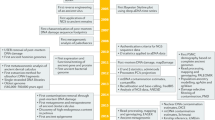
Key Points
-
High-throughput sequencing technologies have revolutionized ancient DNA (aDNA) research by enabling the reconstruction of whole-genome sequences from traces of short and extremely degraded DNA fragments.
-
DNA preservation is highly variable across samples and environments, as well as within single archaeological remains. The current temporal range for whole-genome sequencing covers the past million years.
-
DNA extracts from ancient material are generally metagenomic assemblages that include the DNA from the host and its associated microorganisms, as well as from a range of environmental microorganisms that colonize the sample after its death.
-
Various molecular approaches have been developed to improve access to aDNA in samples and reduce the sequencing costs of paleogenomics. These include extraction methods tailored to ultrashort DNA fragments, target enrichment for library inserts annealing to a panel of nucleic acid probes, and library building procedures targeting each DNA strand individually or incorporating only the most damaged DNA fragments.
-
Analyses of aDNA are prone to contamination by modern DNA molecules, which generally show limited degradation and fragmentation. Therefore, ruling out contamination — for example, exploiting patterns of DNA degradation and monitoring the heterozygosity levels observed at haploid loci — represents the cornerstone of every aDNA study.
-
DNA degradation reactions taking place post-mortem introduce mutation and depth-of-coverage patterns in the sequence data that can be exploited to authenticate paleogenomes and reconstruct genome-wide nucleosome and methylation maps.
Abstract
Research involving ancient DNA (aDNA) has experienced a true technological revolution in recent years through advances in the recovery of aDNA and, particularly, through applications of high-throughput sequencing. Formerly restricted to the analysis of only limited amounts of genetic information, aDNA studies have now progressed to whole-genome sequencing for an increasing number of ancient individuals and extinct species, as well as to epigenomic characterization. Such advances have enabled the sequencing of specimens of up to 1 million years old, which, owing to their extensive DNA damage and contamination, were previously not amenable to genetic analyses. In this Review, we discuss these varied technical challenges and solutions for sequencing ancient genomes and epigenomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Higuchi, R., Bowman, B., Freiberger, M., Ryder, O. A. & Wilson, A. C. DNA sequences from the quagga, an extinct member of the horse family. Nature 312, 282–284 (1984).
Hagelberg, E., Sykes, B. & Hedges, R. Ancient bone DNA amplified. Nature 342, 485 (1989).
Pääbo, S. Ancient DNA: extraction, characterization, molecular cloning, and enzymatic amplification. Proc. Natl Acad. Sci. USA 86, 1939–1943 (1989).
Rasmussen, M. et al. Ancient human genome sequence of an extinct Paleo-Eskimo. Nature 463, 757–762 (2010). This study takes advantage of the relative absence of environmental microorganisms within ancient hairs to characterize the first high-quality ancient human genome.
Miller, W. et al. Sequencing the nuclear genome of the extinct woolly mammoth. Nature 456, 387–390 (2008).
Green, R. E. et al. A draft sequence of the Neandertal genome. Science 328, 710–722 (2010). This paper reports the first draft genome of an archaic hominin and many methodological developments that are still commonly used for characterizing and analysing ancient genomes.
Bos, K. I. et al. A draft genome of Yersinia pestis from victims of the Black Death. Nature 478, 506–510 (2011). This paper reports the first genome isolated from an ancient pathogenic bacterium, confirming the Black Death as a plague epidemic. It revealed that no derived variant is unique to the medieval strain, suggesting that non-genetic factors enhanced the virulence of the pathogen.
Martin, M. D. et al. Reconstructing genome evolution in historic samples of the Irish potato famine pathogen. Nat. Commun. 4, 2172 (2013).
Schuenemann, V. J. et al. Genome-wide comparison of medieval and modern Mycobacterium leprae. Science 341, 179–183 (2013).
Bos, K. I. et al. Pre-Columbian mycobacterial genomes reveal seals as a source of New World human tuberculosis. Nature 514, 494–497 (2014).
Devault, A. M. et al. Second-pandemic strain of Vibrio cholera from the Philadelphia cholera outbreak. N. Engl. J. Med. 370, 334–340 (2014).
Devault, A. M. et al. Ancient pathogen DNA in archaeological samples detected with a microbial detection array. Sci. Rep. 4, 4245 (2014).
