Key Points
-
Improvements in DNA-editing technologies have enabled engineering of microorganisms at the gene, network and genome level, helping to elucidate causal links between genotypes and phenotypes, and facilitating the targeted exploration of complex phenotype landscapes.
-
DNA sequencing and genome engineering are synergistic technologies: understanding genetic and phenotypic diversity establishes a platform for pursuing many goals in biological design.
-
Multiplex genome modification techniques have enabled combinatoric genomic variations across populations, exploring large numbers of genotype–phenotype landscapes in living cells.
-
The combination of DNA synthesis and in vivo genome editing establishes the ability to fundamentally redesign systems from refactored pathways to whole-genome recoding.
-
The future of genome editing will require the combination of de novo synthesis and in vivo genome evolution integrated with functional screens and selections to isolate variants exhibiting a desired phenotype.
Abstract
Next-generation DNA sequencing has revealed the complete genome sequences of numerous organisms, establishing a fundamental and growing understanding of genetic variation and phenotypic diversity. Engineering at the gene, network and whole-genome scale aims to introduce targeted genetic changes both to explore emergent phenotypes and to introduce new functionalities. Expansion of these approaches into massively parallel platforms establishes the ability to generate targeted genome modifications, elucidating causal links between genotype and phenotype, as well as the ability to design and reprogramme organisms. In this Review, we explore techniques and applications in genome engineering, outlining key advances and defining challenges.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Johnson, A. The Hidden Writer (Random House, 1997).
Sanger, F., Nicklen, S. & Coulson, A. R. DNA sequencing with chain-terminating inhibitors. Proc. Natl Acad. Sci. USA 74, 5463–5467 (1977).
Loenen, W. A., Dryden, D. T., Raleigh, E. A., Wilson, G. G. & Murray, N. E. Highlights of the DNA cutters: a short history of the restriction enzymes. Nucleic Acids Res. 42, 3–19 (2014).
Simon, R., Priefer, U. & Puhler, A. A. Broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat. Biotechnol. 1, 784–791 (1983).
Renda, B. A., Hammerling, M. J. & Barrick, J. E. Engineering reduced evolutionary potential for synthetic biology. Mol. Biosyst. 10, 1668–1678 (2014).
Pal, C., Papp, B. & Posfai, G. The dawn of evolutionary genome engineering. Nat. Rev. Genet. 15, 504–512 (2014).
Desai, M. M., Fisher, D. S. & Murray, A. W. The speed of evolution and maintenance of variation in asexual populations. Curr. Biol. 17, 385–394 (2007).
Silver, P. A., Way, J. C., Arnold, F. H. & Meyerowitz, J. T. Synthetic biology: engineering explored. Nature 509, 166–167 (2014).
Neylon, C. Chemical and biochemical strategies for the randomization of protein encoding DNA sequences: library construction methods for directed evolution. Nucleic Acids Res. 32, 1448–1459 (2004).
Bunge, J., Willis, A. & Walsh, F. Estimating the number of species in microbial diversity studies. Annu. Rev. Stat. Appl. 1, 427–445 (2014).
Pace, N. R. A molecular view of microbial diversity and the biosphere. Science 276, 734–740 (1997).
Stemmer, W. P. Rapid evolution of a protein in vitro by DNA shuffling. Nature 370, 389–391 (1994).
Jackel, C., Kast, P. & Hilvert, D. Protein design by directed evolution. Annu. Rev. Biophys. 37, 153–173 (2008).
Das, R. & Baker, D. Macromolecular modeling with Rosetta. Annu. Rev. Biochem. 77, 363–382 (2008).
Esvelt, K. M., Carlson, J. C. & Liu, D. R. A system for the continuous directed evolution of biomolecules. Nature 472, 499–503 (2011). This paper describes a mutagenesis and selection system that links a desired function to viral fitness and enables the continuous, directed evolution of proteins.
Fijalkowska, I. J. & Schaaper, R. M. Mutants in the Exo I motif of Escherichia coli dnaQ: defective proofreading and inviability due to error catastrophe. Proc. Natl Acad. Sci. USA 93, 2856–2861 (1996).
