Key Points
-
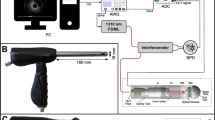
Confocal laser endomicroscopy (CLE) and endocytoscopy are two technically different methods to obtain high-resolution microscopic images during ongoing endoscopy
-
Multiple trials have demonstrated the ability of gastroenterologists to obtain and interpret microscopic images from the upper and lower gastrointestinal tract and the hepatobiliary-pancreatic system during endoscopy
-
In expert hands, on-site microscopy can be used to minimize sampling error and to guide endoscopic therapy
-
CLE has enabled new insights into pathophysiology by its ability to provide tissue microscopy in its native environment
Abstract
Performing real-time microscopy has been a vision of endoscopists since the very early phases of gastrointestinal endoscopy. Confocal endomicroscopy, an adaption of confocal laser scanning microscopy, and endocytoscopy, an adaption of white-light microscopy, have been introduced into the endoscopic armamentarium in the past decade. Both techniques yield on-site histological information. Multiple trials have demonstrated the ability of gastroenterologists to obtain and interpret microscopic images from the upper and lower gastrointestinal tract, and also the hepatobiliary-pancreatic system, during endoscopy. Such microscopic information has been successfully used in expert hands to minimize sampling error by 'smart', microscopically targeted biopsies and to guide endoscopic interventions. However, endomicroscopy is also unique in its ability to dynamically visualize cellular processes in their native environment free of artefacts. This ability enables fundamental insights into mechanisms of human diseases in clinical and translational science.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kudo, S. et al. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest. Endosc. 44, 8–14 (1996).
Brown, S. R., Baraza, W. & Hurlstone, P. Chromoscopy versus conventional endoscopy for the detection of polyps in the colon and rectum. Cochrane Database of Systematic Reviews. Issue 1. Art. No.: CD006439 http://dx.doi.org/10.1002/14651858.CD006439.pub2.
Murthy, S., Goetz, M., Hoffman, A. & Kiesslich, R. Novel colonoscopic imaging. Clin. Gastroenterol. Hepatol. 10, 984–987 (2012).
Hoffman, A. et al. High definition colonoscopy combined with i-Scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy 42, 827–833 (2010).
Goetz, M. Molecular imaging in GI endoscopy. Gastrointest. Endosc. 76, 1207–1209 (2012).
Mahmood, U. Optical molecular imaging approaches in colorectal cancer. Gastroenterology 138, 419–422 (2010).
Wallace, M. B. & Fockens, P. Probe-based confocal laser endomicroscopy. Gastroenterology 136, 1509–1513 (2009).
Kiesslich, R., Goetz, M., Vieth, M., Galle, P. R. & Neurath, M. F. Technology insight: confocal laser endoscopy for in vivo diagnosis of colorectal cancer. Nat. Clin. Pract. Oncol. 4, 480–490 (2007).
De Palma, G. D. & Wallace, M. B., Giovannini, M. Confocal laser endomicroscopy. Gastroenterol. Res. Pract. 2012, 216209 (2012).
Goetz, M., Watson, A. & Kiesslich, R. Confocal laser endomicroscopy in gastrointestinal diseases. J. Biophotonics 4, 498–508 (2011).
Neumann, H. et al. Review article: in vivo imaging by endocytoscopy. Aliment. Pharmacol. Ther. 33, 1183–1193 (2011).
Buchner, A. M. et al. Comparison of probe-based confocal laser endomicroscopy with virtual chromoendoscopy for classification of colon polyps. Gastroenterology 138, 834–842 (2010).
Kudo, S. E. et al. Diagnosis of colorectal lesions with a novel endocytoscopic classification—a pilot study. Endoscopy 43, 869–875 (2011).
Kodashima, S. et al. Ex-vivo study of high-magnification chromoendoscopy in the gastrointestinal tract to determine the optimal staining conditions for endocytoscopy. Endoscopy 38, 1115–1121 (2006).
Wallace, M. B. et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment. Pharmacol. Ther. 31, 548–552 (2010).
Kiesslich, R. et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 127, 706–713 (2004).
Polglase, A. L. et al. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest. Endosc. 62, 686–695 (2005).
Goetz, M. et al. Simultaneous confocal laser endomicroscopy and chromoendoscopy with topical cresyl violet. Gastrointest. Endosc. 70, 959–968 (2009).
Kiesslich, R. et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 127, 706–713 (2004).
Goetz, M. et al. Near-infrared confocal imaging during mini-laparoscopy: a novel rigid endomicroscope with increased imaging plane depth. J. Hepatol. 53, 84–90 (2010).
