Key Points
-
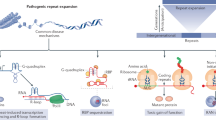
More than 20 diseases are caused by expansions of trinucleotide repeats. The expended repeats can be in either coding or non-coding DNA.
-
Friedreich ataxia is caused by a non-coding repeat expansion that leads to loss of the protein frataxin, accumulation of iron in mitochondria, increased susceptibility to oxidative stress, and a reduction in oxidative phosphorylation that is due to deficiency in mitochondrial proteins. Antioxidant therapy has had promising results and is currently being investigated in further clinical trials.
-
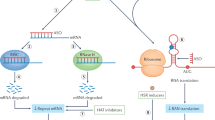
Repeat expansion in the 5′ UTR of the fragile X syndrome gene results in increased DNA methylation, reduced gene transcription and loss of the protein product FMR1. DNA demethylating agents and histone deacetylase (HDAC) inhibitors have had limited success in enhancing transcription in vitro. An antagonist of the group 1 metabotropic glutamate receptor ameliorates the phenotype in a Drosophila model of fragile X syndrome.
-
An expanded CTG repeat in the 3′ UTR of the dystrophia myotonica protein kinase (DMPK) gene probably causes the disease manifestations of dystrophia myotonica 1 by sequestering RNA-binding proteins. Ribozyme therapy to splice out the expanded region has had efficacy both in vitro and in vivo. Bioflavonoids can reduce cell toxicity in vitro, possibly through activation of class III HDACs.
-
Animal and cell-culture models of polyglutamine diseases show protein aggregation, caspase activation, excitotoxicity, oxidative stress and transcriptional dysregulation. Treatments that are based on these findings have shown efficacy in model systems. Combinatorial therapy using drugs with different targets might yield the most beneficial effects. Definitive therapy that is based on inactivation of the mutant gene still has technical barriers to overcome.
-
The triplet repeat expansion disorders have defined genetic defects, well-characterized cell-culture and animal models, and identified disease mechanisms that offer the opportunity for systematic approaches to the development of therapeutics.
Abstract
The underlying genetic mutations for many inherited neurodegenerative disorders have been identified in recent years. One frequent type of mutation is trinucleotide repeat expansion. Depending on the location of the repeat expansion, the mutation might result in a loss of function of the disease gene, a toxic gain of function or both. Disease gene identification has led to the development of model systems for investigating disease mechanisms and evaluating treatments. Examination of experimental findings reveals similarities in disease mechanisms as well as possibilities for treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
La Spada, A. R., Wilson, E. M., Lubahn, D. B., Harding, A. E. & Fischbeck, K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79 (1991).
Cummings, C. J. & Zoghbi, H. Y. Fourteen and counting: unraveling trinucleotide repeat diseases. Hum. Mol. Genet. 9, 909–916 (2000).
Campuzano, V. et al. Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum. Mol. Genet. 6, 1771–1780 (1997).
Wilson, R. B. Frataxin and frataxin deficiency in Friedreich's ataxia. J. Neurol. Sci. 207, 103–105 (2003).
Pandolfo, M. Molecular basis of Friedreich ataxia. Mov. Disord. 16, 815–821 (2001).
Gillis, J. C., Benefield, P. & McTavish, D. Idebenone. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in age-related cognitive disorders. Drugs Aging 5, 133–152 (1994).
Rotig, A., Sidi, D., Munnich, A. & Rustin, P. Molecular insights into Friedreich's ataxia and antioxidant-based therapies. Trends Mol. Med. 8, 221–224 (2002). This is a review of the consequences of frataxin loss and the rationale behind antioxidant therapy.
Artuch, R. et al. Friedreich's ataxia: idebenone treatment in early stage patients. Neuropediatrics 33, 190–193 (2002).
Gutzmann, H. & Hadler, D. Sustained efficacy and safety of idebenone in the treatment of Alzheimer's disease: update on a 2-year double-blind multicentre study. J. Neural Transm. Suppl. 54, 301–310 (1998).
Kelso, G. F. et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem. 276, 4588–4596 (2001).
Jauslin, M. L., Meier, T., Smith, R. A. & Murphy, M. P. Mitochondria-targeted antioxidants protect Friedreich ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 17, 1972–1974 (2003).
