Key Points
-
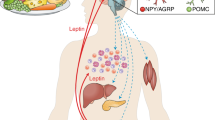
The regulation of body weight involves a homeostatic control system in which hormones that circulate in proportion to body fat stores enter the brain, where they act to reduce food intake and increase energy expenditure. Conditions that cause weight loss reduce the level of these hormones, triggering adaptive increases in hunger and energy efficiency that are sustained until lost weight is regained.
-
Three complementary approaches have been used to advance our understanding of the basic biology of this control system:
-
Forward genetics has helped to identify key molecules in normal and abnormal energy homeostasis.
-
Reverse genetics has helped to clarify the function of these molecules in normal and abnormal energy homeostasis.
-
Physiological studies have generated models for investigating perception and integration of these molecular signals in discrete neuronal pathways.
-
The energy homeostasis system is composed of:
-
Hormones, such as leptin and insulin, that communicate information related to body fat stores to the brain and that coordinate long-term shifts in energy balance to promote weight stability. Other hormones, such as ghrelin and cholecystokinin, facilitate the onset and termination of individual meals.
-
Brain areas, such as the hypothalamic arcuate nucleus, which transduces hormonal input into neurobiological responses that ultimately alter food intake, energy expenditure, endocrine function and glucose metabolism, and such as the lateral hypothalamic area, which receives input from arcuate nucleus neurons and is vital for its transduction into a feeding response. Other areas that are involved in energy homeostasis include the caudate, putamen and nucleus accumbens, which process dopaminergic signals involved in the motivation to eat, and the nucleus of the solitary tract, a hindbrain area that is crucial for processing afferent, meal-related signals that are involved in the perception of satiety.
-
Various genetic and environmental factors can disrupt the function of this homeostatic system and thereby cause obesity. Clarifying the underlying mechanisms is crucial to the development of more effective therapeutic interventions.
Abstract
The homeostatic regulation of adiposity is a physiological concept that originated nearly 70 years ago, the genetic foundations of which have just begun to emerge. A key element of this concept is the existence of one or more humoral mediators, which circulate at levels that reflect body fat stores and which signal through neuronal receptors to elicit appropriate behavioural and metabolic responses over short time periods. Initial insights into adiposity regulation came from the positional cloning of mouse obesity mutations, but the field is now poised to address specific physiological questions using more sophisticated genetic approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hetherington, A. W. & Ranson, S. W. Hypothalamic lesions and adiposity in the rat. Anat. Rec. 78, 149–172 (1940).
Elmquist, J. K., Elias, C. F. & Saper, C. B. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22, 221–232 (1999).
Lorden, J. F. & Caudle, A. Behavioral and endocrinological effects of single injections of monosodium glutamate in the mouse. Neurobehav. Toxicol. Teratol. 8, 509–519 (1986).
Schwartz, M. W., Woods, S. C., Porte, D. Jr, Seeley, R. J. & Baskin, D. G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Tatemoto, K., Carlquist, M. & Mutt, V. Neuropeptide Y — a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 296, 659–660 (1982).
Clark, J. T., Kalra, P. S., Crowley, W. R. & Kalra, S. P. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115, 427–429 (1984).
Woods, S. C., Seeley, R. J., Porte, D. Jr & Schwartz, M. W. Signals that regulate food intake and energy homeostasis. Science 280, 1378–1383 (1998).
Baskin, D. G., Breininger, J. F. & Schwartz, M. W. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes 48, 828–833 (1999).
Sahu, A., Sninsky, C. A., Kalra, P. S. & Kalra, S. P. Neuropeptide-Y concentration in microdissected hypothalamic regions and in vitro release from the medial basal hypothalamus–preoptic area of streptozotocin-diabetic rats with and without insulin substitution therapy. Endocrinology 126, 192–198 (1990).
Wilding, J. P. et al. Increased neuropeptide-Y messenger ribonucleic acid (mRNA) and decreased neurotensin mRNA in the hypothalamus of the obese (ob/ob) mouse. Endocrinology 132, 1939–1944 (1993).
Ostlund, R. E. Jr, Yang, J. W., Klein, S. & Gingerich, R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J. Clin. Endocrinol. Metab. 81, 3909–3913 (1996).
Rosenbaum, M. et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J. Clin. Endocrinol. Metab. 81, 3424–3427 (1996).
McGregor, G. P. et al. Radiommunological measurement of leptin in plasma of obese and diabetic human subjects. Endocrinology 137, 1501–1504 (1996).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Ahima, R. S. et al. Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252 (1996).Fasting induces various neuroendocrine responses. By administering leptin to starving mice, the authors showed that these responses arise partly owing to leptin deficiency.