Wagner, D. M. et al. Yersinia pestis and the Plague of Justinian 541–543 AD: a genomic analysis. Lancet Infect. Dis. 14, 319–326 (2014).
Rasmussen, M. et al. An Aboriginal Australian genome reveals separate human dispersals into Asia. Science 334, 94–98 (2011).
Keller, A. et al. New insights into the Tyrolean Iceman's origin and phenotype as inferred by whole-genome sequencing. Nat. Commun. 3, 698 (2012).
Meyer, M. et al. A high-coverage genome sequence from an archaic Denisovan individual. Science 338, 222–226 (2012). This paper describes a novel method for constructing aDNA libraries using ssDNA templates, which enabled the characterization of the Denisovan genome at a quality rivalling that of modern genomes, starting from only minute amounts of DNA extracts.
Orlando, L. et al. Recalibrating Equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature 499, 74–78 (2013). This study takes advantage of both second-generation (high-throughput, and library- and amplification-dependent) and third-generation (high-throughput, and library- and amplification-independent) sequencing technologies to present the oldest genome sequence hitherto characterized: that of an ~700,000-year-old horse.
Gamba, C. et al. Genome flux and stasis in a five millennium transect of European prehistory. Nat. Commun. 5, 5257 (2014).
Jónsson, H. et al. Speciation with gene flow in equids despite extensive chromosomal plasticity. Proc. Natl Acad. Sci. USA 111, 18655–18660 (2014).
Malaspinas, A. S. et al. Two ancient human genomes reveal Polynesian ancestry among the indigenous Botocudos of Brazil. Curr. Biol. 24, R1035–R1037 (2014).
Olalde, I. et al. Derived immune and ancestral pigmentation alleles in a 7,000-year-old Mesolithic European. Nature 507, 225–228 (2014).
Prüfer, K. et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 505, 43–49 (2014).
Raghavan, M. et al. The genetic prehistory of the New World Arctic. Science 345, 1255832 (2014).
Raghavan, M. et al. Upper Paleolithic Siberian genome reveals dual ancestry of Native Americans. Nature 505, 87–91 (2014).
Rasmussen, M. et al. The genome of a Late Pleistocene human from a Clovis burial site in western Montana. Nature 506, 225–229 (2014).
Schubert, M. et al. Prehistoric genomes reveal the genetic foundation and cost of horse domestication. Proc. Natl Acad. Sci. USA 111, E5661–E5669 (2014).
Seguin-Orlando, A. et al. Genomic structure in Europeans dating back at least 36,200 years. Science 346, 1113–1118 (2014).
Ramirez, O. et al. Genome data from a sixteenth century pig illuminate modern breed relationships. Heredity 114, 175–184 (2015).
Schroeder, H. et al. Genome-wide ancestry of 17th century enslaved Africans from the Caribbean. Proc. Natl Acad. Sci. USA 112, 3669–3673 (2015).
Metzker, M. L. Sequencing technologies — the next generation. Nat. Rev. Genet. 11, 31–46 (2010).
Dabney, J. et al. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc. Natl Acad. Sci. USA 110, 15758–15763 (2013).
Meyer, M. et al. A mitochondrial genome sequence of a hominin from Sima de los Huesos. Nature 505, 403–406 (2014).
Gokhman, D. et al. Reconstructing the DNA methylation maps of the Neandertal and the Denisovan. Science 344, 523–527 (2014).
Pedersen, J. S. et al. Genome-wide nucleosome map and cytosine methylation levels of an ancient human genome. Genome Res. 24, 454–466 (2014). This study exploits DNA degradation patterns in HTS data sets to characterize, for the first time, genome-wide nucleosome and methylation maps from an ancient human and infer ancient gene expression levels and the age at death of the individual.
Ermini, L., Der Sarkissian, C., Willerslev, E. & Orlando, L. Major transitions in human evolution revisited: a tribute to ancient DNA. J. Hum. Evol. 79, 4–20 (2015).
Shapiro, B. & Hofreiter, M. A paleogenomic perspective on evolution and gene function: new insights from ancient DNA. Science 343, 1236573 (2014).
Orlando, L. & Cooper, A. Using ancient DNA to understand evolutionary and ecological processes. Ann. Rev. Ecol. Evol. Syst. 45, 573–598 (2014).