Wardman, P. & Candeias, L. P. Fenton chemistry: an introduction. Radiat. Res. 145, 523–531 (1996).
Ponka, P., Borova, J., Neuwirt, J., Fuchs, O. & Necas, E. A study of intracellular iron metabolism using pyridoxal isonicotinoyl hydrazone and other synthetic chelating agents. Biochim. Biophys. Acta 586, 278–297 (1979).
Richardson, D. R., Mouralian, C., Ponka, P. & Becker, E. Development of potential iron chelators for the treatment of Friedreich's ataxia: ligands that mobilize mitochondrial iron. Biochim. Biophys. Acta 1536, 133–140 (2001).
Sarsero, J. P. et al. Upregulation of expression from the FRDA genomic locus for the therapy of Friedreich ataxia. J. Gene Med. 5, 72–81 (2003).
Ghazizadeh, M. Cisplatin may induce frataxin expression. J. Nippon Med. Sch. 70, 367–371 (2003).
Pieretti, M. et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66, 817–822 (1991).
Chiurazzi, P., Pomponi, M. G., Willemsen, R., Oostra, B. A. & Neri, G. In vitro reactivation of the FMR1 gene involved in fragile X syndrome. Hum. Mol. Genet. 7, 109–113 (1998).
Pascale, E. et al. Modulation of methylation in the FMR1 promoter region after long term treatment with L-carnitine and acetyl-L-carnitine. J. Med. Genet. 40, e76 (2003).
Coffee, B., Zhang, F., Warren, S. T. & Reines, D. Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nature Genet. 22, 98–101 (1999).
Chiurazzi, P. et al. Synergistic effect of histone hyperacetylation and DNA demethylation in the reactivation of the FMR1 gene. Hum. Mol. Genet. 8, 2317–2323 (1999).
Nimchinsky, E. A., Oberlander, A. M. & Svoboda, K. Abnormal development of dendritic spines in FMR1 knock-out mice. J. Neurosci. 21, 5139–5146 (2001).
Irwin, S. A. et al. Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. Am. J. Med. Genet. 111, 140–146 (2002).
Huber, K. M., Gallagher, S. M., Warren, S. T. & Bear, M. F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl Acad. Sci. USA 99, 7746–7750 (2002).
Bear, M. F., Huber, K. M. & Warren, S. T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370–377 (2004). The theory of excessive metabotropic receptor function in fragile X syndrome.
McBride, S. M. et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 45, 753–764 (2005).
Meola, G. & Sansone, V. Treatment in myotonia and periodic paralysis. Rev. Neurol. 160, S55–S69 (2004).
Fu, Y. H. et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 255, 1256–1258 (1992).
Timchenko, N. A. et al. RNA CUG repeats sequester CUGBP1 and alter protein levels and activity of CUGBP1. J. Biol. Chem. 276, 7820–7826 (2001).
Phylactou, L. A., Darrah, C. & Wood, M. J. Ribozyme-mediated trans-splicing of a trinucleotide repeat. Nature Genet. 18, 378–381 (1998).
Furuya, H. et al. Some flavonoids and DHEA-S prevent the cis-effect of expanded CTG repeats in a stable PC12 cell transformant. Biochem. Pharmacol. 69, 503–516 (2005).
Scalbert, A., Johnson, I. T. & Saltmarsh, M. Polyphenols: antioxidants and beyond. Am. J. Clin. Nutr. 81, S215–S217 (2005).
Howitz, K. T. et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 (2003).
Parker, J. A. et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nature Genet. 37, 349–350 (2005). This paper describes HDAC (sirtuin family) activation as a means of suppressing polyglutamine toxicity.
Reddy, P. H. et al. Behavioural abnormalities and selective neuronal loss in HD transgenic mice expressing mutated full-length HD cDNA. Nature Genet. 20, 198–202 (1998).
Sato, T. et al. Transgenic mice harboring a full-length human mutant DRPLA gene exhibit age-dependent intergenerational and somatic instabilities of CAG repeats comparable with those in DRPLA patients. Hum. Mol. Genet. 8, 99–106 (1999).
Katsuno, M. et al. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35, 843–854 (2002). The authors describe the ligand-dependent toxicity of the mutant androgen receptor in Kennedy disease. This emphasizes the need to understand each polyglutamine disorder in the context of the disease protein's normal function.
