Key Points
-
Straight and sharp boundaries often separate groups of cells with distinct functions or fates during animal development. The formation and maintenance of such boundaries is important for the growth and patterning of tissues.
-
Straight and sharp boundaries are challenged by cell rearrangements caused by cell proliferation or tissue deformation.
-
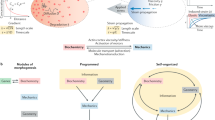
Two basic types of boundaries can be defined: non-lineage boundaries, in which cell identity is plastic and cells can move across gene expression boundaries and adapt their identity to their local neighbours; and lineage (or compartment) boundaries in which cell identity is inherited and straight and sharp boundaries between groups of cells with distinct identities are maintained by cell sorting.
-
Boundaries between the somites of vertebrate embryos are examples of non-lineage boundaries. Mesoderm posterior (Mesp) transcription factors are key players in the pre-patterning that defines the position and identity of somites.
-
Boundaries within the vertebrate hindbrain and the developing Drosophila melanogaster wing are examples of compartment boundaries. Selector genes provide cell identity to compartments in D. melanogaster.
-
Cell signalling is important for maintaining boundaries. Ephrin receptor (Eph)–ephrin signalling is required to maintain compartment boundaries in the vertebrate hindbrain and the non-lineage boundaries between the somites.
-
The deposition of extracellular matrix is a key physical mechanism to maintain somite boundaries.
-
Differential mechanical tension has emerged as a new principle to maintain compartment boundaries in D. melanogaster tissues.
Abstract
The formation and maintenance of boundaries between neighbouring groups of embryonic cells is vital for development because groups of cells with distinct functions must often be kept physically separated. Furthermore, because cells at the boundary often take on important signalling functions by acting as organizing centres, boundary shape and integrity can also control the outcome of many downstream patterning events. Recent experimental findings and theoretical descriptions have shed new light on classic questions about boundaries. In particular, in the past couple of years the role of forces acting in epithelial tissues to maintain boundaries has emerged as a new principle in understanding how early pattern is made into permanent anatomy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wolpert, L. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 25, 1–47 (1969).
Meinhardt, H. Cell determination boundaries as organizing regions for secondary embryonic fields. Dev. Biol. 96, 375–385 (1983).
Fraser, S., Keynes, R. & Lumsden, A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature 344, 431–435 (1990).
Garcia-Bellido, A., Ripoll, P. & Morata, G. Developmental compartmentalisation of the wing disk of Drosophila. Nature New Biol. 245, 251–253 (1973).
Julich, D., Mould, A. P., Koper, E. & Holley, S. A. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph–ephrin signaling. Development 136, 2913–2921 (2009). Live imaging and genetic mosaics in zebrafish of fluorescently tagged integrin receptors show that integrin clustering at the somite boundary precedes fibronectin accumulation and is driven by ephrin B2 activation, thereby restricting fibronectin matrix formation to the boundary interface.
Kemp, H. A., Cooke, J. E. & Moens, C. B. EphA4 and EfnB2a maintain rhombomere coherence by independently regulating intercalation of progenitor cells in the zebrafish neural keel. Dev. Biol. 327, 313–326 (2009). A study that investigated the role of Eph–ephrin-mediated cell affinity within and between segments in the zebrafish hindbrain neuroepithelium using live imaging. Interestingly, both EphA4 and EfnB2a proteins function particularly during cell divisions in the neuroepithelium, when demands for cell affinity are likely to be high.
Landsberg, K. P. et al. Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr. Biol. 19, 1950–1955 (2009). This study applies physical approaches and quantitative imaging to demonstrate and quantify an increase in mechanical tension along the A–P boundary of D. melanogaster wing imaginal discs. Moreover, mathematical modelling shows that a local increase in tension is sufficient to maintain straight and sharp compartment boundaries.
Monier, B., Pelissier-Monier, A., Brand, A. H. & Sanson, B. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nature Cell Biol. 12, 60–65 (2010). This paper uses a combination of CALI and live imaging to demonstrate that Myosin II is required to maintain parasegment boundaries of D. melanogaster embryos.
