Key Points
-
The study of floral evolution provides a simple system to investigate the link between environmental adaptation, phenotypic determination and genetic change.
-
The methods of molecular genetics offer powerful comparative tools for unravelling the evolution of floral adaptations.
-
Flower colour is a simple phenotype with well-characterized pathways of biochemical and molecular determination, which greatly facilitates comparative analysis.
-
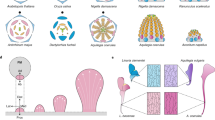
The morning glory genus Ipomoea is well suited to comparative molecular research on floral evolution owing to the great range of floral phenotypes that have arisen in the genus in the past 30 million years.
-
Differences in floral colour among Ipomoea species seem to be largely the result of shifts in gene expression rather than the result of changes in enzymatic genes.
-
Over longer periods of evolutionary time (≥50 million years) gene duplication has been the source of important new innovations in the flavonoid biosynthesis.
-
Transposons are an important source of allelic diversity in flavonoid genes in Ipomoea species.
-
Ecological research with Ipomoea provides a direct link between phenotypic selection and the environment by showing that bumblebee pollinators discriminate between pigmented and white floral morphs, leading to asymmetries in genetic transmission that should favour white genes when they are expressed at low frequency.
-
The study of floral adaptation at all levels of biological organization requires mixing historical inference with direct experimentation.
Abstract
Flowers have long fascinated humans. The scientific study of floral biology unifies many diverse areas of research, ranging from systematics to ecology, and from genetics to molecular biology. Despite this unity, few plant species offer the experimental versatility to encompass all levels of biological investigation in a single system. An exception is the morning glory genus Ipomoea, in which a broad picture of floral evolution, ranging from ecology to molecular biology, is emerging.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Correns, C. Mendel's law concerning the behavior of progeny of varietal hybrids. Berichte der Deutschen Botanischen Gesellschaft 18, 158–168 (1900).
de Vries, H. Concerning the law of segregation of hybrids. Comptes Rendus de l'Academie des Sciences 130, 845–847 (1900).
Watson, J. D. & Crick, F. C. Genetical implications of the structure of deoxyribonucleic acid. Nature 171, 964 (1953).
Daborn, P. J. et al. A single P450 allele associated with insecticide resistance in Drosophila. Science 297, 2253–2256 (2002).
Bradshaw, A. D. & McNeilly, T. Evolution and Pollution (Arnold, London, 1981).
Steinberg, M. H. Disorders of Hemoglobin (Cambridge Univ. Press, UK, 2001).
Watt, W. Adaptation, Constraint, and Neutrality: Mechanistic Case Studies with Butterflies and their General Implications (Cambridge Univ. Press, Cambridge, in the press).
Yokoyama, S. Molecular evolution of vertebrate visual pigments. Prog. Retin. Eye Res. 19, 385–419 (2000).
Doebley, J. & Lukens, L. Transcriptional regulators and the evolution of plant form. Plant Cell 10, 1075–1082 (1998).
Wang, R. L., Stec, J., Hey, L., Lukens, L. & Doebley, J. The limits of selection during maize domestication. Nature 398, 236–239 (1999).
Clark, A. Limits to Prediction of Phenotypes from Knowledge of Genotypes 205–222 (Plenum, New York, 2000).
Clegg, M. T. & Durbin, M. L. Flower color variation: a model for the experimental study of evolution. Proc. Natl Acad. Sci. USA 97, 7016–7023 (2000). A summary of genetic and molecular studies on flower colour evolution in Ipomoea . This paper makes a case for why flower colour is a good model for the study of evolution and phenotypic adaptation.
Beldade, P. & Brakefield, P. M. The genetics and evo–devo of butterfly wing patterns. Nature Rev. Genet. 3, 442–452 (2002).
Austin, D. F. & Huaman, Z. A synopsis of Ipomoea (Convolvulaceae) in the Americas. Taxon 45, 3–38 (1996).