Stephens, T. W. et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377, 530–532 (1995).
Erickson, J. C., Clegg, K. E. & Palmiter, R. D. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature 381, 415–421 (1996).Mice that lack Npy have normal daily food intake and intact feeding responses to food deprivation and to leptin administration, findings that challenge the importance of this peptide in feeding.
Bannon, A. W. et al. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 868, 79–87 (2000).
Palmiter, R. D., Erickson, J. C., Hollopeter, G., Baraban, S. C. & Schwartz, M. W. Life without neuropeptide Y. Recent Prog. Horm. Res. 53, 163–199 (1998).
Lewandoski, M. Conditional control of gene expression in the mouse. Nature Rev. Genet. 2, 743–755 (2001).
Sindelar, D. K., Mystkowski, P., Marsh, D. J., Palmiter, R. D. & Schwartz, M. W. Attenuation of diabetic hyperphagia in neuropeptide Y-deficient mice. Diabetes 51, 778–783 (2002).
Marsh, D. J. et al. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nature Genet. 21, 119–122 (1999).
Hollopeter, G., Erickson, J. C., Seeley, R. J., Marsh, D. J. & Palmiter, R. D. Response of neuropeptide Y-deficient mice to feeding effectors. Regul. Pept. 75–76, 383–389 (1998).
Eberle, A. N. The Melanotropins. Chemistry, Physiology and Mechanism of Action (Karger, Basel, Switzerland, 1988).
Castle, W. E. & Little, C. C. On a modified Mendelian ratio among yellow mice. Science 32, 868–870 (1910).
Barsh, G. S., Ollmann, M. M., Wilson, B. D., Miller, K. A. & Gunn, T. M. Molecular pharmacology of Agouti protein in vitro and in vivo. Ann. NY Acad. Sci. 885, 143–152 (1999).
Ollmann, M. M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–138 (1997).Reports the cloning of Agrp and that it acts as an antagonist of neuronal melanocortin receptors (as does agouti). Mice that overexpress agouti or Agrp were also created. They both developed obesity, but only agouti mutants had a yellow coat colour.
Shutter, J. R. et al. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 11, 593–602 (1997).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).Mice that lack Mc4r develop a hyperphagic obesity syndrome similar to that of agouti lethal yellow mice, but without coat colour effects. Heterozygous mice have an intermediate phenotype, which suggests that the loss of one Mc4r allele is sufficient to affect energy homeostasis.
Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997).
Farooqi, I. S. et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Invest. 106, 271–279 (2000).
Vaisse, C. et al. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J. Clin. Invest. 106, 253–262 (2000).
Hahn, T. M., Breininger, J. F., Baskin, D. G. & Schwartz, M. W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature Neurosci. 1, 271–272 (1998).Npy and Agrp are expressed in the same neurons, and fasting induces expression of both peptides in these cells. As Agrp is not produced elsewhere in the brain, these results identified Npy/Agrp neurons as a unique neuronal cell type.
Kristensen, P. et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393, 72–76 (1998).
Seeley, R. J. et al. Behavioral, endocrine, and hypothalamic responses to involuntary overfeeding. Am. J. Physiol. 271, R819–R823 (1996).
Schwartz, M. W. et al. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46, 2119–2123 (1997).
Hagan, M. M. et al. Role of the CNS melanocortin system in the response to overfeeding. J. Neurosci. 19, 2362–2367 (1999).
Hara, J. et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30, 345–354 (2001).
Bagnol, D. et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J. Neurosci. 19, RC26 (1999).
Cowley, M. A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).By creating mice that express GFP from the Pomc promoter, arcuate nucleus Pomc cells were identified in hippocampal brain slices and studied electrophysiologically. This work showed that leptin increases the firing rate of these neurons.
Mountjoy, K. G., Mortrud, M. T., Low, M. J., Simerly, R. B. & Cone, R. D. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endocrinol. 8, 1298–1308 (1994).
Butler, A. A. et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141, 3518–3521 (2000).
Chen, A. S. et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nature Genet. 26, 97–102 (2000).
Shimada, M., Tritos, N. A., Lowell, B. B., Flier, J. S. & Maratos-Flier, E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 396, 670–674 (1998).
Legradi, G., Emerson, C. H., Ahima, R. S., Flier, J. S. & Lechan, R. M. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology 138, 2569–2576 (1997).