Orlando, L. & Willerslev, E. An epigenetic window into the past? Science 345, 511–512 (2014).
Höss, M., Jaruga, P., Zastawny, T. H., Dizdaroglu, M. & Pääbo, S. DNA damage and DNA sequence retrieval from ancient tissues. Nucleic Acids Res. 24, 1304–1307 (1996).
Hansen, A. J., Willerslev, E., Wiuf, C., Mourier, T. & Arctander, P. Statistical evidence for miscoding lesions in ancient DNA templates. Mol. Biol. Evol. 18, 262–265 (2001).
Hofreiter, M., Jaenicke, V., Serre, D., von Haeseler, A. & Pääbo, S. DNA sequences from multiple amplifications reveal artifacts induced by cytosine deamination in ancient DNA. Nucleic Acids Res. 29, 4793–4799 (2001).
Stiller, M. et al. Patterns of nucleotide misincorporations during enzymatic amplification and direct large-scale sequencing of ancient DNA. Proc. Natl Acad. Sci. USA 103, 13578–13584 (2006).
Gilbert, M. T. et al. Recharacterization of ancient DNA miscoding lesions: insights in the era of sequencing-by-synthesis. Nucleic Acids Res. 35, 1–10 (2007).
Briggs, A. et al. Patterns of damage in genomic DNA sequences from a Neandertal. Proc. Natl Acad. Sci. USA 104, 14616–14621 (2007). This study characterizes typical nucleotide misincorporation and fragmentation patterns using HTS data from aDNA extracts, which have been subsequently used as essential authentication criteria.
Orlando, L. et al. True single-molecule DNA sequencing of a Pleistocene horse bone. Genome Res. 21, 1705–1719 (2011).
Sawyer, S. et al. Temporal patterns of nucleotide misincorporations and DNA fragmentation in ancient DNA. PLoS ONE 7, e34131 (2012).
Overballe-Petersen, S., Orlando, L. & Willerslev, E. Next-generation sequencing offers new insights into DNA degradation. Trends Biotechnol. 30, 364–368 (2012).
Jónsson, H. et al. mapDamage2.0: fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics 29, 1682–1684 (2013).
Hansen, A. J. et al. Crosslinks rather than strand breaks determine access to ancient DNA sequences from frozen sediments. Genet. 173, 1175–1179 (2006).
Heyn, P. et al. Road blocks on paleogenomes — polymerase extension profiling reveals the frequency of blocking lesions in ancient DNA. Nucleic Acids Res. 38, e161 (2010).
Poinar, H. N., Kuch, M., McDonald, G., Martin, P. & Pääbo, S. Nuclear gene sequences from a late Pleistocene sloth coprolithe. Curr. Biol. 13, 1150–1152 (2003).
Poinar, H. N. et al. Metagenomics to paleogenomics: large-scale sequencing of mammoth DNA. Science 311, 393–394 (2006). This study reports the first genetic analysis of ancient specimens based on a HTS technology, paving the way for whole-genome sequencing from ancient specimens.
Allentoft, M. E. et al. The half-life of DNA in bone: measuring decay kinetics in 158 dated fossils. Proc. Biol. Sci. 279, 4724–4733 (2012).
Smith, C. I., Chamberlain, A. T., Riley, M. S., Stringer, C. & Collins, M. J. The thermal history of human fossils and the likelihood of successful DNA amplification. J. Hum. Evol. 45, 203–217 (2003).
Schwarz, C. et al. New insights from old bones: DNA preservation and degradation in permafrost preserved mammoth remains. Nucleic Acids Res. 37, 3215–2129 (2009).
Ginolhac, A. et al. Improving the performance of true single molecule sequencing for ancient DNA. BMC Genomics 13, 177 (2012).
Der Sarkissian, C. et al. Shotgun microbial profiling of fossil remains. Mol. Ecol. 23, 1780–1798 (2014).
Damgaard, P. et al. Improving access to endogenous DNA in ancient bones and teeth. BioRxiv http://dx.doi.org/10.1101/014985 (2015).
Salamon, M., Tuross, N., Arensburg, B. & Weiner, S. Relatively well preserved DNA is present in the crystal aggregates of fossil bones. Proc. Natl Acad. Sci. USA 102, 13783–13788 (2005).
