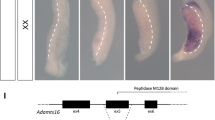
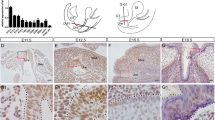
Abstract
The rising incidence of genitourinary (GU) defects among newborns establishes the need and opportunity to focus research efforts on the amelioration of this growing public-health concern. Sadly, our inability to explain the causes of GU defects can be directly attributed to our lack of understanding of GU gene function. Recently, mouse models have been used to provide new insights into the mechanisms that underlie congenital GU malformations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Giwercman, A., Carlsen, E., Keiding, N. & Skakkenbaek, N. E. Evidence for increasing incidence of abnormalities of the human testis: a review. Environ. Health Perspect. 2, 65–71 (1993).
Paulozzi, L. J., Erickson, D. & Jackson, R. J. Hypospadias trends in two US surveillance systems. Pediatrics 100, 831–834 (1997).
Gallentine, M. L., Morey, A. F. & Thompson, I. M. Hypospadias: a contemporary epidemiologic assessment. Urology 57, 788–790 (2001).
Sadler, T. W. Langman's Medical Embryology 7th edn 299–310 (Lippincott, Williams and Wilkins, New York, 1995).
Arey, L. B. Developmental Anatomy 6th edn 330–339 (Saunders Company, Philadelphia, 1954).
Murakami, R. A histological study of the development of the penis of wild-type and androgen-insensitive mice. J. Anat. 153, 223–231 (1987).
Wolf, C. et al. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodine, chlozolinate, p,p′-DDE, and ketaconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol. Ind. Health 15, 94–118 (1999).
Ostby, J., Monosson, E., Kelce, W. R. & Gray, L. E. Envrionmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation in the male rat. Toxicol. Ind. Health 15, 48–64 (1999).
Schardein, J. L. Chemically Induced Birth Defects 271–339 (Dekker, New York, 1993).
Clark, R. L. et al. External genitalia abnormalities in male rats exposed to finasteride, a 5-α reductase inhibitor. Teratology 42, 91–100 (1990).
Prahalada, S. et al. Effects of finasteride, a type-2 5-α reductase inhibitor on fetal development of the rhesus monkey (Macaca mulatta). Teratology 55, 119–131 (1997).
Kelce, W. R. & Wilson, E. M. Environmental antiandrogens: developmental effects, molecular mechanisms, and clinical implications. J. Mol. Med. 75, 198–207 (1997).
Kaloo, N. B., Gearhart, J. P. & Barrack, J. R. Sexually dimorphic expression of estrogen receptors, but not of androgen receptors in human fetal external genitalia. J. Clin. Endocrinol. Metab. 77, 692–698 (1993).
Labrie, C. et al. Combination of an antiandrogen and a 5 α-reductase inhibitor: a further step towards total androgen blockade? Endocrinology 129, 566–568 (1991).
Svensson, H., Reychman, M., Troeng, T. & Aberg, M. Staged reconstruction of hypospadias with chordee: outcome and costs. Scan. J. Plast. Reconst. Surg. Hand Surg. 31, 51–55 (1997).
Perriton, C. L., Powles, N., Chiang, C., Maconochie, M. K. & Cohn, M. J. Sonic hedgehog signalling from the urethral plate epithelium controls external genitalia development. Dev. Biol. 247, 26–46 (2002).
Capecchi, M. R. Choose your target. Nature Genet. 26, 159–161 (2000).
Anagnostopoulos, A. V., Mobraaten, L. E., Sharp, J. J. & Davisson, M. T. Transgenic and knockout databases: behavioral profiles of mouse mutants. Physiol. Behav. 73, 675–689 (2001).
Baskin, L. S. Hypospadias and urethral development. J. Urol. 163, 951–956 (2000).
Minelli, A. Homology, limbs and genitalia. Evol. Dev. 4, 127–132 (2002).
Suzuki, K. et al. Embryonic development of the mouse genitalia: insights into a unique mode of organogenesis. Evol. Dev. 4, 133–141 (2002).
Haraguchi, R. et al. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development 127, 2471–2479 (2000).
Haraguchi, R. et al. Unique functions of Sonic hedgehog during external genitalia development. Development 128, 4241–4250 (2001).
Morgan, E. A., Nguyen, S. B., Scott, V. L. & Stadler, H. S. Loss of Fgf-8 and Bmp-7 signaling in Hoxa13 mutant mice cause hypospadia. Development (in the press).
de Santa Barbara, P. & Roberts, D. J. Tail gut endoderm and gut/genitourinary/tail development: new tissue-specific role for Hoxa13. Development 129, 551–561 (2002).
Stern, A. M. et al. The hand–foot–uterus syndrome: a new hereditary disorder characterized by hand and foot dysplasia, dermatoglyphic, abnormalities, and partial duplication of the female genital tract. J. Pediatr. 77, 109–116 (1970).
Mortlock, D. P. & Innis, J. W. Mutation of HOXA13 in hand–foot–genital syndrome. Nature Genet. 156, 179–180 (1997).
Fromental-Ramain, C. et al. Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development 122, 2997–3011 (1997).
Post, L. C. & Innis, J. W. Infertility in adult hypodactyly mice is associated with hypospadia of distal reproductive structures. Biol. Reprod. 61, 1402–1408 (1999).
Warot, X., Fromental-Ramain, C., Fraulob, V., Chambon, P. & Dollé, P. Gene dosage-dependent effects of Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive system and urogenital tracts. Development 124, 4781–4791 (1997).