Lawrence, P. A. A clonal analysis of segment development in Oncopeltus (Hemiptera). J. Embryol. Exp. Morphol. 30, 681–699 (1973).
Kornberg, T., Siden, I., O'Farrell, P. & Simon, M. The engrailed locus of Drosophila: in situ localization of transcripts reveals compartment-specific expression. Cell 40, 45–53 (1985).
Mann, R. S. & Morata, G. The developmental and molecular biology of genes that subdivide the body of Drosophila. Annu. Rev. Cell Dev. Biol. 16, 243–271 (2000).
Franklin, V. et al. Regionalisation of the endoderm progenitors and morphogenesis of the gut portals of the mouse embryo. Mech. Dev. 125, 587–600 (2008).
Tremblay, K. D. & Zaret, K. S. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev. Biol. 280, 87–99 (2005).
Kulesa, P. M. & Fraser, S. E. Cell dynamics during somite boundary formation revealed by time-lapse analysis. Science 298, 991–995 (2002).
Dequeant, M. L. & Pourquie, O. Segmental patterning of the vertebrate embryonic axis. Nature Rev. Genet. 9, 370–382 (2008).
Oginuma, M., Niwa, Y., Chapman, D. L. & Saga, Y. Mesp2 and Tbx6 cooperatively create periodic patterns coupled with the clock machinery during mouse somitogenesis. Development 135, 2555–2562 (2008). This paper uses high-resolution in situ hybridization and genetic perturbation to examine the relationship between MESP2, TBX6 and Notch signalling in mouse embryos and shows that MESP2 coordinates the input between periodic Notch signalling and spatially dependent Tbx6 expression to generate segments.
Lawrence, P. A., Green, S. M. & Johnston, P. Compartmentalization and growth of the Drosophila abdomen. J. Embryol. Exp. Morphol. 43, 233–245 (1978).
Morata, G. & Lawrence, P. A. Anterior and posterior compartments in the head of Drosophila. Nature 274, 473–474 (1978).
Steiner, E. Establishment of compartments in the developing leg imaginal discs of Drosophila melanogaster. Rouxs Arch. Dev. Biol. 180, 9–30 (1976).
Struhl, G. Developmental compartments in the proboscis of Drosophila. Nature 270, 723–725 (1977).
Jimenez-Guri, E. et al. Clonal analysis in mice underlines the importance of rhombomeric boundaries in cell movement restriction during hindbrain segmentation. PLoS ONE 5, e10112 (2010).
Langenberg, T. & Brand, M. Lineage restriction maintains a stable organizer cell population at the zebrafish midbrain-hindbrain boundary. Development 132, 3209–3216 (2005).
Zervas, M., Millet, S., Ahn, S. & Joyner, A. L. Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron 43, 345–357 (2004).
Altabef, M., Clarke, J. D. & Tickle, C. Dorso-ventral ectodermal compartments and origin of apical ectodermal ridge in developing chick limb. Development 124, 4547–4556 (1997).
Arques, C. G., Doohan, R., Sharpe, J. & Torres, M. Cell tracing reveals a dorsoventral lineage restriction plane in the mouse limb bud mesenchyme. Development 134, 3713–3722 (2007).
Pearse, R. V., Scherz, P. J., Campbell, J. K. & Tabin, C. J. A cellular lineage analysis of the chick limb bud. Dev. Biol. 310, 388–400 (2007).
Qiu, Q., Chen, H. & Johnson, R. L. Lmx1b-expressing cells in the mouse limb bud define a dorsal mesenchymal lineage compartment. Genesis 47, 224–233 (2009).
Smith, D. M. & Tabin, C. J. Clonally related cells are restricted to organ boundaries early in the development of the chicken gut to form compartment boundaries. Dev. Biol. 227, 422–431 (2000).
Inoue, T. et al. Role of cadherins in maintaining the compartment boundary between the cortex and striatum during development. Development 128, 561–569 (2001).
Zeltser, L. M., Larsen, C. W. & Lumsden, A. A new developmental compartment in the forebrain regulated by Lunatic fringe. Nature Neurosci. 4, 683–684 (2001).
Garcia-Bellido, A. Genetic control of wing disc development in Drosophila. Ciba Found. Symp. 0, 161–182 (1975).