McDonald, J. A. & Mabry, T. J. Phylogenetic systematics of new-world Ipomoea (Convolvulaceae) based on chloroplast DNA restriction site variation. Plant Syst. Evol. 180, 243–259 (1992).
Manos, P. S., Miller, R. E. & Wilkin, P. Phylogenetic analysis of Ipomoea, Argyreia, Stictocardia, and Turbina suggests a generalized model of morphological evolution in morning glories. Syst. Bot. 26, 585–602 (2001).
Miller, R. E., Rausher, M. D. & Manos, P. S. Phylogenetic systematics of Ipomoea (Convolvulaceae) based on ITS and waxy sequences. Syst. Bot. 24, 209–227 (1999). A thorough study using sequence data from both ribosomal internal transcribed spacer (ITS) and a single-copy gene (Waxy) to elucidate the phylogenetics of Ipomoea . Work by this group should lead to a better understanding of the relationships in the genus Ipomoea.
Iida, S., Hoshino, A., Johzuka-Hisatomi, Y., Habu, Y. & Inagaki, Y. in Molecular Strategies in Biological Evolution Vol. 870 265–274 (Annals of the New York Academy of Sciences, New York, 1999). A summary of what is known about transposable elements in two species of Ipomoea and the role of such elements in generating new phenotypic traits.
Imai, Y. The genetics of Pharbitis purpurea. J. Coll. Agric. Imperial Univ. Tokyo 9, 119–122 (1927).
Imai, Y. Analysis of flower colour in Pharbitis nil. J. Genet. 24, 203–224 (1931).
Imai, Y. Linkage studies in Pharbitis nil. Genetica 16, 467–475 (1934).
Barker, E. E. Heredity studies in the morning glory (Ipomoea purpurea L. Roth.) Bull. Cornell Univ. Agric. Experiment Station 392, 121–154 (1917).
Glover, D. E., Durbin, M. L., Huttley, G. A. & Clegg, M. T. Genetic diversity in the common morning glory. Plant Spec. Biol. 11, 41–50 (1996).
Brown, B. A. & Clegg, M. T. Influence of flower color polymorphism on genetic transmission in a natural population of the common morning glory, Ipomoea purpurea. Evolution 38, 796–803 (1984).
Ennos, R. A. & Clegg, M. T. Flower color variation in the morning glory, Ipomoea purpurea. J. Hered. 74, 247–250 (1983).
Schoen, D. J. & Clegg, M. T. The influence of flower color on outcrossing rate and male reproductive success in Ipomoea purpurea. Evolution 39, 1242–1249 (1985).
Epperson, B. K. & Clegg, M. T. Frequency-dependent variation for outcrossing rate among flower-color morphs of Ipomoea purpurea. Evolution 41, 1302–1311 (1987).
Rausher, M. D. & Fry, J. D. Effects of a locus affecting floral pigmentation in Ipomoea purpurea on female fitness components. Genetics 134, 1237–1247 (1993).
Fisher, R. A. Average excess and average effect of a gene substitution. Ann. Eugen. 11, 53–63 (1941).
Holsinger, K. E. Pollination Biology and the Evolution of Mating Systems in Flowering Plants 107–149 (Plenum, New York, 1996).
Rausher, M. D., Augustine, D. & Vanderkooi, A. Absence of pollen discounting in a genotype of Ipomoea purpurea exhibiting increased selfing. Evolution 47, 1688–1695 (1993).
Chang, S. M. & Rausher, M. D. The role of inbreeding depression in maintaining the mixed mating system of the common morning glory, Ipomoea purpurea. Evolution 53, 1366–1376 (1999).
Mojonnier, L. E. & Rausher, M. D. A floral color polymorphism in the common morning glory (Ipomoea purpurea): the effects of overdominance in seed size. Evolution 51, 608–614 (1997).
Durbin, M. L., McCaig, B. & Clegg, M. T. Molecular evolution of the chalcone synthase multigene family in the morning glory genome. Plant Mol. Biol. 42, 79–92 (2000).