Adler, C. J., Haak, W., Donlon, D. & Cooper, A. Survival and recovery of DNA from ancient teeth and bones. J. Archaeol. Sci. 38, 956–964 (2011).
Seguin-Orlando, A. et al. Ligation bias in illumina next-generation DNA libraries: implications for sequencing ancient genomes. PLoS ONE 8, e78575 (2013).
Dabney, J. & Meyer, M. Length and GC-biases during sequencing library amplification: a comparison of various polymerase-buffer systems with ancient and modern DNA sequencing libraries. Biotechniques 87–94 (2012).
Young, A. L. et al. A new strategy for genome assembly using short sequence reads and reduced representation libraries. Genome Res. 20, 249–256 (2010).
Seguin-Orlando, A. et al. Amplification of TruSeq ancient DNA libraries with AccuPrime Pfx: consequences on nucleotide misincorporation and methylation patterns. STAR 1, STAR2015112054892315Y.0000000005 (2015).
Star, B. et al. Palindromic sequence artifacts generated during next generation sequencing library preparation from historic and ancient DNA. PLoS ONE 9, e89676 (2014).
Gansauge, M. T. & Meyer, M. Single-stranded DNA library preparation for the sequencing of ancient or damaged DNA. Nat. Protoc. 8, 737–748 (2013).
Gilbert, M. T. et al. Whole-genome shotgun sequencing of mitochondria from ancient hair shafts. Science 317, 1927–1930 (2007).
Gansauge, M. T. & Meyer, M. Selective enrichment of damaged DNA molecules for ancient genome sequencing. Genome Res. 24, 1543–1549 (2014).
Briggs, A. et al. Targeted retrieval and analysis of five Neandertal mtDNA genomes. Science 325, 318–321 (2009).
Maricic, T., Whitten, M. & Pääbo, S. Multiplexed DNA sequence capture of mitochondrial genomes using PCR products. PLoS ONE 5, e14004 (2010).
Haak, W. et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature http://dx.doi.org/10.1038/nature14317 (2015).
Rohland, N., Harney, E., Mallick, S., Nordenfelt, S. & Reich, D. Partial uracil–DNA–glycosylase treatment for screening of ancient DNA. Phil. Trans. R. Soc. B 370, 20130624 (2015).
Burbano, H. A. et al. Targeted investigation of the Neandertal genome by array-based sequence capture. Science 328, 723–725 (2010). This paper reports the first characterization of an ancient exome using target enrichment approaches on microarrays.
Fu, Q. et al. A revised timescale for human evolution based on ancient mitochondrial genomes. Curr. Biol. 23, 553–559 (2013).
Vilstrup, J. T. et al. Mitochondrial phylogenomics of modern and ancient equids. PLoS ONE 8, e55950 (2013).
Castellano, S. et al. Patterns of coding variation in the complete exomes of three Neandertals. Proc. Natl Acad. Sci. USA 111, 6666–6671 (2014).
Fu, Q. et al. DNA analysis of an early modern human form Tianyuan Cave, China. Proc. Natl Acad. Sci. USA 110, 2223–2227 (2013). This paper describes a target enrichment procedure exploiting millions of DNA probes cleaved from user-designed DNA microarrays to characterize the almost complete sequence of the non-repetitive fraction of chromosome 21 for an ~40,000-year-old human.
Carpenter, M. L. et al. Pulling out the 1%: whole-genome capture for the targeted enrichment of ancient DNA sequencing libraries. Am. J. Hum. Genet. 93, 852–864 (2013). This paper reports the first whole-genome target enrichment method, which makes use of self-generated RNA probes. The method substantially reduces the operational cost of target enrichment and allows genetic analyses of specimens with only minute amounts of aDNA templates.
Enk, J. M. et al. Ancient whole genome enrichment using baits built from modern DNA. Mol. Biol. Evol. 31, 1292–1295 (2014).
Avila-Arcos, C. et al. Comparative performance of two whole-genome capture methodologies on ancient DNA Illumina libraries. Methods Ecol. Evol. http://dx.doi.org/10.1111/2041-210X.12353 (2015).
Briggs, A. et al. Removal of deaminated cytosines and detection of in vivo methylation in ancient DNA. Nucleic Acids Res. 38, e87 (2010). This paper presents an enzymatic procedure based on the treatment of DNA extracts with USER mix, which can considerably reduce the sequencing error rate of ancient genomes by limiting the effect of nucleotide misincorporations at damaged sites.