Opperman, T., Murli, S., Smith, B. T. & Walker, G. C. A model for a umuDC-dependent prokaryotic DNA damage checkpoint. Proc. Natl Acad. Sci. USA 96, 9218–9223 (1999).
Carlson, J. C., Badran, A. H., Guggiana-Nilo, D. A. & Liu, D. R. Negative selection and stringency modulation in phage-assisted continuous evolution. Nat. Chem. Biol. 10, 216–222 (2014).
Sorensen, S. J., Bailey, M., Hansen, L. H., Kroer, N. & Wuertz, S. Studying plasmid horizontal transfer in situ: a critical review. Nat. Rev. Microbiol. 3, 700–710 (2005).
Bouma, J. E. & Lenski, R. E. Evolution of a bacteria/plasmid association. Nature 335, 351–352 (1988).
Betenbaugh, M. J., Beaty, C. & Dhurjati, P. Effects of plasmid amplification and recombinant gene expression on the growth kinetics of recombinant E. coli. Biotechnol. Bioengineer. 33, 1425–1436 (1989).
Gersbach, C. A. Genome engineering: the next genomic revolution. Nat. Methods 11, 1009–1011 (2014).
Gaj, T., Gersbach, C. A. & Barbas, C. F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405 (2013).
Kim, H. & Kim, J. S. A guide to genome engineering with programmable nucleases. Nat. Rev. Genet. 15, 321–334 (2014).
Miller, J. C. et al. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat. Biotechnol. 25, 778–785 (2007).
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012). This seminal paper uncovers the Cas9 endonuclease guided by dual RNAs for site-specific DNA cleavage and presents the use of a single chimeric RNA for programming.
Mojica, F. J., Diez-Villasenor, C., Garcia-Martinez, J. & Almendros, C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology 155, 733–740 (2009).
Fu, Y. et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 31, 822–826 (2013).
Ran, F. A. et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389 (2013).
Tsai, S. Q. et al. Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat. Biotechnol. 32, 569–576 (2014).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Jinek, M. et al. RNA-programmed genome editing in human cells. eLife 2, e00471 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Bikard, D. et al. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 41, 7429–7437 (2013).
Gilbert, L. A. et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451 (2013).
Citorik, R. J., Mimee, M. & Lu, T. K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 32, 1141–1145 (2014).
Bikard, D. et al. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol. 32, 1146–1150 (2014).
Gantz, V. M. & Bier, E. Genome editing. The mutagenic chain reaction: a method for converting heterozygous to homozygous mutations. Science 348, 442–444 (2015).
DiCarlo, J. E., Chavez, A., Dietz, S. L., Esvelt, K. M. & Church, G. M. RNA-guided gene drives efficiently reversibly bias inheritance wild yeast. BioRxiv http://dx.doi.org/10.1101/013896 (2015).
Ryan, O. W. et al. Selection of chromosomal DNA libraries using a multiplex CRISPR system. eLife 3, e03703 (2014).
Kabadi, A. M., Ousterout, D. G., Hilton, I. B. & Gersbach, C. A. Multiplex CRISPR/Cas9-based genome engineering from a single lentiviral vector. Nucleic Acids Res. 42, e147 (2014).
Konermann, S. et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517, 583–588 (2015).
Costantino, N. & Court, D. L. Enhanced levels of lambda Red-mediated recombinants in mismatch repair mutants. Proc. Natl Acad. Sci. USA 100, 15748–15753 (2003).
Court, D. L., Sawitzke, J. A. & Thomason, L. C. Genetic engineering using homologous recombination. Annu. Rev. Genet. 36, 361–388 (2002).
Binder, S., Siedler, S., Marienhagen, J., Bott, M. & Eggeling, L. Recombineering in Corynebacterium glutamicum combined with optical nanosensors: a general strategy for fast producer strain generation. Nucleic Acids Res. 41, 6360–6369 (2013).
Wang, Y. et al. Bacillus subtilis genome editing using ssDNA with short homology regions. Nucleic Acids Res. 40, e91 (2012).