Stadler, H. S., Higgins, K. M. & Capecchi, M. R. Loss of Eph-receptor expression correlates with loss of cell adhesion and chondrogenic capacity in Hoxa13 mutant limbs. Development 128, 4177–4188 (2001).
Hartman, C. & Tabin, C. J. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development 127, 3141–3159 (2000).
Hamblet, N. S. et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation, and neural tube closure. Development 129, 5827–5838 (2002).
Holmberg, J., Clarke, D. L. & Frisen, J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature 408, 203–206 (2000).
Podlasek, C. A., Duboule, D. & Bushman, W. Male accessory sex organ morphogenesis is altered by loss of function of Hoxd13. Dev. Dyn. 208, 454–465 (1997).
Oefelein, M., Chin-Chance, C. & Bushman, W. Expression of the homeotic gene Hoxd13 in the developing and adult mouse prostate. J. Urol. 155, 342–346 (1996).
Zelster, L., Desplan, C. & Heintz, N. Hoxb-13: a new Hox gene in a distant region of the Hox B cluster maintains colinearity. Development 122, 2475–2484 (1996).
Steers, W. D. 5 α-reductase activity in the prostate. Urology 58, 17–24 (2001).
Sreenath, T., Orosz, A., Fujita, K. & Bieberich, C. J. Androgen-independent expression of Hoxb13 in the mouse prostate. Prostate 41, 203–207 (1999).
Podlasek, C. A. et al. Hoxa10 deficient mice exhibit abnormal development of the accessory sex organs. Dev. Dyn. 214, 1–12 (1999).
Baskin, L. S. et al. Urethral seam formation and hypospadias. Cell Tissue Res. 305, 379–387 (2001).
Anderson, C. A. & Clark, R. L. External genitalia of the rat: normal development and the histogenesis of 5 α-reductase inhibitor-induced abnormalities. Teratology 42, 483–496 (1990).
Miller, C., Degenhardt, K. & Sassoon, D. A. Fetal exposure to DES results in de-regulation of Wnt-7a during uterine morphogenesis. Nature Genet. 20, 228–230 (1998).
Liang, M., Benson, G. V., Lim, H., Dey, S. K. & Maas, R. L. Abdominal B (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in Mullerian duct by synthetic estrogen diethylstilbestrol (DES). Dev. Biol. 197, 141–154 (1998).
Acknowledgements
The author thanks D. Stadler for the critical reading of this manuscript and S. Nguyen, E. Morgan and V. Scott for expert technical assistance. A portion of this work was supported by grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Disorders, and the March of Dimes Birth Defects Foundation (Basil O'Connor Starter Scholar Research Award Grant).
Author information
Authors and Affiliations
Glossary
- CORONAL HYPOSPADIA
-
A malformation of the distal portion of the penis in which the urinary opening is misplaced.
- FINASTERIDE
-
An inhibitor of the enzyme 5-β reductase that converts testosterone to dihydroxytestosterone (a potent androgen used during the masculinization of the male fetus).
- FOCAL SCULPTING
-
A developmental process that removes cells or tissues by programmed cell death, which facilitates the formation or differentiation of an embryonic region.
- HYPOPLASIA
-
The underdevelopment of a tissue or organ.
- HYPOSPADIA
-
A developmental defect that affects the growth and closure of the ventral urethra, meatus or scrotum.
- OS-CLITORIS
-
A structure found in female mice, which differs from the human clitoris by the presence of an ossified region of endochondral and membranous bone. Homologous to the os-penis in male mice.
- OS-PENIS
-
A structure found in male mice, which differs from the human penis by the presence of an ossified region of endochondral and membranous bone. Homologous to the os-clitoris in female mice.
- PARALOGUES
-
Genes that are related by duplication in a genome, which have evolved new functions
- PERITONEAL HYPOSPADIA
-
A malformation of the base of the external genitalia and body wall, which remains open in affected individuals.
- STRATIFICATION
-
The layered differentiated state of epithelial cells that line many of the genitourinary structures.
- TERATOGEN
-
An agent that causes the malformation of a developing fetus; for example, chemicals, viruses and ionizing radiation.
- TUNEL ASSAY
-
(Terminal dUTP nick-end-labelling assay). An enzymatic process that labels the 3′ ends of DNA in a cell that is undergoing programmed cell death.
- URETHRAL EPITHELIUM
-
(UE). A centrally located layer of cells in the developing genital tubercle that secretes many of the growth factors that are required for normal genitourinary development.
- URETHRAL HYPOSPADIA
-
A defect in closure of the primary shaft of the penis in which multiple openings form.
- VINCLOZOLIN
-
A protectant nonsystemic dicarboximide fungicide that exhibits antiandrogenic activity in mammals. It is used mainly on oilseed rape and peas in the United Kingdom, and on vines, fruit and vegetables in the United States.
Rights and permissions
About this article
Cite this article
Stadler, H. Modelling genitourinary defects in mice: an emerging genetic and developmental system. Nat Rev Genet 4, 478–482 (2003). https://doi.org/10.1038/nrg1083
Issue Date:
DOI: https://doi.org/10.1038/nrg1083
This article is cited by
-
Mutation screening of BMP4, BMP7, HOXA4 and HOXB6 genes in Chinese patients with hypospadias
European Journal of Human Genetics (2007)
-
Molecular genetic analysis of a de novo balanced translocation t(6;17)(p21.31;q11.2) associated with hypospadias and anorectal malformation
Human Genetics (2006)
-
The making of a male
Nature Reviews Genetics (2004)