Mason, V. C., Li, G., Helgen, K. M. & Murphy, W. J. Efficient cross-species capture hybridization and next-generation sequencing of mitochondrial genomes from noninvasively sampled museum specimens. Genome Res. 21, 1695–1704 (2011).
Zhang, H. et al. Morphological and genetic evidence for early Holocene cattle management in northeastern China. Nat. Commun. 4, 2755 (2013).
Fabre, P. H. et al. Rodents of the Caribbean: origin and diversification of hutias unraveled by next-generation museomics. Biol. Lett. http://dx.doi.org/10.1098/rsbl.2014.0266 (2014).
Foote, A. D. et al. Tracking niche variation over millennial timescales in sympatric killer whale lineages. Proc. Biol. Sci. 280, 20131481 (2013).
Schuenemann, V. J. et al. Targeted enrichment of ancient pathogens yielding the pPCP1 plasmid of Yersinia pestis from victims of the Black Death. Proc. Natl Acad. Sci. USA 108, E746–E452 (2011).
Kircher, M., Sawyer, S. & Meyer, M. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 40, e3 (2012).
Avila-Arcos, M. C. et al. Application and comparison of large-scale solution-based DNA capture-enrichment methods on ancient DNA. Sci. Rep. 1, 73 (2011).
Hodges, E. et al. Hybrid selection of discrete genomic intervals on custom-designed microarrays for massively parallel sequencing. Nat. Protoc. 4, 960–974 (2009).
Bos, K. I. et al. Parallel detection of ancient pathogens via array-based DNA capture. Phil. Trans. R. Soc. B 370, 20130375 (2015).
Schubert, M. et al. Characterization of ancient and modern genomes by SNP detection and phylogenomic and metagenomic analysis using PALEOMIX. Nat. Protoc. 9, 1056–1082 (2013). This paper presents a fully automated pipeline performing all sequence analyses associated with re-sequencing genomic projects, phylogenomic inference and metagenomic profiling. It is applicable to both modern and ancient sequence data sets.
Lindgreen, S. AdapterRemoval: easy cleaning of next-generation sequencing reads. BMC Res. Notes 5, 337 (2012).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
McKenna, A. et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Kozlov, A. M., Aberer, A. J. & Stamatakis, A. ExaML version 3: a tool for phylogenomic analyses on supercomputers. Bioinformatics http://dx.doi.org/10.1093/bioinformatics/btv184 (2015).
Segata, N. et al. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat. Methods 9, 811–814 (2012).
Schubert, M. et al. Improving ancient DNA read mapping against modern reference genomes. BMC Genomics 13, 178 (2012).
Kerpedjev, P., Frellsen, J., Lindgreen, S. & Krogh, A. Adaptable probabilistic mapping of short reads using position specific scoring matrices. BMC Bioinformatics 15, 100 (2014).
Ginolhac, A., Rasmussen, M., Gilbert, M. T., Willerslev, E. & Orlando, L. mapDamage: testing for damage patterns in ancient DNA sequences. Bioinformatics 27, 2153–2155 (2011).
Skoglund, P. et al. Separating endogenous ancient DNA from modern day contamination in a Siberian Neandertal. Proc. Natl Acad. Sci. USA 111, 2229–2234 (2014).
Lindgreen, S., Krogh, A. & Pedersen, J. S. SNPest: a probabilistic graphical model for estimating genotypes. BMC Res. Notes 7, 698 (2014).
Skoglund, P. et al. Origins and genetic legacy of Neolithic farmers and hunter-gatherers in Europe. Science 336, 466–469 (2012).
García-Garcerà, M. et al. Fragmentation of contaminant and endogenous DNA in ancient samples determined by shotgun sequencing; prospects for human paleogenomics. PLoS ONE 6, e24161 (2011).
Sánchez-Quinto, F. et al. Genomic affinities of two 7,000-year-old Iberian hunter-gatherers. Curr. Biol. 22, 1494–1499 (2012).
Green, R. E. et al. The Neandertal genome and ancient DNA authenticity. EMBO J. 28, 2494–2502 (2009).
Reich, D. et al. Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature 468, 1053–1060 (2010).
Llamas, B. et al. High-resolution analysis of cytosine methylation in ancient DNA. PLoS ONE 7, e30226 (2012).