Sawitzke, J. A. et al. Recombineering: in vivo genetic engineering in E. coli, S. enterica, and beyond. Methods Enzymol. 421, 171–199 (2007).
Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marraffini, L. A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 31, 233–239 (2013).
Farzadfard, F. & Lu, T. K. Genomically encoded analog memory with precise in vivo DNA writing in living cell populations. Science 346, 1256272 (2014). This work describes the generation of target mutations through the in vivo production of ssDNA and co-expression of recombineering machinery. The accumulation of these mutations in the population in response to various input signals acts as a genetic memory device.
Brakmann, S. & Grzeszik, S. An error-prone T7 RNA polymerase mutant generated by directed evolution. ChemBioChem 2, 212–219 (2001).
Bebenek, K., Abbotts, J., Wilson, S. H. & Kunkel, T. A. Error-prone polymerization by HIV-1 reverse transcriptase. Contribution of template-primer misalignment, miscoding, and termination probability to mutational hot spots. J. Biol. Chem. 268, 10324–10334 (1993).
Wang, H. H. et al. Programming cells by multiplex genome engineering and accelerated evolution. Nature 460, 894–898 (2009). This paper describes the use of efficient oligonucleotide-mediated recombineering to create targeted, multi-site diversity across the E. coli genome.
Gallagher, R. R., Li, Z., Lewis, A. O. & Isaacs, F. J. Rapid editing and evolution of bacterial genomes using libraries of synthetic DNA. Nat. Protoc. 9, 2301–2316 (2014).
Gregg, C. J. et al. Rational optimization of tolC as a powerful dual selectable marker for genome engineering. Nucleic Acids Res. 42, 4779–4790 (2014).
Carr, P. A. et al. Enhanced multiplex genome engineering through co-operative oligonucleotide co-selection. Nucleic Acids Res. 40, e132 (2012).
Wang, H. H. et al. Genome-scale promoter engineering by coselection MAGE. Nat. Methods 9, 591–593 (2012).
Bonde, M. T. et al. Direct mutagenesis of thousands of genomic targets using microarray-derived oligonucleotides. ACS Synth. Biol. 4, 17–22 (2015).
Mansell, T. J., Warner, J. R. & Gill, R. T. Trackable multiplex recombineering for gene-trait mapping in E. coli. Methods Mol. Biol. 985, 223–246 (2013).
Warner, J. R., Reeder, P. J., Karimpour-Fard, A., Woodruff, L. B. & Gill, R. T. Rapid profiling of a microbial genome using mixtures of barcoded oligonucleotides. Nat. Biotechnol. 28, 856–862 (2010).
DiCarlo, J. E. et al. Yeast oligo-mediated genome engineering (YOGE). ACS Synth. Biol. 2, 741–749 (2013).
Pines, G., Freed, E. F., Winkler, J. D. & Gill, R. T. Bacterial recombineering: genome engineering via phage-based homologous recombination. ACS Synth. Biol. http://dx.doi.org/10.1021/acssynbio.5b00009 (2015).
Wang, H. H., Xu, G., Vonner, A. J. & Church, G. Modified bases enable high-efficiency oligonucleotide-mediated allelic replacement via mismatch repair evasion. Nucleic Acids Res. 39, 7336–7347 (2011).
Gibson, D. G. et al. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science 319, 1215–1220 (2008).
Kodumal, S. J. et al. Total synthesis of long DNA sequences: synthesis of a contiguous 32-kb polyketide synthase gene cluster. Proc. Natl Acad. Sci. USA 101, 15573–15578 (2004).
Huang, J., Koide, A., Makabe, K. & Koide, S. Design of protein function leaps by directed domain interface evolution. Proc. Natl Acad. Sci. USA 105, 6578–6583 (2008).
Temme, K., Zhao, D. & Voigt, C. A. Refactoring the nitrogen fixation gene cluster from Klebsiella oxytoca. Proc. Natl Acad. Sci. USA 109, 7085–7090 (2012). In this study, the essential genes in a heterologous biosynthetic pathway are identified, removed from their native context, and placed under well-characterized regulatory elements to facilitate more predictable pathway engineering.