Legradi, G. et al. Arcuate nucleus ablation prevents fasting-induced suppression of ProTRH mRNA in the hypothalamic paraventricular nucleus. Neuroendocrinology 68, 89–97 (1998).
Fekete, C. et al. Association of cocaine- and amphetamine-regulated transcript-immunoreactive elements with thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and its role in the regulation of the hypothalamic–pituitary–thyroid axis during fasting. J. Neurosci. 20, 9224–9234 (2000).
Fekete, C. et al. α-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J. Neurosci. 20, 1550–1558 (2000).
Legradi, G. & Lechan, R. M. Agouti-related protein containing nerve terminals innervate thyrotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology 140, 3643–3652 (1999).
Nillni, E. A. et al. Leptin regulates prothyrotropin-releasing hormone biosynthesis. Evidence for direct and indirect pathways. J. Biol. Chem. 275, 36124–36133 (2000).
Kobayashi, H. et al. A novel homozygous missense mutation of melanocortin-4 receptor (MC4R) in a Japanese woman with severe obesity. Diabetes 51, 243–246 (2002).
Clement, K. et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392, 398–401 (1998).
Montague, C. T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387, 903–908 (1997).
Harris, M. et al. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J. Clin. Invest. 107, 111–120 (2001).
Szczypka, M. S. et al. Viral gene delivery selectively restores feeding and prevents lethality of dopamine-deficient mice. Neuron 22, 167–178 (1999).
Szczypka, M. S. et al. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron 30, 819–828 (2001).Brain regions that are responsible for the phenotype of dopamine-deficient mice were investigated using a viral gene-therapy approach. Production of dopamine in the caudate–putamen of these animals restored feeding responses, which are markedly impaired by dopamine deficiency.
Drago, J. et al. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc. Natl Acad. Sci. USA 91, 12564–12568 (1994).
Accili, D. et al. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc. Natl Acad. Sci. USA 93, 1945–1949 (1996).
Rubinstein, M. et al. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell 90, 991–1001 (1997).
Xu, M. et al. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell 79, 729–742 (1994).
Baik, J. H. et al. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377, 424–428 (1995).
Zhou, Q. Y. & Palmiter, R. D. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83, 1197–1209 (1995).
Szczypka, M. S. et al. Feeding behavior in dopamine-deficient mice. Proc. Natl Acad. Sci. USA 96, 12138–12143 (1999).
Berridge, K. C. & Robinson, T. E. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 28, 309–369 (1998).
Matsui, M. et al. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc. Natl Acad. Sci. USA 97, 9579–9584 (2000).
Yamada, M. et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature 410, 207–212 (2001).
Woods, S. C., Lotter, E. C., McKay, L. D. & Porte, D. Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282, 503–505 (1979).
Bruning, J. C. et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 (2000).
Marks, J. L., Porte, D. Jr, Stahl, W. L. & Baskin, D. G. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology 127, 3234–3236 (1990).
Wilcox, B. J. et al. Insulin binding in the hypothalamus of lean and genetically obese Zucker rats. Peptides 10, 1159–1164 (1989).
Schwartz, M. W. et al. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology 130, 3608–3616 (1992).
Friedman, J. M. & Halaas, J. L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998).
Tartaglia, L. A. The leptin receptor. J. Biol. Chem. 272, 6093–6096 (1997).
Cheatham, B. & Kahn, C. R. Insulin action and the insulin signaling network. Endocr. Rev. 16, 117–142 (1995).
Spanswick, D., Smith, M. A., Groppi, V. E., Logan, S. D. & Ashford, M. L. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390, 521–525 (1997).
Spanswick, D., Smith, M. A., Mirshamsi, S., Routh, V. H. & Ashford, M. L. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nature Neurosci. 3, 757–758 (2000).In electrophysiological recordings in rat hypothalamic slices, insulin was shown to hyperpolarize and inhibit firing of glucose-responsive neurons in normal rats, but not in obese rats with a mutant leptin receptor.
Niswender, K. D. et al. Key enzyme in leptin-induced anorexia. Nature 413, 794–795 (2001).This paper reports that leptin activates PI3K in the rat hypothalamus, and that pre-treatment with PI3K inhibitors blocks the anorexic action of leptin. This implicates hypothalamic signalling through PI3K as a key mediator of leptin action in vivo and indicates that insulin and leptin might signal through converging mechanisms in arcuate nucleus neurons.
Kahn, B. B. & Flier, J. S. Obesity and insulin resistance. J. Clin. Invest. 106, 473–481 (2000).