Smith, O. et al. Genomic methylation patterns in archaeological barley show de-methylation as a time-dependent diagenetic process. Sci. Rep. 4, 5559 (2014).
d'Abbadie, M. et al. Molecular breeding of polymerases for amplification of ancient DNA. Nat. Biotech. 25, 939–943 (2007).
da Fonseca, R. R. et al. The origin and evolution of maize in the American Southwest. Nat. Plants 1, 14003 (2015).
Pickrell, J. K. & Reich, D. Toward a new history and geography of human genes informed by ancient DNA. Trends Genet. 30, 377–389 (2014).
Fordyce, S. L. et al. Deep sequencing of RNA from ancient maize kernels. PLoS ONE 8, e50961 (2013).
Allaby, R. G. et al. Using archaeogenomic and computational approaches to unravel the history of local adaptation in crops. Phil. Trans. R. Soc. B 370, 20130377 (2015).
Cappellini, E. et al. Proteomic analysis of a Pleistocene mammoth femur reveals more than one hundred ancient bone proteins. 11, 917–926 (2012).
Cappellini, E., Collins, M. J. & Gilbert, M. T. Unlocking ancient protein palimpsests. Science 343, 1320–1322 (2014).
Warinner, C. et al. Direct evidence of milk consumption from ancient human dental calculus. Sci. Rep. 4, 7104 (2014).
Adler, C. J. et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat. Genet. 45, 450–455 (2013).
Warinner, C. et al. Pathogens and host immunity in the ancient human oral cavity. Nat. Genet. 46, 336–344 (2014).
Shapiro, B. How to Clone a Mammoth — the Science of De-extinction (Princeton University Press, 2014).
Lazaridis, I. et al. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature 513, 409–413 (2014).
Fu, Q. et al. Genome sequence of a 45,000-year-old modern human from western Siberia. Nature 514, 445–449 (2014).
Krause, J. et al. The complete mitochondrial DNA genome of an unknown hominin from southern Siberia. Nature 464, 894–897 (2010).
Orlando, L. A. 400,000-year-old mitochondrial genome questions phylogenetic relationships amongst archaic hominins. Bioessays 36, 598–605 (2014).
Scally, A. & Durbin, R. Revising the human mutation rate: implications for understanding human evolution. Nat. Rev. Genet. 13, 745–753 (2012).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664 (2009).
Malaspinas, A. S. et al. bammds: a tools for assessing the ancestry of low-depth whole-genome data using multidimensional scaling (MDS). Bioinformatics 30, 2962–2964 (2014).
Skoglund, P., Sjodin, P., Skoglund, T., Lascoux, M. & Jakobsson, M. Investigating population history using temporal genetic differentiation. Mol. Biol. Evol. 31, 2516–2527 (2014).
Durand, E. Y., Patterson, N., Reich, D. & Slatkin, M. Testing for ancient admixture between closely related populations. Mol. Biol. Evol. 28, 2239–2252 (2011).
Patterson, N. et al. Ancient admixture in human history. Genetics. 192, 1065–1093 (2012).
Eriksson, A. & Manica, A. Effect of ancient population structure on the degree of polymorphism shared between modern human populations and ancient hominins. Proc. Natl Acad. Sci. USA 109, 13956–13960 (2012).
Sankararaman, S., Patterson, N., Heng, L., Pääbo, S. & Reich, D. The date of interbreeding between Neandertals and modern humans. PLoS Genet. 8, e1002947 (2012).
Acknowledgements
This work was supported by the Danish Council for Independent Research, Natural Sciences (FNU, 4002-00152B and 0602-02383B); the Danish National Research Foundation (DNFR94); the Lundbeck Foundation (R52-5062); a Marie Curie Career Integration Grant (FP7 CIG-293845); and the “Chaires d'Attractivité 2014” IDEX, University of Toulouse, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Osseous materials
-
Calcified animal tissues, such as bones and teeth.
- Second-generation sequencing
-
High-throughput short-read DNA sequencing platforms that require library construction and thus modification of the DNA before sequencing. Most commonly represented by the Illumina, GS-FLX (454), ABI SOLiD and Ion Torrent series.
- Resonance structures
-
Dynamic, alternative forms of molecular groups, such as nucleotide bases, that result from electron delocalization within the molecule.