Chan, L. Y., Kosuri, S. & Endy, D. Refactoring bacteriophage T7. Mol. Syst. Biol. 1, 2005.0018 (2005).
Smanski, M. J. et al. Functional optimization of gene clusters by combinatorial design and assembly. Nat. Biotechnol. 32, 1241–1249 (2014).
Horwitz, A. A. et al. Efficient multiplexed integration of synergistic alleles and metabolic pathways in yeasts via CRISPR-Cas. Cell Syst. http://dx.doi.org/10.1016/j.cels.2015.02.001 (2015).
Isaacs, F. J. et al. Engineered riboregulators enable post-transcriptional control of gene expression. Nat. Biotechnol. 22, 841–847 (2004).
Isaacs, F. J., Dwyer, D. J. & Collins, J. J. RNA synthetic biology. Nat. Biotechnol. 24, 545–554 (2006).
Na, D. et al. Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nat. Biotechnol. 31, 170–174 (2013).
Bayer, T. S. & Smolke, C. D. Programmable ligand-controlled riboregulators of eukaryotic gene expression. Nat. Biotechnol. 23, 337–343 (2005).
Qi, L. S. & Arkin, A. P. A versatile framework for microbial engineering using synthetic non-coding RNAs. Nat. Rev. Microbiol. 12, 341–354 (2014).
Drinnenberg, I. A. et al. RNAi in budding yeast. Science 326, 544–550 (2009).
Crook, N. C., Schmitz, A. C. & Alper, H. S. Optimization of a yeast RNA interference system for controlling gene expression and enabling rapid metabolic engineering. ACS Synth. Biol. 3, 307–313 (2014).
Hutchison, C. A. et al. Global transposon mutagenesis and a minimal Mycoplasma genome. Science 286, 2165–2169 (1999).
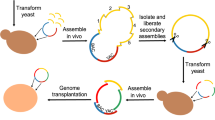
Gibson, D. G. et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329, 52–56 (2010). This work describes the creation of a synthetic genome from synthesized DNA fragments using a combination of in vitro assembly, propagation in E. coli and recombination cloning in S. cerevisiae . The resultant genome was successfully transplanted into a recipient cell to generate a cell run by the synthetic genome.
Bornscheuer, U. T. & Pohl, M. Improved biocatalysts by directed evolution and rational protein design. Curr. Opin. Chem. Biol. 5, 137–143 (2001).
Goodman, D. B., Church, G. M. & Kosuri, S. Causes and effects of N-terminal codon bias in bacterial genes. Science 342, 475–479 (2013).
Posfai, G. et al. Emergent properties of reduced-genome Escherichia coli. Science 312, 1044–1046 (2006). This study uses recombination and selection methods to remove parts of the E. coli genome in a targeted and planned manner, and evaluates the resultant phenotypes of E. coli with reduced genomes.
Lartigue, C. et al. Genome transplantation in bacteria: changing one species to another. Science 317, 632–638 (2007).
Fleishman, S. J. et al. RosettaScripts: a scripting language interface to the Rosetta macromolecular modeling suite. PLoS ONE 6, e20161 (2011).
Bock, A. et al. Selenocysteine: the 21st amino acid. Mol. Microbiol. 5, 515–520 (1991).
Srinivasan, G., James, C. M. & Krzycki, J. A. Pyrrolysine encoded by UAG in Archaea: charging of a UAG-decoding specialized tRNA. Science 296, 1459–1462 (2002).
Ambrogelly, A., Palioura, S. & Soll, D. Natural expansion of the genetic code. Nat. Chem. Biol. 3, 29–35 (2007).
Noren, C. J., Anthony-Cahill, S. J., Griffith, M. C. & Schultz, P. G. A general method for site-specific incorporation of unnatural amino acids into proteins. Science 244, 182–188 (1989).
van Hest, J. C. M., Kiick, K. L. & Tirrell, D. A. Efficient incorporation of unsaturated methionine analogues into proteins in vivo. J. Am. Chem. Soc. 122, 1282–1288 (2000).
Chin, J. W. Expanding and reprogramming the genetic code of cells and animals. Annu. Rev. Biochem. 83, 379–408 (2014).
Liu, C. C. & Schultz, P. G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 79, 413–444 (2010).
Wang, L., Brock, A., Herberich, B. & Schultz, P. G. Expanding the genetic code of Escherichia coli. Science 292, 498–500 (2001). This paper describes the development of an OTS that is capable of site-specifically incorporating a nsAA into proteins in E. coli.
Neumann, H., Wang, K., Davis, L., Garcia-Alai, M. & Chin, J. W. Encoding multiple unnatural amino acids via evolution of a quadruplet-decoding ribosome. Nature 464, 441–444 (2010).
Anderson, J. C. et al. An expanded genetic code with a functional quadruplet codon. Proc. Natl Acad. Sci. USA 101, 7566–7571 (2004).
O'Donoghue, P., Ling, J., Wang, Y. S. & Soll, D. Upgrading protein synthesis for synthetic biology. Nat. Chem. Biol. 9, 594–598 (2013).
Park, H. S. et al. Expanding the genetic code of Escherichia coli with phosphoserine. Science 333, 1151–1154 (2011).
Heinemann, I. U. et al. Enhanced phosphoserine insertion during Escherichia coli protein synthesis via partial UAG codon reassignment and release factor 1 deletion. FEBS Lett. 586, 3716–3722 (2012).
Johnson, D. B. et al. RF1 knockout allows ribosomal incorporation of unnatural amino acids at multiple sites. Nat. Chem. Biol. 7, 779–786 (2011).
Johnson, D. B. et al. Release factor one is nonessential in Escherichia coli. ACS Chem. Biol. 7, 1337–1344 (2012).
Mukai, T. et al. Codon reassignment in the Escherichia coli genetic code. Nucleic Acids Res. 38, 8188–8195 (2010).
Lajoie, M. J. et al. Genomically recoded organisms expand biological functions. Science 342, 357–360 (2013).
Isaacs, F. J. et al. Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science 333, 348–353 (2011). References 101 and 102 describe genome engineering technologies and the construction of a recoded strain of E. coli (that is, a GRO) in which all TAG codons have been converted to TAA.
Annaluru, N. et al. Total synthesis of a functional designer eukaryotic chromosome. Science 344, 55–58 (2014).
Dymond, J. S. et al. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design. Nature 477, 471–476 (2011). This study describes the design and construction of partially synthetic yeast chromosomes that incorporate multiple design features aimed at improving the ease of future evolution and engineering.
Ma, N. J., Moonan, D. W. & Isaacs, F. J. Precise manipulation of bacterial chromosomes by conjugative assembly genome engineering. Nat. Protoc. 9, 2285–2300 (2014).
Lajoie, M. J. et al. Probing the limits of genetic recoding in essential genes. Science 342, 361–363 (2013).
Ivanova, N. N. et al. Stop codon reassignments in the wild. Science 344, 909–913 (2014).
Hammerling, M. J. et al. Bacteriophages use an expanded genetic code on evolutionary paths to higher fitness. Nat. Chem. Biol. 10, 178–180 (2014).
Mandell, D. J. et al. Biocontainment of genetically modified organisms by synthetic protein design. Nature 518, 55–60 (2015).
Rovner, A. J. et al. Recoded organisms engineered to depend on synthetic amino acids. Nature 518, 89–93 (2015).
Umehara, T. et al. N-acetyl lysyl-tRNA synthetases evolved by a CcdB-based selection possess N-acetyl lysine specificity in vitro and in vivo. FEBS Lett. 586, 729–733 (2012).
Ling, J., Reynolds, N. & Ibba, M. Aminoacyl-tRNA synthesis and translational quality control. Annu. Rev. Microbiol. 63, 61–78 (2009).
Tanrikulu, I. C. et al. Discovery of Escherichia coli methionyl-tRNA synthetase mutants for efficient labeling of proteins with azidonorleucine in vivo. Proc. Natl Acad. Sci. USA 106, 15285–15290 (2009).