Coleman, K. G., Poole, S. J., Weir, M. P., Soeller, W. C. & Kornberg, T. The invected gene of Drosophila: sequence analysis and expression studies reveal a close kinship to the engrailed gene. Genes Dev. 1, 19–28 (1987).
Garcia-Bellido, A. & Santamaria, P. Developmental analysis of the wing disc in the mutant engrailed of Drosophila melanogaster. Genetics 72, 87–104 (1972).
Blair, S. S., Brower, D. L., Thomas, J. B. & Zavortink, M. The role of apterous in the control of dorsoventral compartmentalization and PS integrin gene expression in the developing wing of Drosophila. Development 120, 1805–1815 (1994).
von Baer, K. E. Über die Entwicklungsgeschichte der Thiere (Königsberg, 1828).
Orr, H. Contribution to the embryology of the lizard. J. Morphol. 1, 311–372 (1887).
Vaage, S. The segmentation of the primitive neural tube in chick embryos (Gallus domesticus). A morphological, histochemical and autoradiographical investigation. Ergeb. Anat. Entwicklungsgesch. 41, 3–87 (1969).
Keynes, R. & Lumsden, A. Segmentation and the origin of regional diversity in the vertebrate central nervous system. Neuron 4, 1–9 (1990).
Mellitzer, G., Xu, Q. & Wilkinson, D. G. Eph receptors and ephrins restrict cell intermingling and communication. Nature 400, 77–81 (1999).
Xu, Q., Mellitzer, G., Robinson, V. & Wilkinson, D. G. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature 399, 267–271 (1999).
Kiecker, C. & Lumsden, A. Compartments and their boundaries in vertebrate brain development. Nature Rev. Neurosci. 6, 553–564 (2005).
Alexander, T., Nolte, C. & Krumlauf, R. Hox genes and segmentation of the hindbrain and axial skeleton. Annu. Rev. Cell Dev. Biol. 25, 431–456 (2009).
Keynes, R. J. & Stern, C. D. Segmentation in the vertebrate nervous system. Nature 310, 786–789 (1984).
Takahashi, Y. et al. Mesp2 initiates somite segmentation through the Notch signalling pathway. Nature Genet. 25, 390–396 (2000).
Saga, Y., Hata, N., Koseki, H. & Taketo, M. M. Mesp2: a novel mouse gene expressed in the presegmented mesoderm and essential for segmentation initiation. Genes Dev. 11, 1827–1839 (1997).
Nakajima, Y., Morimoto, M., Takahashi, Y., Koseki, H. & Saga, Y. Identification of Epha4 enhancer required for segmental expression and the regulation by Mesp2. Development 133, 2517–2525 (2006).
Schroter, C. & Oates, A. C. Segment number and axial identity in a segmentation clock period mutant. Curr. Biol. 20, 1254–1258 (2010).
Cooke, J. The problem of periodic patterns in embryos. Phil. Trans. R. Soc. Lond. B 295, 509–524 (1981).
Morimoto, M., Takahashi, Y., Endo, M. & Saga, Y. The Mesp2 transcription factor establishes segmental borders by suppressing Notch activity. Nature 435, 354–359 (2005).
Takahashi, Y., Inoue, T., Gossler, A. & Saga, Y. Feedback loops comprising Dll1, Dll3 and Mesp2, and differential involvement of Psen1 are essential for rostrocaudal patterning of somites. Development 130, 4259–4268 (2003).
Takahashi, Y., Yasuhiko, Y., Kitajima, S., Kanno, J. & Saga, Y. Appropriate suppression of Notch signaling by Mesp factors is essential for stripe pattern formation leading to segment boundary formation. Dev. Biol. 304, 593–603 (2007).
Yasuhiko, Y. et al. Tbx6-mediated Notch signaling controls somite-specific Mesp2 expression. Proc. Natl Acad. Sci. USA 103, 3651–3656 (2006).
Yasuhiko, Y. et al. Functional importance of evolutionally conserved Tbx6 binding sites in the presomitic mesoderm-specific enhancer of Mesp2. Development 135, 3511–3519 (2008).