Reiner, A., Dragatsis, I., Zeitlin, S. & Goldowitz, D. Wild-type huntingtin plays a role in brain development and neuronal survival. Mol. Neurobiol. 28, 259–276 (2003).
Cattaneo, E. Dysfunction of wild-type huntingtin in Huntington disease. News Physiol. Sci. 18, 34–37 (2003).
Cummings, C. J. et al. Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nature Genet. 19, 148–154 (1998).
Chai, Y., Koppenhafer, S. L., Shoesmith, S. J., Perez, M. K. & Paulson, H. L. Evidence for proteasome involvement in polyglutamine disease: localization to nuclear inclusions in SCA3/MJD and suppression of polyglutamine aggregation in vitro. Hum. Mol. Genet. 8, 673–682 (1999).
Kazemi-Esfarjani, P. & Benzer, S. Genetic suppression of polyglutamine toxicity in Drosophila. Science 287, 1837–1840 (2000).
Wyttenbach, A. et al. Effects of heat shock, heat shock protein 40 (HDJ-2), and proteasome inhibition on protein aggregation in cellular models of Huntington's disease. Proc. Natl Acad. Sci. USA 97, 2898–2903 (2000).
Sittler, A. et al. Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington's disease. Hum. Mol. Genet. 10, 1307–1315 (2001).
Heiser, V. et al. Identification of benzothiazoles as potential polyglutamine aggregation inhibitors of Huntington's disease by using an automated filter retardation assay. Proc. Natl Acad. Sci. USA 99, 16400–16406 (2002). This paper describes the application of high-throughput screening for polyglutamine disorders and shows that inhibitors of aggregation are therapeutic targets.
Smith, D. L. et al. Inhibition of polyglutamine aggregation in R6/2 HD brain slices-complex dose-response profiles. Neurobiol. Dis. 8, 1017–1026 (2001).
Sanchez, I., Mahlke, C. & Yuan, J. Pivotal role of oligomerization in expanded polyglutamine neurodegenerative disorders. Nature 421, 373–379 (2003).
Tanaka, M. et al. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nature Med. 10, 148–154 (2004). The authors discuss an approach to inhibiting protein aggregation using a saccharide–polyglutamine interaction. The safety profile of this chemical seems exceptionally favourable.
Khoshnan, A., Ko, J. & Patterson, P. H. Effects of intracellular expression of anti-huntingtin antibodies of various specificities on mutant huntingtin aggregation and toxicity. Proc. Natl Acad. Sci. USA 99, 1002–1007 (2002).
Kazantsev, A. et al. A bivalent Huntingtin binding peptide suppresses polyglutamine aggregation and pathogenesis in Drosophila. Nature Genet. 30, 367–376 (2002).
Kahlem, P., Terre, C., Green, H. & Djian, P. Peptides containing glutamine repeats as substrates for transglutaminase-catalyzed cross-linking: relevance to diseases of the nervous system. Proc. Natl Acad. Sci. USA 93, 14580–14585 (1996).
Karpuj, M. V. et al. Transglutaminase aggregates huntingtin into nonamyloidogenic polymers, and its enzymatic activity increases in Huntington's disease brain nuclei. Proc. Natl Acad. Sci. USA 96, 7388–7393 (1999).
Dedeoglu, A. et al. Therapeutic effects of cystamine in a murine model of Huntington's disease. J. Neurosci. 22, 8942–8950 (2002).
Karpuj, M. V. et al. Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nature Med. 8, 143–149 (2002). This article provides an example of effective treatment after the development of disease manifestations. It also describes activity of the drug outside its pharmacological target.
Lesort, M., Lee, M., Tucholski, J. & Johnson, G. V. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J. Biol. Chem. 278, 3825–3830 (2003).
Bailey, C. D. & Johnson, G. V. Tissue transglutaminase contributes to disease progression in the R6/2 Huntington's disease mouse model via aggregate-independent mechanisms. J. Neurochem. 92, 83–92 (2005).
Wellington, C. L. & Hayden, M. R. Caspases and neurodegeneration: on the cutting edge of new therapeutic approaches. Clin. Genet. 57, 1–10 (2000).
