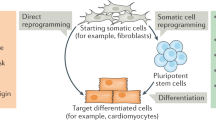
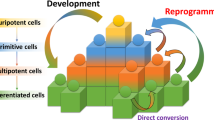
Abstract
No-one can have failed to notice the splash that induced pluripotent stem (iPS) cells have made in the few years since somatic cells were first reprogrammed to pluripotency. But what is their real promise, where should research efforts be focused, and are we at a stage where we can replace embryonic stem cells? Four pioneering iPS cell researchers offer their personal insights into these and other questions of current debate. As well expressing hope for the improved understanding and treatment of human disease, they urge caution over safety and propose the establishment of iPS cell banks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Banito, A. et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 23, 2134–2139 (2009).
Hong, H. et al. Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature 460, 1132–1135 (2009).
Utikal, J. et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature 460, 1145–1148 (2009).
Marión, R. M. et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature 460, 1149–1153 (2009).
Li, H. et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature 460, 1136–1139 (2009).
Kawamura, T. et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 460, 1140–1144 (2009).
Zhao, Y. et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell 3, 475–479 (2008).
Ebert, A. E. et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457, 277–280 (2009).
Lee, G. et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461, 402–406 (2009).
Raya, A. et al. Disease-corrected haematopoetic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature 460, 53–59 (2009).
Park, I.-H. et al. Disease-specific induced pluripotent stem cells. Cell 134, 877–886 (2008).
Dimos, J. T., Rodolfa, K. T. et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321, 1218–1221 (2008).
Stadtfeld, M. et al. Induced pluripotent stem cells generated without viral integration. Science 322, 945–949 (2009).
Yu, J. et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 (2009).
Woltjen, K. et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458, 466–470 (2009).
Okita, K. et al. Generation of induced mouse pluripotent stem cells without viral vectors. Science 322, 949–953 (2008).
Miura, K. et al. Variation in the safety of induced pluripotent stem cell lines. Nature Biotech. 27, 743–745 (2009).
Hanna, J. et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318, 1920–1930 (2007).
Hotta, A. et al. Isolation of human iPS cells using EOS lentiviral vectors to select for pluripotency. Nature Methods 6, 370–376 (2009).
Wichterle, H. et al. Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385–397 (2002).
Ying, Q. L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Konrad Hochedlinger is a member of the scientific advisory board of iPierian.
Related links
Related links
FURTHER INFORMATION
Juan Carlos Izpisúa Belmonte's homepages
Juan Carlos Izpisúa Belmonte's homepages
Glossary
- Bivalent histone modification
-
The co-occurrence of histone tail methylation marks that are associated with transcriptional activation and repression. Bivalency is observed in mammalian embryonic stem cells at developmentally important genes.
- DNA methylome
-
The pattern of DNA methylation across the genome. Many DNA methylation marks are set up during early mammalian development and are erased in the germ line.
- Neurosphere
-
A cluster of neurogenic cells that is generated from a single neural stem cell or progenitor cell when it is cultured in a semi-solid medium that contains appropriate neurotrophic growth factors.
- p53
-
A transcription factor encoded by TP53 that is known to be a tumour suppressor and to be involved in the regulation of many cellular pathways, including the cell cycle.
- Reprogramming factors
-
Transcription factors that enable reprogramming to pluripotency when expressed together in adult somatic cells. The four factors originally used were OCT4, SOX2, Krüppel-like factor 4 and MYC.
- Teratoma
-
A tumour consisting of several cell types.
Rights and permissions
About this article
Cite this article
Belmonte, J., Ellis, J., Hochedlinger, K. et al. Induced pluripotent stem cells and reprogramming: seeing the science through the hype. Nat Rev Genet 10, 878–883 (2009). https://doi.org/10.1038/nrg2700
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg2700
This article is cited by
-
Untangling the Biology of Genetic Cardiomyopathies with Pluripotent Stem Cell Disease Models
Current Cardiology Reports (2017)
-
The Current Status of iPS Cells in Cardiac Research and Their Potential for Tissue Engineering and Regenerative Medicine
Stem Cell Reviews and Reports (2014)
-
Stability of genomic imprinting in human induced pluripotent stem cells
BMC Genetics (2013)
-
Efficient expansion of human keratinocyte stem/progenitor cells carrying a transgene with lentiviral vector
Stem Cell Research & