansen, Novartis and Shire.
Rights and permissions
About this article
Cite this article
Öhman, L., Törnblom, H. & Simrén, M. Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nat Rev Gastroenterol Hepatol 12, 36–49 (2015). https://doi.org/10.1038/nrgastro.2014.200
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2014.200
This article is cited by
-
Acacia fiber or probiotic supplements to relieve gastrointestinal complaints in patients with constipation-predominant IBS: a 4-week randomized double-blinded placebo-controlled intervention trial
European Journal of Nutrition (2024)
-
Dietary fat induced chylomicron-mediated LPS translocation in a bicameral Caco-2cell model
Lipids in Health and Disease (2023)
-
Current and Future Therapeutic Options for Irritable Bowel Syndrome with Diarrhea and Functional Diarrhea
Digestive Diseases and Sciences (2023)
-
Fecal microbiota transplantation in the treatment of irritable bowel syndrome: a single-center prospective study in Japan
BMC Gastroenterology (2022)
-
Mucosal Microbiota: Closer to the Pathology, Closer to the Truth?
Digestive Diseases and Sciences (2022)