Kawamura, A. et al. Groucho-associated transcriptional repressor ripply1 is required for proper transition from the presomitic mesoderm to somites. Dev. Cell 9, 735–744 (2005).
Kawamura, A., Koshida, S. & Takada, S. Activator-to-repressor conversion of T-box transcription factors by the Ripply family of Groucho/TLE-associated mediators. Mol. Cell. Biol. 28, 3236–3244 (2008).
Moreno, T. A., Jappelli, R., Izpisua Belmonte, J. C. & Kintner, C. Retinoic acid regulation of the Mesp-Ripply feedback loop during vertebrate segmental patterning. Dev. Biol. 315, 317–330 (2008).
Morimoto, M. et al. The negative regulation of Mesp2 by mouse Ripply2 is required to establish the rostro-caudal patterning within a somite. Development 134, 1561–1569 (2007).
Takahashi, J. et al. Analysis of Ripply1/2-deficient mouse embryos reveals a mechanism underlying the rostro-caudal patterning within a somite. Dev. Biol. 342, 134–145 (2010).
Oginuma, M. et al. The oscillation of Notch activation, but not its boundary, is required for somite border formation and rostral-caudal patterning within a somite. Development 137, 1515–1522 (2010).
Oates, A. C., Gorfinkiel, N., Gonzalez-Gaitan, M. & Heisenberg, C. P. Quantitative approaches in developmental biology. Nature Rev. Genet. 10, 517–530 (2009).
Oates, A. C., Rohde, L. A. & Ho, R. K. Generation of segment polarity in the paraxial mesoderm of the zebrafish through a T-box-dependent inductive event. Dev. Biol. 283, 204–214 (2005).
Moreno, T. A. & Kintner, C. Regulation of segmental patterning by retinoic acid signaling during Xenopus somitogenesis. Dev. Cell 6, 205–218 (2004).
Nikaido, M. et al. Tbx24, encoding a T-box protein, is mutated in the zebrafish somite-segmentation mutant fused somites. Nature Genet. 31, 195–199 (2002).
Palmeirim, I., Dubrulle, J., Henrique, D., Ish-Horowicz, D. & Pourquie, O. Uncoupling segmentation and somitogenesis in the chick presomitic mesoderm. Dev. Genet. 23, 77–85 (1998).
Burgess, R., Rawls, A., Brown, D., Bradley, A. & Olson, E. N. Requirement of the paraxis gene for somite formation and musculoskeletal patterning. Nature 384, 570–573 (1996).
Nomura-Kitabayashi, A. et al. Hypomorphic Mesp allele distinguishes establishment of rostrocaudal polarity and segment border formation in somitogenesis. Development 129, 2473–2481 (2002).
Blair, S. S. & Ralston, A. Smoothened-mediated Hedgehog signalling is required for the maintenance of the anterior-posterior lineage restriction in the developing wing of Drosophila. Development 124, 4053–4063 (1997).
Dahmann, C. & Basler, K. Opposing transcriptional outputs of Hedgehog signaling and engrailed control compartmental cell sorting at the Drosophila A/P boundary. Cell 100, 411–422 (2000).
Rodriguez, I. & Basler, K. Control of compartmental affinity boundaries by hedgehog. Nature 389, 614–618 (1997).
Shen, J. & Dahmann, C. The role of Dpp signaling in maintaining the Drosophila anteroposterior compartment boundary. Dev. Biol. 279, 31–43 (2005).
Micchelli, C. A. & Blair, S. S. Dorsoventral lineage restriction in wing imaginal discs requires Notch. Nature 401, 473–476 (1999).
Rauskolb, C., Correia, T. & Irvine, K. D. Fringe-dependent separation of dorsal and ventral cells in the Drosophila wing. Nature 401, 476–480 (1999).
Cooke, J. E., Kemp, H. A. & Moens, C. B. EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr. Biol. 15, 536–542 (2005).
Durbin, L. et al. Eph signaling is required for segmentation and differentiation of the somites. Genes Dev. 12, 3096–3109 (1998).
Nieto, M. A., Gilardi-Hebenstreit, P., Charnay, P. & Wilkinson, D. G. A receptor protein tyrosine kinase implicated in the segmental patterning of the hindbrain and mesoderm. Development 116, 1137–1150 (1992).