Ahima, R. S., Prabakaran, D. & Flier, J. S. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J. Clin. Invest. 101, 1020–1027 (1998).
Strubbe, J. H., Porte, D. Jr & Woods, S. C. Insulin responses and glucose levels in plasma and cerebrospinal fluid during fasting and refeeding in the rat. Physiol. Behav. 44, 205–208 (1988).
Flynn, M. C., Scott, T. R., Pritchard, T. C. & Plata-Salaman, C. R. Mode of action of OB protein (leptin) on feeding. Am. J. Physiol. 275, R174–R179 (1998).
Leibowitz, S. F. & Alexander, J. T. Analysis of neuropeptide Y-induced feeding: dissociation of Y1 and Y2 receptor effects on natural meal patterns. Peptides 12, 1251–1260 (1991).
Smith, G. P. & Gibbs, J. Satiating effect of cholecystokinin. Ann. NY Acad. Sci. 713, 236–241 (1994).
Nagata, A. et al. G protein-coupled cholecystokinin-B/gastrin receptors are responsible for physiological cell growth of the stomach mucosa in vivo. Proc. Natl Acad. Sci. USA 93, 11825–11830 (1996).
Pommier, B. et al. Deletion of CCK2 receptor in mice results in an upregulation of the endogenous opioid system. J. Neurosci. 22, 2005–2011 (2002).
Kopin, A. S. et al. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J. Clin. Invest. 103, 383–391 (1999).
Miyasaka, K. et al. Energy metabolism and turnover are increased in mice lacking the cholecystokinin-B receptor. J. Nutr. 132, 739–741 (2002).
Dauge, V. & Lena, I. CCK in anxiety and cognitive processes. Neurosci. Biobehav. Rev. 22, 815–825 (1998).
Figlewicz, D. P., Stein, L. J., West, D., Porte, D. Jr & Woods, S. C. Intracisternal insulin alters sensitivity to CCK-induced meal suppression in baboons. Am. J. Physiol. 250, R856–R860 (1986).
Matson, C. A. & Ritter, R. C. Long-term CCK–leptin synergy suggests a role for CCK in the regulation of body weight. Am. J. Physiol. 276, R1038–R1045 (1999).
McMinn, J. E., Sindelar, D. K., Havel, P. J. & Schwartz, M. W. Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology 141, 4442–4448 (2000).
Grill, H. J. et al. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143, 239–246 (2002).
Grill, H. J. & Smith, G. P. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. Am. J. Physiol. 254, R853–R856 (1988).
Grill, H. J. & Kaplan, J. M. Interoceptive and integrative contributions of forebrain and brainstem to energy balance control. Int. J. Obes. Relat. Metab. Disord. 25 (Suppl. 5), S73–S77 (2001).
Kojima, M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 (1999).
Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001).Ghrelin was shown to potently stimulate food intake after icv infusion through a mechanism that involves the activation of arcuate nucleus Agrp/Npy neurons, indicating that ghrelin is a physiological stimulator of feeding that acts through a mechanism opposite to that of insulin and leptin, which inhibit Agrp/Npy neurons.
Cummings, D. E. et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50, 1714–1719 (2001).
Wren, A. M. et al. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 86, 5992 (2001).
de Bono, M. & Bargmann, C. I. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell 94, 679–689 (1998).
Kimura, K. D., Tissenbaum, H. A., Liu, Y. & Ruvkun, G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942–946 (1997).
Tissenbaum, H. A. & Ruvkun, G. An insulin-like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans. Genetics 148, 703–717 (1998).
Rulifson, E., Kim, S. K. & Nusse, R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–1120 (2002).
Acknowledgements
We thank members of our laboratories for helpful advice and discussion, and M. Baskin for help in preparing the figures. G.S.B. is an Associate Investigator of the Howard Hughes Medical Institute; G.S.B. and M.W.S. are supported by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Jax
Locuslink
OMIM
FURTHER INFORMATION
Glossary
- FORWARD GENETICS
-
A genetic analysis that proceeds from phenotype to genotype by positional cloning or candidate-gene analysis.
- REVERSE GENETICS
-
Genetic analysis that proceeds from genotype to phenotype through gene-manipulation techniques.
- ARCUATE NUCLEUS
-
A region of the hypothalamus that lies at its most ventral portion and surrounds the third ventricle.