Inagaki, Y. et al. Genomic organization of the genes encoding dihydroflavonol 4- reductase for flower pigmentation in the Japanese and common morning glories. Gene 226, 181–188 (1999).
Durbin, M. L., Lundy, K. E., Morrell, P. E., Torres–Martinez, C. L. & Clegg, M. T. Genes that determine flower color: the role of regulatory changes in the evolution of phenotypic adaptations. Mol. Phyl. Evol. (2003).
Quattrocchio, F., Wing, J. F., van der Woude, K., Mol, J. N. M. & Koes, R. Analysis of bHLH and MYB domain proteins: species-specific regulatory differences are caused by divergent evolution of target anthocyanin genes. Plant J. 13, 475–488 (1998).
Quattrocchio, F. et al. Molecular analysis of the anthocyanin2 gene of petunia and its role in the evolution of flower color. Plant Cell 11, 1433–1444 (1999).
Spelt, C., Quattrocchio, F., Mol, J. N. M. & Koes, R. anthocyanin1 of petunia encodes a basic helix-loop-helix protein that directly activates transcription of structural anthocyanin genes. Plant Cell 12, 1619–1631 (2000).
Spelt, C., Quattrocchio, F., Mol, J. & Koes, R. ANTHOCYANIN1 of petunia controls pigment synthesis, vacuolar pH, and seed coat development by genetically distinct mechanisms. Plant Cell 14, 2121–2135 (2002).
Mol, J., Grotewold, E. & Koes, R. How genes paint flowers and seeds. Trends Plant Sci. 3, 212–217 (1998). A synopsis of the work on regulation of the flavonoid pathway in several diverse species. The authors do an excellent job of comparing and contrasting what is known about regulation of the flavonoid pathway with a goal of identifying common mechanisms.
Koes, R. E., Spelt, C. E., Mol, J. N. M. & Gerats, A. G. M. The chalcone synthase multigene family of Petunia hybrida (V30): sequence homology, chromosomal localization and evolutionary aspects. Plant Mol. Biol. 10, 159–169 (1987).
Stafford, H. A. Flavonoid evolution: an enzymatic approach. Plant Physiol. 96, 680–685 (1991).
Koes, R., Quattrocchio, F. & Mol, J. The flavonoid biosynthetic pathway in plants: function and evolution. BioEssays 16, 123–132 (1993).
Kidwell, M. G. & Lisch, D. R. Perspective: transposable elements, parasitic DNA, and genome evolution. Evolution 55, 1–24 (2001). A thorough analysis of the role of transposable elements in the evolution of genomes and the creation of new traits.
McClintock, B. The origin and behavior of mutable loci in maize. Proc. Natl Acad. Sci. USA 36, 344–355 (1950).
Habu, Y., Hisatomi, Y. & Iida, S. Molecular characterization of the mutable flaked allele for flower variegation in the common morning glory. Plant J. 16, 371–376 (1998).
Durbin, M. L., Denton, A. L. & Clegg, M. T. Dynamics of mobile element activity in chalcone synthase loci in the common morning glory (Ipomoea purpurea). Proc. Natl Acad. Sci. USA 98, 5084–5089 (2001).
Hoshino, A., Johzuka-Hisatomi, Y. & Iida, S. Gene duplication and mobile genetic elements in the morning glories. Gene 265, 1–10 (2001).
Schemske, D. W. & Bradshaw, H. D. Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus). Proc. Natl Acad. Sci. USA 96, 11910–11915 (1999). The work in Mimulus provides further evidence of how pollinator preference can influence phenotype and the evolution of the genus.
Barrier, M., Robichaux, R. H. & Purugganan, M. D. Accelerated regulatory gene evolution in an adaptive radiation. Proc. Natl Acad. Sci. USA 98, 10208–10213 (2001). This work shows that rapid evolution in regulatory genes is correlated with adaptive evolution.