Goldberg, Y. P. et al. Cleavage of huntingtin by apopain, a proapoptotic cysteine protease, is modulated by the polyglutamine tract. Nature Genet. 13, 442–449 (1996).
Wellington, C. L. et al. Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract. J. Biol. Chem. 273, 9158–9167 (1998).
Wellington, C. L. et al. Inhibiting caspase cleavage of huntingtin reduces toxicity and aggregate formation in neuronal and nonneuronal cells. J. Biol. Chem. 275, 19831–19838 (2000).
Ona, V. O. et al. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature 399, 263–267 (1999).
Chen, M. et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nature Med. 6, 797–801 (2000). The authors describe the amelioration of disease manifestations in an HD model with a drug that inhibits caspase.
Wang, X. et al. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc. Natl Acad. Sci. USA 100, 10483–10487 (2003).
Bonelli, R. M., Hodl, A. K., Hofmann, P. & Kapfhammer, H. P. Neuroprotection in Huntington's disease: a 2-year study on minocycline. Int. Clin. Psychopharmacol. 19, 337–342 (2004).
Keene, C. D. et al. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington's disease. Proc. Natl Acad. Sci. USA 99, 10671–10676 (2002).
Coyle, J. T. & Schwarcz, R. Lesion of striatal neurones with kainic acid provides a model for Huntington's chorea. Nature 263, 244–246 (1976).
Beal, M. F. et al. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature 321, 168–171 (1986).
Cepeda, C. et al. NMDA receptor function in mouse models of Huntington disease. J. Neurosci. Res. 66, 525–539 (2001).
Zeron, M. M. et al. Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington's disease. Neuron 33, 849–860 (2002).
Nicniocaill, B., Haraldsson, B., Hansson, O., O'Connor, W. T. & Brundin, P. Altered striatal amino acid neurotransmitter release monitored using microdialysis in R6/1 Huntington transgenic mice. Eur. J. Neurosci. 13, 206–210 (2001).
Li, J. Y., Plomann, M. & Brundin, P. Huntington's disease: a synaptopathy? Trends Mol. Med. 9, 414–420 (2003).
Cha, J. H. et al. Altered neurotransmitter receptor expression in transgenic mouse models of Huntington's disease. Phil. Trans. R. Soc. Lond. B 354, 981–989 (1999).
O'Suilleabhain, P. & Dewey, R. B. Jr. A randomized trial of amantadine in Huntington disease. Arch. Neurol. 60, 996–998 (2003).
Lucetti, C. et al. Amantadine in Huntington's disease: open-label video-blinded study. Neurol. Sci. 23, S83–S84 (2002).
Verhagen Metman, L. et al. Huntington's disease: a randomized, controlled trial using the NMDA-antagonist amantadine. Neurology 59, 694–699 (2002).
Murman, D. L. et al. Cognitive, behavioral, and motor effects of the NMDA antagonist ketamine in Huntington's disease. Neurology 49, 153–161 (1997).
Shoulson, I. et al. A controlled clinical trial of baclofen as protective therapy in early Huntington's disease. Ann. Neurol. 25, 252–259 (1989).
Kremer, B. et al. Influence of lamotrigine on progression of early Huntington disease: a randomized clinical trial. Neurology 53, 1000–1011 (1999).
Seppi, K. et al. Riluzole in Huntington's disease (HD): an open label study with one year follow up. J. Neurol. 248, 866–869 (2001).
Li, L. et al. Role of NR2B-type NMDA receptors in selective neurodegeneration in Huntington disease. Neurobiol. Aging 24, 1113–1121 (2003).
Li, L., Murphy, T. H., Hayden, M. R. & Raymond, L. A. Enhanced striatal NR2B-containing N-methyl-D-aspartate receptor-mediated synaptic currents in a mouse model of Huntington disease. J. Neurophysiol. 92, 2738–2746 (2004).
Arning, L. et al. NR2A and NR2B receptor gene variations modify age of onset in Huntington's disease. Neurogenetics 6, 25–28 (2005).
Chen, H. S. & Lipton, S. A. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J. Physiol. 499, 27–46 (1997).
Beister, A. et al. The N-methyl-D-aspartate antagonist memantine retards progression of Huntington's disease. J. Neural Transm. 68, S117–S122 (2004).