Hoess, R. H., Wierzbicki, A. & Abremski, K. The role of the loxP spacer region in P1 site-specific recombination. Nucleic Acids Res. 14, 2287–2300 (1986).
Enyeart, P. J. et al. Generalized bacterial genome editing using mobile group II introns and Cre-lox. Mol. Syst. Biol. 9, 685 (2013).
Dymond, J. S. et al. Teaching synthetic biology, bioinformatics and engineering to undergraduates: the interdisciplinary Build-a-Genome course. Genetics 181, 13–21 (2009).
Fischer, S. R. History of Writing (Reaktion Books Ltd, 2001).
Dill, K. A. & MacCallum, J. L. The protein-folding problem, 50 years on. Science 338, 1042–1046 (2012).
Lewis, N. E., Nagarajan, H. & Palsson, B. O. Constraining the metabolic genotype–phenotype relationship using a phylogeny of in silico methods. Nat. Rev. Microbiol. 10, 291–305 (2012).
Martin, C. H., Nielsen, D. R., Solomon, K. V. & Prather, K. L. Synthetic metabolism: engineering biology at the protein and pathway scales. Chem. Biol. 16, 277–286 (2009).
Mattanovich, D. & Borth, N. Applications of cell sorting in biotechnology. Microb. Cell Fact. 5, 12 (2006).
Raman, S., Rogers, J. K., Taylor, N. D. & Church, G. M. Evolution-guided optimization of biosynthetic pathways. Proc. Natl Acad. Sci. USA 111, 17803–17808 (2014).
Zhang, F., Carothers, J. M. & Keasling, J. D. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat. Biotechnol. 30, 354–359 (2012).
Zhang, F. & Keasling, J. Biosensors and their applications in microbial metabolic engineering. Trends Microbiol. 19, 323–329 (2011).
Auslander, S. et al. A general design strategy for protein-responsive riboswitches in mammalian cells. Nat. Methods 11, 1154–1160 (2014).
Breaker, R. R. Prospects for riboswitch discovery and analysis. Mol. Cell 43, 867–879 (2011).
Mitchell, L. A. et al. Versatile genetic assembly system (VEGAS) to assemble pathways for expression in S. cerevisiae. Nucleic Acids Res. http://dx.doi.org/10.1093/nar/gkv466 (2015).
Daniel, R., Rubens, J. R., Sarpeshkar, R. & Lu, T. K. Synthetic analog computation in living cells. Nature 497, 619–623 (2013).
Kosuri, S. & Church, G. M. Large-scale de novo DNA synthesis: technologies and applications. Nat. Methods 11, 499–507 (2014).
Roy, S. & Caruthers, M. Synthesis of DNA/RNA and their analogs via phosphoramidite and H-phosphonate chemistries. Molecules 18, 14268–14284 (2013).
Tian, J. et al. Accurate multiplex gene synthesis from programmable DNA microchips. Nature 432, 1050–1054 (2004). In this paper, array-based DNA synthesis, oligonucleotide selection, and assembly are described. These processes enable parallel gene synthesis.
Cleary, M. A. et al. Production of complex nucleic acid libraries using highly parallel in situ oligonucleotide synthesis. Nat. Methods 1, 241–248 (2004).
Kosuri, S. et al. Scalable gene synthesis by selective amplification of DNA pools from high-fidelity microchips. Nat. Biotechnol. 28, 1295–1299 (2010).
Quan, J. et al. Parallel on-chip gene synthesis and application to optimization of protein expression. Nature Biotechnol. 29, 449–452 (2011).
Heller, M. J. DNA microarray technology: devices, systems, and applications. Annu. Rev. Biomed. Eng. 4, 129–153 (2002).
Stemmer, W. P., Crameri, A., Ha, K. D., Brennan, T. M. & Heyneker, H. L. Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene 164, 49–53 (1995).
Bang, D. & Church, G. M. Gene synthesis by circular assembly amplification. Nat. Methods 5, 37–39 (2008).