Sajjadi, F. G. & Pasquale, E. B. Five novel avian Eph-related tyrosine kinases are differentially expressed. Oncogene 8, 1807–1813 (1993).
Scales, J. B., Winning, R. S., Renaud, C. S., Shea, L. J. & Sargent, T. D. Novel members of the eph receptor tyrosine kinase subfamily expressed during Xenopus development. Oncogene 11, 1745–1752 (1995).
Durbin, L. et al. Anteroposterior patterning is required within segments for somite boundary formation in developing zebrafish. Development 127, 1703–1713 (2000).
Barrios, A. et al. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr. Biol. 13, 1571–1582 (2003).
Sato, Y., Yasuda, K. & Takahashi, Y. Morphological boundary forms by a novel inductive event mediated by Lunatic fringe and Notch during somitic segmentation. Development 129, 3633–3644 (2002).
Tanaka, M. & Tickle, C. Tbx18 and boundary formation in chick somite and wing development. Dev. Biol. 268, 470–480 (2004).
Nakaya, Y., Kuroda, S., Katagiri, Y. T., Kaibuchi, K. & Takahashi, Y. Mesenchymal-epithelial transition during somitic segmentation is regulated by differential roles of Cdc42 and Rac1. Dev. Cell 7, 425–438 (2004).
Watanabe, T. et al. Tet-on inducible system combined with in ovo electroporation dissects multiple roles of genes in somitogenesis of chicken embryos. Dev. Biol. 305, 625–636 (2007). This paper uses a combination of grafting and temporal activation of gene expression in chick embryos to show that epithelialization of the caudal somite boundary cells is driven by activated ephrin B2 repression of CDC42 activity.
Watanabe, T., Sato, Y., Saito, D., Tadokoro, R. & Takahashi, Y. EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc. Natl Acad. Sci. USA 106, 7467–7472 (2009).
Gibson, M. C., Patel, A. B., Nagpal, R. & Perrimon, N. The emergence of geometric order in proliferating metazoan epithelia. Nature 442, 1038–1041 (2006).
Farhadifar, R., Roper, J. C., Aigouy, B., Eaton, S. & Julicher, F. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr. Biol. 17, 2095–2104 (2007).
O'Brochta, D. A. & Bryant, P. J. A zone of non-proliferating cells at a lineage restriction boundary in Drosophila. Nature 313, 138–141 (1985).
Major, R. J. & Irvine, K. D. Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. Development 132, 3823–3833 (2005).
Blair, S. S. Mechanisms of compartment formation: evidence that non-proliferating cells do not play a critical role in defining the D/V lineage restriction in the developing wing of Drosophila. Development 119, 339–351 (1993).
Guthrie, S., Butcher, M. & Lumsden, A. Patterns of cell division and interkinetic nuclear migration in the chick embryo hindbrain. J. Neurobiol. 22, 742–754 (1991).
Lumsden, A. & Keynes, R. Segmental patterns of neuronal development in the chick hindbrain. Nature 337, 424–428 (1989).
Heyman, I., Kent, A. & Lumsden, A. Cellular morphology and extracellular space at rhombomere boundaries in the chick embryo hindbrain. Dev. Dyn. 198, 241–253 (1993).
Stellabotte, F., Dobbs-McAuliffe, B., Fernandez, D. A., Feng, X. & Devoto, S. H. Dynamic somite cell rearrangements lead to distinct waves of myotome growth. Development 134, 1253–1257 (2007).
Daggett, D. F., Domingo, C. R., Currie, P. D. & Amacher, S. L. Control of morphogenetic cell movements in the early zebrafish myotome. Dev. Biol. 309, 169–179 (2007).
Hollway, G. E. et al. Whole-somite rotation generates muscle progenitor cell compartments in the developing zebrafish embryo. Dev. Cell 12, 207–219 (2007).
Henry, C. A., Hall, L. A., Burr Hille, M., Solnica-Krezel, L. & Cooper, M. S. Somites in zebrafish doubly mutant for knypek and trilobite form without internal mesenchymal cells or compaction. Curr. Biol. 10, 1063–1066 (2000).