Nicoletti, F. et al. Group-I metabotropic glutamate receptors: hypotheses to explain their dual role in neurotoxicity and neuroprotection. Neuropharmacology 38, 1477–1484 (1999).
Schiefer, J. et al. The metabotropic glutamate receptor 5 antagonist MPEP and the mGluR2 agonist LY379268 modify disease progression in a transgenic mouse model of Huntington's disease. Brain Res. 1019, 246–254 (2004).
Popoli, P. et al. Blockade of striatal adenosine A2A receptor reduces, through a presynaptic mechanism, quinolinic acid-induced excitotoxicity: possible relevance to neuroprotective interventions in neurodegenerative diseases of the striatum. J. Neurosci. 22, 1967–1975 (2002).
Lastres-Becker, I. et al. Effects of cannabinoids in the rat model of Huntington's disease generated by an intrastriatal injection of malonate. Neuroreport 14, 813–816 (2003).
Borlongan, C. V. et al. Systemic 3-nitropropionic acid: behavioral deficits and striatal damage in adult rats. Brain Res. Bull. 36, 549–556 (1995).
Gu, M. et al. Mitochondrial defect in Huntington's disease caudate nucleus. Ann. Neurol. 39, 385–389 (1996).
Browne, S. E. et al. Oxidative damage and metabolic dysfunction in Huntington's disease: selective vulnerability of the basal ganglia. Ann. Neurol. 41, 646–653 (1997).
Beal, M. F. & Ferrante, R. J. Experimental therapeutics in transgenic mouse models of Huntington's disease. Nature Rev. Neurosci. 5, 373–384 (2004). A review of the pathogenesis of HD in the context of mouse models and the efficacy of therapeutic agents tested in these models.
Dedeoglu, A. et al. Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington's disease transgenic mice. J. Neurochem. 85, 1359–1367 (2003).
Verbessem, P. et al. Creatine supplementation in Huntington's disease: a placebo-controlled pilot trial. Neurology 61, 925–930 (2003).
Huntington Study Group. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington's disease. Neurology 57, 397–404 (2001).
Ranen, N. G. et al. A controlled trial of idebenone in Huntington's disease. Mov. Disord. 11, 549–554 (1996).
Okazawa, H. Polyglutamine diseases: a transcription disorder? Cell. Mol. Life Sci. 60, 1427–1439 (2003).
McCampbell, A. et al. CREB-binding protein sequestration by expanded polyglutamine. Hum. Mol. Genet. 9, 2197–2202 (2000).
Dunah, A. W. et al. Sp1 and TAFII130 transcriptional activity disrupted in early Huntington's disease. Science 296, 2238–2243 (2002).
Taylor, J. P. et al. Aberrant histone acetylation, altered transcription, and retinal degeneration in a Drosophila model of polyglutamine disease are rescued by CREB-binding protein. Genes Dev. 17, 1463–1468 (2003).
Grewal, S. I. & Moazed, D. Heterochromatin and epigenetic control of gene expression. Science 301, 798–802 (2003).
Steffan, J. S. et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413, 739–743 (2001). This is a description of reduced acetyltransferase activity and histone acetylation in polyglutamine disease, and how this can be overcome by HDAC inhibitors with consequent amelioration of toxicity.
Igarashi, S. et al. Inducible PC12 cell model of Huntington's disease shows toxicity and decreased histone acetylation. Neuroreport 14, 565–568 (2003).
Hockly, E. et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington's disease. Proc. Natl Acad. Sci. USA 100, 2041–2046 (2003).
Ferrante, R. J. et al. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice. J. Neurosci. 23, 9418–9427 (2003). This article discusses the beneficial effects of HDAC inhibitors in an HD mouse model.
Gottlicher, M. Valproic acid: an old drug newly discovered as inhibitor of histone deacetylases. Ann. Hematol. 83 (Suppl. 1), 91–92 (2004).
Burlina, A. B., Ogier, H., Korall, H. & Trefz, F. K. Long-term treatment with sodium phenylbutyrate in ornithine transcarbamylase-deficient patients. Mol. Genet. Metab. 72, 351–355 (2001).
Kelly, W. K., O'Connor, O. A. & Marks, P. A. Histone deacetylase inhibitors: from target to clinical trials. Expert Opin. Investig. Drugs 11, 1695–1713 (2002).