- Pre-digestion
-
Exposure of ancient calcified materials to a short initial digestion aimed at removing substantial fractions of exogenous contaminants.
- 454
-
The initial generation of GS-FLX sequencing platforms based on pyrosequencing, before their acquisition and renaming by Roche.
- T/A ligation
-
A common DNA ligation technology that relies on complementary pairing of thymine and adenine overhangs at the 3′ ends of the adaptors and inserts to be ligated, respectively.
- Shotgun sequencing
-
The sequencing of fragmented DNA in the absence of any selection strategy.
- Primer extension capture
-
(PEC). An enrichment technology based on the ligation of short 5′-biotinylated oligonucleotides (including a 12-nucleotide-long spacer followed by a primer of 18–25 nucleotides that is designed to match a particular region of interest) to single-stranded target molecules. This is followed by a single round of polymerase-based extension so as to increase the length over which the molecules are hybridized.
- Tiled probes
-
Probes that overlap in their positioning on the target so as to ensure that every target position is covered by more than one different probe.
- Chimeric DNA libraries
-
Recombination between libraries containing different template molecules during library PCR amplification, resulting in hybrid (chimeric) sequences that do not represent true biological sequences.
- Double-indexed DNA libraries
-
DNA libraries in which short (for example, 8 bp long) unique nucleotide indexes are incorporated within both adaptors used during library construction. Indexes are bordered by known sequences that serve to prime index sequencing reactions and also enable library attachment to the surface of the sequencing flow cell.
- Mate pairs
-
Pairs of sequences derived from both ends of a DNA library.
- Edit distance
-
The number of sequence mismatch counts between reads and targets.
- Probabilistic aligners
-
Mapping algorithms that can accommodate non-uniform distributions of sequencing errors along reads, generally leading to improved alignments between reads and reference genomes.
- Thermal age
-
The predicted time that it would have taken an archaeological sample to produce the observed degree of DNA degradation were the sample exposed to a constant temperature of 10 °C since deposition. Thermal age has been proposed to adjust the chronological age of a sample to its thermal history and to help in predicting the likelihood of DNA surviving in archaeological remains.
- Haplotypes
-
The DNA sequences of haploid chromosomes.
- Derived alleles
-
Alleles that are evolutionarily derived in a lineage of interest and that are not represented in an ancestral population or species.
- Ancestral alleles
-
Alleles in the ancestral state before a mutation took place in a descending population, species or lineage.
- Nearly fixed
-
Fixed alleles are those that are derived and present in all individuals in a descendent population or species. Nearly fixed alleles therefore represent those that are present in nearly all individuals (thus close to fixation, for example, showing allelic frequencies of 99% in the population).
- Epialleles
-
Allelic variants showing identical genetic sequences but different epigenetic marks, such as different methylation patterns.
- Ghost population
-
An unsampled population that exchanges migrants with other sampled populations and that can be identified based on admixture signatures left in descending populations.
- Introgressive block lengths
-
Population admixture introduces a mosaic of ancestry blocks along the genome, the lengths of which decrease with each subsequent generation owing to recombination. Introgressive block lengths can therefore be exploited to determine the date of admixture events.
- CTCF regions
-
Genomic regions targeted by CCCTC-binding factor (CTCF) and involved in regulating the three-dimensional structure of chromatin and transcription by mediating long-range interactions between genomic sequences.
- Admixture
-
Interbreeding of individuals from multiple population origins, resulting in the introduction of DNA from one population into the genomes of a second population.
Rights and permissions
About this article
Cite this article
Orlando, L., Gilbert, M. & Willerslev, E. Reconstructing ancient genomes and epigenomes. Nat Rev Genet 16, 395–408 (2015). https://doi.org/10.1038/nrg3935
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3935
This article is cited by
-
SPIN enables high throughput species identification of archaeological bone by proteomics
Nature Communications (2022)
-
Mitogenomic diversity and stable isotopes provide insights into the maternal genetic history, mobility patterns, and diet of early medieval individuals from the Eastern Italian Alps
Archaeological and Anthropological Sciences (2022)
-
Genomic reconstruction of fossil and living microorganisms in ancient Siberian permafrost
Microbiome (2021)
-
Environmental palaeogenomic reconstruction of an Ice Age algal population
Communications Biology (2021)
-
DNA methylation profiling in mummified human remains from the eighteenth-century
Scientific Reports (2021)