Julich, D., Geisler, R. & Holley, S. A. Integrinα5 and δ/notch signaling have complementary spatiotemporal requirements during zebrafish somitogenesis. Dev. Cell 8, 575–586 (2005).
Koshida, S. et al. Integrinα5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev. Cell 8, 587–598 (2005).
Kragtorp, K. A. & Miller, J. R. Integrin α5 is required for somite rotation and boundary formation in Xenopus. Dev. Dyn. 236, 2713–2720 (2007).
Martins, G. G. et al. Dynamic 3D cell rearrangements guided by a fibronectin matrix underlie somitogenesis. PLoS ONE 4, e7429 (2009).
Rifes, P. et al. Redefining the role of ectoderm in somitogenesis: a player in the formation of the fibronectin matrix of presomitic mesoderm. Development 134, 3155–3165 (2007).
Georges-Labouesse, E. N., George, E. L., Rayburn, H. & Hynes, R. O. Mesodermal development in mouse embryos mutant for fibronectin. Dev. Dyn. 207, 145–156 (1996).
Steinberg, M. S. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science 141, 401–408 (1963).
Godt, D. & Tepass, U. Drosophila oocyte localization is mediated by differential cadherin-based adhesion. Nature 395, 387–391 (1998).
Gonzalez-Reyes, A. & St. Johnston, D. Patterning of the follicle cell epithelium along the anterior-posterior axis during Drosophila oogenesis. Development 125, 2837–2846 (1998).
Nose, A., Nagafuchi, A. & Takeichi, M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell 54, 993–1001 (1988).
Shinza-Kameda, M., Takasu, E., Sakurai, K., Hayashi, S. & Nose, A. Regulation of layer-specific targeting by reciprocal expression of a cell adhesion molecule, capricious. Neuron 49, 205–213 (2006).
Milan, M., Weihe, U., Perez, L. & Cohen, S. M. The LRR proteins capricious and Tartan mediate cell interactions during DV boundary formation in the Drosophila wing. Cell 106, 785–794 (2001).
Mao, Y., Kerr, M. & Freeman, M. Modulation of Drosophila retinal epithelial integrity by the adhesion proteins capricious and tartan. PLoS ONE 3, e1827 (2008).
Harris, A. K. Is cell sorting caused by differences in the work of intercellular adhesion? A critique of the Steinberg hypothesis. J. Theor. Biol. 61, 267–285 (1976).
Brodland, G. W. The differential interfacial tension hypothesis (DITH): a comprehensive theory for the self-rearrangement of embryonic cells and tissues. J. Biomech. Eng. 124, 188–197 (2002).
Lecuit, T. & Lenne, P. F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nature Rev. Mol. Cell Biol. 8, 633–644 (2007).
Major, R. J. & Irvine, K. D. Localization and requirement for Myosin II at the dorsal-ventral compartment boundary of the Drosophila wing. Dev. Dyn. 235, 3051–3058 (2006).
Brodland, G. W. & Chen, H. H. The mechanics of heterotypic cell aggregates: insights from computer simulations. J. Biomech. Eng. 122, 402–407 (2000).
Rauzi, M., Verant, P., Lecuit, T. & Lenne, P. F. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nature Cell Biol. 10, 1401–1410 (2008).
Uehata, M. et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389, 990–994 (1997).
Winter, C. G. et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105, 81–91 (2001).
Simone, R. P. & DiNardo, S. Actomyosin contractility and Discs large contribute to junctional conversion in guiding cell alignment within the Drosophila embryonic epithelium. Development 137, 1385–1394 (2010).
Wei, S. Y. et al. Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Dev. Cell 8, 493–504 (2005).