Dunbar, K. B., Montgomery, E. A. & Canto, M. I. The learning curve of in vivo confocal laser endomicroscopy for prediction of Barrett's esophagus [abstract]. Gastroenterology 134 (Suppl. 1), A62–A63 (2008).
Kiesslich, R. et al. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology 132, 874–882 (2007).
Sanduleanu, S. et al. A. In vivo diagnosis and classification of colorectal neoplasia by chromoendoscopy-guided confocal laser endomicroscopy. Clin. Gastroenterol. Hepatol. 8, 371–378 (2010).
Kuiper, T. et al. New classification for probe-based confocal laser endomicroscopy in the colon. Endoscopy 43, 1076–1081 (2011).
Kiesslich, R. et al. In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. Clin. Gastroenterol. Hepatol. 4, 979–987 (2006).
Dunbar, K. B., Okolo, P. 3rd, Montgomery, E. & Canto, M. I. Confocal laser endomicroscopy in Barrett's esophagus and endoscopically inapparent Barrett's neoplasia: a prospective, randomized, double-blind, controlled, crossover trial. Gastrointest. Endosc. 70, 645–654 (2009).
Pohl, H. et al. Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett's oesophagus. Gut 57, 1648–1653 (2008).
Bajbouj, M. et al. Probe-based confocal laser endomicroscopy compared with standard four-quadrant biopsy for evaluation of neoplasia in Barrett's esophagus. Endoscopy 42, 435–440 (2010).
Wang, P. et al. Classification of histological severity of Helicobacter pylori-associated gastritis by confocal laser endomicroscopy. World J. Gastroenterol. 16, 5203–5210 (2010).
Kiesslich, R. et al. Diagnosing Helicobacter pylori in vivo by confocal laser endoscopy. Gastroenterology 128, 2119–2123 (2005).
Guo, Y. T. et al. Diagnosis of gastric intestinal metaplasia with confocal laser endomicroscopy in vivo: a prospective study. Endoscopy 40, 547–553 (2008).
Li, W. B. et al. Characterization and identification of gastric hyperplastic polyps and adenomas by confocal laser endomicroscopy. Surg. Endosc. 24, 517–524 (2010).
Li, W. B. et al. Diagnostic value of confocal laser endomicroscopy for gastric superficial cancerous lesions. Gut 60, 299–306 (2011).
Ji, R., Zuo, X. L., Li, C. Q., Zhou, C. J. & Li, Y. Q. Confocal endomicroscopy for in vivo prediction of completeness after endoscopic mucosal resection. Surg. Endosc. 25, 1933–1938 (2011).
Jeon, S. R. et al. Optical biopsies by confocal endomicroscopy prevent additive endoscopic biopsies before endoscopic submucosal dissection in gastric epithelial neoplasias: a prospective, comparative study. Gastrointest. Endosc. 74, 772–780 (2011).
Buchner, A. M. et al. Comparison of probe-based confocal laser endomicroscopy with virtual chromoendoscopy for classification of colon polyps. Gastroenterology 138, 834–842 (2010).
Shahid, M. W. et al. Diagnostic accuracy of probe-based confocal laser endomicroscopy in detecting residual colorectal neoplasia after EMR: a prospective study. Gastrointest. Endosc. 75, 525–533 (2012).
Kuiper, T., van den Broek, F. J., van Eeden, S., Fockens, P. & Dekker, E. Feasibility and accuracy of confocal endomicroscopy in comparison with narrow-band imaging and chromoendoscopy for the differentiation of colorectal lesions. Am. J. Gastroenterol. 107, 543–550 (2012).
Meining, A. et al. Detection of cholangiocarcinoma in vivo using miniprobe-based confocal fluorescence microscopy. Clin. Gastroenterol. Hepatol. 6, 1057–1060 (2008).
Loeser, C. S., Robert, M. E., Mennone, A., Nathanson, M. H. & Jamidar, P. Confocal endomicroscopic examination of malignant biliary strictures and histologic correlation with lymphatics. J. Clin. Gastroenterol. 45, 246–252 (2011).
Talreja, J. P. et al. Interpretation of probe-based confocal laser endomicroscopy of indeterminate biliary strictures: is there any interobserver agreement? Dig. Dis. Sci. 57, 3299–3302 (2012).
Meining, A. et al. Classification of probe-based confocal laser endomicroscopy findings in pancreaticobiliary strictures. Endoscopy 44, 251–257 (2012).
Konda, V. J. et al. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos). Gastrointest. Endosc. 74, 1049–1060 (2011).
Inoue, H. et al. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: a pilot trial. Endoscopy 38, 891–895 (2006).
Kumagai, Y. et al. Endocytoscopic observation for esophageal squamous cell carcinoma: can biopsy histology be omitted? Dis. Esophagus 22, 505–512 (2009).