- LATERAL HYPOTHALAMIC AREA
-
(LHA). Hypothalamic region, adjacent to the perifornical area, that produces melanin concentrating hormone and hypocretin/orexin. Lesions of the LHA cause decreased feeding.
- NEUROPEPTIDES
-
Secreted peptides produced by neurons in the brain or spinal cord that are used for cell–cell communication, often as neurotransmitters.
- PARACRINE
-
A form of cell–cell communication that depends on a secreted substance that acts over a short distance and does not enter the circulation.
- INVERSE AGONIST
-
A ligand for a G-protein-coupled receptor that, on receptor binding, decreases the affinity of the ligand–receptor complex for the Gα protein subunit, thereby decreasing receptor activity.
- HYPERPHAGIA
-
Increased feeding.
- ANORECTIC
-
The quality of inhibiting food intake.
- OREXIGENIC
-
The quality of stimulating food intake.
- AFFERENT NEURON
-
A neuron that provides input, usually from axonal projections, to other neurons or brain regions.
- SYMPATHETIC NERVOUS SYSTEM
-
The division of the autonomic nervous system that stimulates heart rate, blood pressure and heat production.
- THERMOGENESIS
-
The production of heat that occurs when the body burns calories, such as during exercise.
- GONADOTROPHIC AXIS
-
The system whereby the hypothalamus causes release of hormones from the pituitary gland that control reproductive function.
- SOMATOTROPHIC AXIS
-
The system by which the hypothalamus causes release of growth hormone from the pituitary gland.
- ADRENAL GLAND
-
An organ above the kidney that synthesizes certain steroid hormones and releases adrenaline. The portion of the adrenal gland that synthesizes cortisol (corticosterone in rodents) is stimulated by adrenocorticotrophic hormone released from the pituitary gland.
- THYROXINE
-
The main circulating hormone produced by the thyroid gland that stimulates metabolic rate.
- CRE–LOX P
-
A site-specific recombination system. Two short DNA sequences (lox sites) are engineered to flank the target DNA. Activation of the Cre-recombinase enzyme catalyses recombination between the lox sites, leading to excision of the intervening sequence.
- STRIATUM
-
The portion of the basal ganglia that includes the caudate nucleus, putamen and nucleus accumbens.
- BASAL GANGLIA
-
A group of interconnected nuclei in the forebrain and midbrain that includes the striatum (putamen and caudate nucleus), globus pallidus, subthalamic nucleus, ventral tegmental area and substantia nigra.
- DOPAMINE
-
An important neurotransmitter produced by the substantia nigra.
- SUBSTANTIA NIGRA
-
A region of the ventral midbrain that contains pigment and sends afferent dopamine-releasing neurons to the striatum.
- CATECHOLAMINE
-
A neurotransmitter that is derived from tyrosine, such as dopamine, adrenaline or noradrenaline.
- APHAGIA
-
Failure to eat.
- VENTRAL TEGMENTAL AREA
-
A region of the ventral midbrain that sends afferent dopamine-releasing neurons to the nucleus accumbens.
- TRANSNEURONAL TRACING
-
An experimental approach for mapping neuronal circuits.
- HYPERPOLARIZATION
-
A decrease in the electrical potential across the plasma membrane from its resting value, usually associated with reduced neuronal firing.
- DAUER
-
Juvenile nematode in which development is arrested during unsuitable conditions and resumes when conditions improve.
- TONIC SUPPRESSION
-
The steady-state inhibition of a process that is regulated physiologically by disinhibition.
Rights and permissions
About this article
Cite this article
Barsh, G., Schwartz, M. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet 3, 589–600 (2002). https://doi.org/10.1038/nrg862
Issue Date:
DOI: https://doi.org/10.1038/nrg862
This article is cited by
-
Obesity treatment effect in Danish children and adolescents carrying Melanocortin-4 Receptor mutations
International Journal of Obesity (2021)
-
Concurrent changes in photoperiod-induced seasonal phenotypes and hypothalamic CART peptide-containing systems in night-migratory redheaded buntings
Brain Structure and Function (2020)
-
Gpr17 deficiency in POMC neurons ameliorates the metabolic derangements caused by long-term high-fat diet feeding
Nutrition & Diabetes (2019)
-
Seasonal consumption of polyphenol-rich fruits affects the hypothalamic leptin signaling system in a photoperiod-dependent mode
Scientific Reports (2018)
-
Polymorphisms in Genes Involved in the Leptin-Melanocortin Pathway are Associated with Obesity-Related Cardiometabolic Alterations in a Southern Chilean Population
Molecular Diagnosis & Therapy (2018)