Gibson, D. G. Synthesis of DNA fragments in yeast by one-step assembly of overlapping oligonucleotides. Nucleic Acids Res. 37, 6984–6990 (2009).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Itaya, M., Tsuge, K., Koizumi, M. & Fujita, K. Combining two genomes in one cell: stable cloning of the Synechocystis PCC6803 genome in the Bacillus subtilis 168 genome. Proc. Natl Acad. Sci. USA 102, 15971–15976 (2005).
Acknowledgements
The authors thank M. Lajoie, the Isaacs laboratory and the anonymous reviewers for helpful comments on this paper. A.D.H. acknowledges support from US National Cancer Institute (NCI) award 1F30CA196191-01. P.M. is supported by the Raymond and Beverly Sackler Institute for Biological, Physical and Engineering Sciences. F.J.I. gratefully acknowledges support from the US Defense Advanced Research Projects Agency (N66001-12-C-4020, N66001-12-C-4211); US Department of Energy (152339.5055249.100); Gen9 Inc., DuPont Inc., and the Arnold and Mabel Beckman Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Directed evolution
-
A process that utilizes mutagenesis and a user-defined selective pressure to develop populations that are enriched for mutants exhibiting a desired function or behaviour.
- Nuclease-based genome editing
-
The use of engineered nucleases with a DNA-targeting mechanism (for example, a zinc-finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN) or a clustered regularly interspaced short palindromic repeat (CRISPR)) for targeted mutagenesis in vivo.
- CRISPR–Cas
-
A bacterial adaptive immune system comprising clustered regularly interspaced short palindromic repeats (CRISPRs) and CRISPR-associated (Cas) endonucleases that has been repurposed for genome editing.
- Recombineering
-
(Recombination-mediated genetic engineering). A technique that utilizes homologous recombination to alter genetic loci using synthetic single-stranded DNA and double-stranded DNA.
- Bioprospecting
-
Searching and sourcing useful genes from other organisms to specifically confer a desired phenotype (for example, medically or industrially relevant pathways and small molecules).
- Refactoring
-
Reorganization of biological systems or pathways with the goal of improving the ease and predictability of future engineering efforts. This often entails the removal of native regulation, separation of overlapping genetic elements and the use of well-characterized regulatory elements.
- Codon optimization
-
A multifactorial process that utilizes synonymous codons to reflect the preferred codon frequency in order to optimize protein expression in a target species.
- Gene synthesis
-
De novo construction of user-defined double-stranded DNA.
- Genetic code expansion
-
Modification of the genetic code to enable site-directed incorporation of amino acids beyond the canonical 20 into proteins.
- Non-standard amino acid
-
(nsAA). An amino acid beyond the canonical 20 present in the genetic code. nsAAs generally fall into two classes: post-translational modifications (for example, phosphoserine); and synthetic amino acids that do not exist in nature.
- Orthogonal translation systems
-
(OTSs). Used in genetic code expansion to link incorporation of a non-standard amino acid (nsAA) to a target codon. These systems are composed of a tRNA that is selectively charged with a given nsAA by a cognate aminoacyl-tRNA synthetase (aaRS). Other possible components of OTSs include mutant elongation factor Tu and ribosomes designed to accommodate the given nsAA.
- Genomically recoded organism
-
(GRO). An organism in which codons have been reassigned to create an alternate genetic code. For example, an Escherichia coli strain in which all instances of the TAG stop codon have been mutated to TAA and release factor 1 (RF1) has been deleted, enabling the use of TAG as an open coding channel.
Rights and permissions
About this article
Cite this article
Haimovich, A., Muir, P. & Isaacs, F. Genomes by design. Nat Rev Genet 16, 501–516 (2015). https://doi.org/10.1038/nrg3956
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3956
This article is cited by
-
The case for biotech on Mars
Nature Biotechnology (2020)
-
ZeBRα a universal, multi-fragment DNA-assembly-system with minimal hands-on time requirement
Scientific Reports (2019)
-
Millstone: software for multiplex microbial genome analysis and engineering
Genome Biology (2017)
-
Beyond editing to writing large genomes
Nature Reviews Genetics (2017)
-
Vibrio natriegens as a fast-growing host for molecular biology
Nature Methods (2016)