Brunet, A. et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 (2004).
Emerich, D. F. et al. Implants of polymer-encapsulated human NGF-secreting cells in the nonhuman primate: rescue and sprouting of degenerating cholinergic basal forebrain neurons. J. Comp. Neurol. 349, 148–164 (1994).
Emerich, D. F. et al. Implants of encapsulated human CNTF-producing fibroblasts prevent behavioral deficits and striatal degeneration in a rodent model of Huntington's disease. J. Neurosci. 16, 5168–5181 (1996).
Bemelmans, A. P. et al. Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington's disease, as demonstrated by adenoviral gene transfer. Hum. Gene Ther. 10, 2987–2997 (1999).
Perez-Navarro, E., Canudas, A. M., Akerund, P., Alberch, J. & Arenas, E. Brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 prevent the death of striatal projection neurons in a rodent model of Huntington's disease. J. Neurochem. 75, 2190–2199 (2000).
McBride, J. L. et al. Structural and functional neuroprotection in a rat model of Huntington's disease by viral gene transfer of GDNF. Exp. Neurol. 181, 213–223 (2003).
Kells, A. P. et al. AAV-mediated gene delivery of BDNF or GDNF is neuroprotective in a model of Huntington disease. Mol. Ther. 9, 682–688 (2004).
Canals, J. M. et al. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease. J. Neurosci. 24, 7727–7739 (2004).
Cepeda, C. et al. Increased GABAergic function in mouse models of Huntington's disease: reversal by BDNF. J. Neurosci. Res. 78, 855–867 (2004).
Popovic, N., Maingay, M., Kirik, D. & Brundin, P. Lentiviral gene delivery of GDNF into the striatum of R6/2 Huntington mice fails to attenuate behavioral and neuropathological changes. Exp. Neurol. 193, 65–74 (2005).
Zuccato, C. et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science 293, 493–498 (2001).
Sopher, B. L. et al. Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration. Neuron 41, 687–699 (2004).
Bloch, J. et al. Neuroprotective gene therapy for Huntington's disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study. Hum. Gene Ther. 15, 968–975 (2004).
Dunnett, S. B. et al. Striatal transplantation in a transgenic mouse model of Huntington's disease. Exp. Neurol. 154, 31–40 (1998).
van Dellen, A., Deacon, R., York, D., Blakemore, C. & Hannan, A. J. Anterior cingulate cortical transplantation in transgenic Huntington's disease mice. Brain Res. Bull. 56, 313–318 (2001).
Freeman, T. B. et al. Transplanted fetal striatum in Huntington's disease: phenotypic development and lack of pathology. Proc. Natl Acad. Sci. USA 97, 13877–13882 (2000).
Bachoud-Levi, A. C. et al. Motor and cognitive improvements in patients with Huntington's disease after neural transplantation. Lancet 356, 1975–1979 (2000).
Gaura, V. et al. Striatal neural grafting improves cortical metabolism in Huntington's disease patients. Brain 127, 65–72 (2004).
McBride, J. L. et al. Human neural stem cell transplants improve motor function in a rat model of Huntington's disease. J. Comp. Neurol. 475, 211–219 (2004).
Lescaudron, L., Unni, D. & Dunbar, G. L. Autologous adult bone marrow stem cell transplantation in an animal model of huntington's disease: behavioral and morphological outcomes. Int. J. Neurosci. 113, 945–956 (2003).
Ende, N. & Chen, R. Human umbilical cord blood cells ameliorate Huntington's disease in transgenic mice. J. Med. 32, 231–240 (2001).
Yamamoto, A., Lucas, J. J. & Hen, R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell 101, 57–66 (2000). The authors show that blockade of expression of the mutant protein leads to a disappearance of inclusions and a reversal of the behavioural phenotype. This indicates that continuous expression of the mutant protein is necessary for the disease.
Caplen, N. J. et al. Rescue of polyglutamine-mediated cytotoxicity by double-stranded RNA-mediated RNA interference. Hum. Mol. Genet. 11, 175–184 (2002).
Xia, H. et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nature Med. 10, 816–820 (2004). A discussion of in vivo use of RNAi as a potential therapy for dominant neurodegenerative disorders.