Glover, B. J. & Martin, C. The role of petal cell shape and pigmentation in pollination success in Antirrhinum majus. Heredity 80, 778–784 (1998).
Comba, L. et al. The role of genes influencing the corolla in pollination of Antirrhinum majus. Plant Cell Environ. 23, 639–647 (2000).
Martin, C., Prescott, A., Mackay, S., Bartlett, J. & Vrijlandt, E. Control of anthocyanin biosynthesis in flowers of Antirrhinum majus. Plant J. 1, 37–49 (1991).
Epperson, B. K. & Clegg, M. T. Genetics of flower color polymorphism in the common morning glory (Ipomoea purpurea). J. Hered. 79, 64–68 (1988).
Epperson, B. K. & Clegg, M. T. Unstable white flower color genes and their derivatives in the morning glory. J. Hered. 83, 405–409 (1992).
Saitou, N. & Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987).
Tropf, S., Lanz, T., Rensing, S. A., Schroder, J. & Schroder, G. Evidence that stilbene synthases have developed from chalcone synthases several times in the course of evolution. J. Mol. Evol. 38, 610–618 (1994).
Tropf, S., Karcher, B., Schroder, G. & Schroder, J. Reaction-mechanisms of homodimeric plant polyketide synthases (stilbene and chalcone synthase): a single active-site for the condensing reaction is sufficient for synthesis of stilbenes, chalcones, and 6′-deoxychalcones. J. Biol. Chem. 270, 7922–7928 (1995).
Johzuka-Hisatomi, Y., Hoshino, A., Mori, T., Habu, Y. & Iida, S. Characterization of the chalcone synthase genes expressed in flowers of the common and Japanese morning glories. Genes Genet. Sys. 74, 141–147 (1999).
Acknowledgements
This research was partially supported by a grant from the Alfred P. Sloan Foundation.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- FLORAL TUBE
-
The tube-like, proximal-most region of Ipomoea petals.
- FLORAL LIMB
-
The expanded, distal-most region of Ipomoea petals.
- ANTHOCYANIN
-
Soluble glycoside pigments that produce the blue-to-red colour in flowers and other plant tissues.
- MATING SYSTEM MODIFIER LOCI
-
Genes that alter the system of mating and the statistical rules of genetic transmission in populations. An example is a gene that increases the frequency of self-fertilization.
- POLLEN DISCOUNTING
-
Situation in which the transmission of genes through pollen is reduced below a random expectation.
- OVERDOMINANCE
-
Phenotypic expression is greater in the heterozygote than in either homozygote. This can result in an increased fitness of the heterozygote and lead to the maintenance of both alleles in the population.
- PLEIOTROPIC
-
Responsible for several distinct and seemingly unrelated phenotypic effects.
- FLAVONOID BIOSYNTHESIS
-
The production of a group of aromatic compounds that includes many common pigments such as flavones and anthocyanins.
- TRANSPOSABLE ELEMENT
-
A segment of genetic material that is capable of changing its location in the genome of an organism.
Rights and permissions
About this article
Cite this article
Clegg, M., Durbin, M. Tracing floral adaptations from ecology to molecules. Nat Rev Genet 4, 206–215 (2003). https://doi.org/10.1038/nrg1023
Issue Date:
DOI: https://doi.org/10.1038/nrg1023
This article is cited by
-
Association of flower color with pollen reward may explain increased bumblebee visitation to the Scotch broom yellow morph
Plant Ecology (2021)
-
A R2R3-MYB gene-based marker for the non-darkening seed coat trait in pinto and cranberry beans (Phaseolus vulgaris L.) derived from ‘Wit-rood boontje’
Theoretical and Applied Genetics (2020)
-
A MITE Insertion in the Promoter Region of Anthocyanidin Synthase from Morus alba L.
Plant Molecular Biology Reporter (2018)
-
Genome sequence and analysis of the Japanese morning glory Ipomoea nil
Nature Communications (2016)
-
How important are transposons for plant evolution?
Nature Reviews Genetics (2013)