Acknowledgements
The authors would like to thank Y. Saga, Y. Takahashi and S. Holley for comments on the manuscript. We thank D. Umetsu for help in preparing Figure 2. We apologize to all authors whose primary work we could not cite owing to space limitations. Work in the laboratory of C.D. is supported by the Max Planck Society, the Deutsche Forschungsgemeinschaft and the Human Frontiers Science Program. A.C.O. is supported by the Max Planck Society and the European Research Council under the European Communities Seventh Framework Programme (FP7/2007-2013) / ERC grant no. 207634. M.B. is supported by the TU Dresden, the Deutsche Forschungsgemeinschaft (SFB 655 and CRTD) and the European Union (ZF Health).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Glossary
- French flag model
-
A tissue-patterning scenario in which a gradient of secreted signal causes a concentration-dependent activation of three target genes in non-overlapping and abutting domains across a field of initially undifferentiated cells. The idea comes from Lewis Wolpert, and the name refers to the three fields of colour on the French flag.
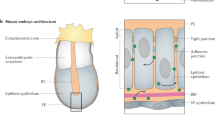
- Paraxial mesoderm
-
The bilaterally symmetrical tissue extending from the tail to the head of the vertebrate embryo that forms somites and their derivatives, such as bone, muscle, tendons and skin.
- Mesenchymal-to-epithelial transition
-
The process whereby a mesenchymal population of cells rearrange their local positions and cell polarity to build an epithelium.
- Telencephalon
-
The most anterior segment of the vertebrate brain. It gives rise to the forebrain and, in mammals, the neocortex.
- Zona limitans intrathalamica
-
A zone that divides the dorsal and ventral thalamus of the forebrain.
- Wing imaginal disc
-
An epithelial tissue that gives rise to the wings and parts of the body wall of adult flies. It is subdivided by the anteroposterior and dorsoventral compartment boundaries.
- Tension
-
A force relating to the stretching of an object; the opposite of compression.
- Hox gene family
-
A family of homeobox DNA-binding domain-containing transcription factors that were initially identified by their function in homeotic transformations.
- Rhombomere
-
The basic unit of segmental organization in the hindbrain. The rhombomere has lineage or compartment boundaries.
- Basic helix–loop–helix
-
A family of transcription factors that are characterized by their basic helix–loop–helix DNA binding and dimerization domain structure.
- Clock and wavefront
-
A mechanism for segmentally patterning the vertebrate paraxial mesoderm, involving a cellular oscillator, the clock, in the cells of the presomitic mesoderm, and a wavefront of differentiation that arrests the clock as it moves across the presomitic mesoderm.
- Integrin
-
A cell surface transmembrane protein that binds fibronectin; integrins are usually associated with focal adhesion complexes.
- Fibronectin
-
An extracellular matrix glycoprotein that is capable of forming fibrils, a ligand for integrins.
- Ephrin reverse signalling
-
The activity of ephrin cell surface proteins, initially thought to be ligands only, to transduce a signal from the Eph-type receptor tyrosine kinase.
- Cadherin
-
A cell surface transmembrane calcium-dependent cell-adhesion protein that is capable of homophilic binding. Cadherins are usually associated with adherens junctions in epithelial tissue.
- Chromophore-assisted laser inactivation
-
The use of high-intensity laser light delivered to subcellular locations with fluorescently tagged proteins of interest to inactivate them through the local release of free radicals from the stimulated chromophore.
- Adherens junctions
-
Multiprotein membrane complexes that mediate adhesion between epithelial cells. Adherens junctions contain cadherins, α- and β-catenins, and p120, the cytoplasmic faces of which connect to the actin cytoskeleton.
Rights and permissions
About this article
Cite this article
Dahmann, C., Oates, A. & Brand, M. Boundary formation and maintenance in tissue development. Nat Rev Genet 12, 43–55 (2011). https://doi.org/10.1038/nrg2902
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg2902
This article is cited by
-
Peritoneal mesometrial resection with lymphadenectomy following prior hysterectomy in intermediate/high-risk endometrial cancer: feasibility and safety
Archives of Gynecology and Obstetrics (2024)
-
Cell invasion during competitive growth of polycrystalline solidification patterns
Nature Communications (2023)
-
4-bit adhesion logic enables universal multicellular interface patterning
Nature (2022)
-
Totale mesometriale Resektion (TMMR) + therapeutische Lymphonodektomie (tLNE) beim Zervixkarzinom FIGO IB – IIAIIA
Der Gynäkologe (2022)
-
Asymptotic Behavior of a Nonlocal Advection System with Two Populations
Journal of Dynamics and Differential Equations (2022)