Sasajima, K. et al. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest. Endosc. 63, 1010–1017 (2006).
Kudo, S. E. et al. Diagnosis of colorectal lesions with a novel endocytoscopic classification—a pilot study. Endoscopy 43, 869–875 (2011).
Mori, Y. et al. Comprehensive diagnostic ability of endocytoscopy compared with biopsy for colorectal neoplasms: a prospective randomized noninferiority trial. Endoscopy 45, 98–105 (2013).
Goetz, M., Ansems, J. V., Galle, P. R., Schuchmann, M. & Kiesslich, R. In vivo real-time imaging of the liver with confocal endomicroscopy permits visualization of the temporospatial patterns of hepatocyte apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G764–G772 (2011).
Bojarski, C. et al. In vivo diagnosis of acute intestinal graft-versus-host disease by confocal endomicroscopy. Endoscopy 41, 433–438 (2009).
Watson, A. J. et al. Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology 129, 902–912 (2005).
Kiesslich, R. et al. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology 133, 1769–1778 (2007).
Liu, J. J. et al. Mind the gaps: confocal endomicroscopy showed increased density of small bowel epithelial gaps in inflammatory bowel disease. J. Clin. Gastroenterol. 45, 240–245 (2011).
Liu, J. J. et al. Increased epithelial gaps in the small intestines of patients with inflammatory bowel disease: density matters. Gastrointest. Endosc. 73, 1174–1180 (2011).
Kiesslich, R. et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 61, 1146–1153 (2012).
Moussata, D. et al. Confocal laser endomicroscopy is a new imaging modality for recognition of intramucosal bacteria in inflammatory bowel disease in vivo. Gut 60, 26–33 (2011).
Lim, L. G. et al. A. Confocal endomicroscopy in the evaluation of gastric and duodenal epithelial gaps in patients with Crohn's disease and ulcerative colitis [abstract]. Gut 59 (Suppl. 3) A261 (2010).
Turcotte, J. F. et al. Breaks in the wall: increased gaps in the intestinal epithelium of irritable bowel syndrome patients identified by confocal laser endomicroscopy (with videos). Gastrointest. Endosc. 77, 624–630 (2013).
Meining, A. & Wallace, M. B. Endoscopic imaging of angiogenesis in vivo. Gastroenterology 134, 915–918 (2008).
Ji, R. et al. Mucosal barrier defects in gastric intestinal metaplasia: in vivo evaluation by confocal endomicroscopy. Gastrointest. Endosc. 75, 980–987 (2012).
Lin, K. Y., Maricevich, M., Bardeesy, N., Weissleder, R. & Mahmood, U. In vivo quantitative microvasculature phenotype imaging of healthy and malignant tissues using a fiber-optic confocal laser microprobe. Transl. Oncol. 1, 84–94 (2008).
Tous, R. et al. The anatomy of an optical biopsy semantic retrieval system. IEEE MultiMedia 19, 16–27 (2012).
Rex, D. K. et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest. Endosc. 73, 419–422 (2011).
Atrya, R. & Goetz, M. Molecular imaging in gastroenterology. Nat. Rev. Gastroenterol. Hepatol. http://dx.doi.org/10.1038/nrgastro.2013.123.
Author information
Authors and Affiliations
Contributions
M. Goetz contributed to all aspects of this manuscript. N. P. Malek made a substantial contribution to discussion of content and reviewed/editing the manuscript before submission. R. Kiesslich researched data for the article, made a substantial contribution to discussion of content, and reviewed/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M. Goetz receives research support from Pentax and Optiscan. R. Kiesslich receives research support from Pentax. N. Malek declares no competing interests.
Rights and permissions
About this article
Cite this article
Goetz, M., Malek, N. & Kiesslich, R. Microscopic imaging in endoscopy: endomicroscopy and endocytoscopy. Nat Rev Gastroenterol Hepatol 11, 11–18 (2014). https://doi.org/10.1038/nrgastro.2013.134
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2013.134
This article is cited by
-
Single multimode fibre for in vivo light-field-encoded endoscopic imaging
Nature Photonics (2023)
-
Chromoendoscopy in Combination with Random Biopsies for Patients with Pathogenic CDH1 Mutations Undergoing Endoscopic Surveillance
Journal of Gastrointestinal Cancer (2023)
-
Convolutional neural network-based system for endocytoscopic diagnosis of early gastric cancer
BMC Gastroenterology (2022)
-
Novel endoscopic optical diagnostic technologies in medical trial research: recent advancements and future prospects
BioMedical Engineering OnLine (2021)
-
Phonon imaging in 3D with a fibre probe
Light: Science & Applications (2021)