Harper, S. Q. et al. RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc. Natl Acad. Sci. USA 102, 5820–5825 (2005). This paper shows that RNAi can accomplish disease allele-specific silencing in vivo.
Ravina, B. M. et al. Neuroprotective agents for clinical trials in Parkinson's disease: a systematic assessment. Neurology 60, 1234–1240 (2003).
Acknowledgements
This work was supported in part by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute of General Medical Sciences (NIGMS) at the US National Institutes of Health. N.A.D. is supported under a clinical pharmacology research associate training fellowship awarded by NIGMS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
MedlinePlus
OMIM
Spinal and bulbar muscular atrophy
FURTHER INFORMATION
FRAXA—The Fragile X Research Foundation
Glossary
- OPEN-LABEL TRIAL
-
A clinical trial in which both subjects and investigators know which drug is being tested and the doses that are being used.
- DOUBLE-BLIND TRIAL
-
A study in which neither the investigator nor the subject know whether a medication or placebo is being used for any given subject, so as to prevent subjective bias on the part of the subject or investigator.
- PLACEBO-CONTROLLED
-
The use of an inactive substance or treatment that seems to be the same as, and is given in the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared with the effects of the placebo.
- PHASE II CLINICAL TRIAL
-
A study that is carried out to obtain more safety data and preliminary data on the effectiveness of the drug for a particular indication in patients with the disease or condition. Phase II studies help to determine the feasibility of larger-scale definitive trials.
- PHASE I CLINICAL TRIAL
-
An initial clinical study that involves small numbers of healthy human volunteers and small doses to assess safety, metabolism and excretion of a drug.
- LYMPHOBLASTS
-
Immature white blood cells.
- DENDRITES
-
Short and typically highly branched extensions of the neuronal cell body that form synaptic contacts with the terminals of other neurons and allow the transmission of nerve impulses between cells.
- MYOTONIA
-
The failure of muscle to relax immediately after voluntary contraction has stopped.
- ANTICIPATION
-
The tendency of certain diseases to have an earlier age of onset and increasing severity in successive generations.
- PROTEASOME
-
A cytosolic protein complex that degrades proteins that have been marked for destruction by the ubiquitylation pathway.
- CORTEX
-
The superficial layer of grey matter that is involved in higher functions, including initiation of voluntary movements, cognition and emotion.
- STRIATUM
-
The region of the brain that receives excitatory input from the cortex, thalamus and midbrain. It has a pivotal role in modulating motor activity and higher cognitive function.
- STRIATAL PROJECTION NEURONS
-
These are medium sized, GABA-containing neurons of the striatum that project to the substantia nigra and have an important role in the regulation of movement.
- PHASE III CLINICAL TRIAL
-
A study that is intended to gather the extra information about effectiveness and safety that is needed to evaluate the overall benefit–risk relationship of the drug. Phase III studies also provide a basis for extrapolating the results to the general population.
- RESVERATROL
-
A natural compound that is found in grapes, mulberries, peanuts and other plants or food products, especially red wine, that has antioxidant, antimutagen and anti-inflammatory properties.
- PET SCAN
-
A positron emission tomography scan. This is an imaging technique that relies on the detection of γ-rays that are emitted from tissues after the administration of a natural biochemical substance into which positron-emitting isotopes have been incorporated.
Rights and permissions
About this article
Cite this article
Di Prospero, N., Fischbeck, K. Therapeutics development for triplet repeat expansion diseases. Nat Rev Genet 6, 756–766 (2005). https://doi.org/10.1038/nrg1690
Issue Date:
DOI: https://doi.org/10.1038/nrg1690
This article is cited by
-
A plant-based mutant huntingtin model-driven discovery of impaired expression of GTPCH and DHFR
Cellular and Molecular Life Sciences (2022)
-
Insulin-like growth factor 2 (IGF2) protects against Huntington’s disease through the extracellular disposal of protein aggregates
Acta Neuropathologica (2020)
-
DNA mismatch repair in trinucleotide repeat instability
Science China Life Sciences (2017)
-
Inhibition of Protein Misfolding/Aggregation Using Polyglutamine Binding Peptide QBP1 as a Therapy for the Polyglutamine Diseases
Neurotherapeutics (2013)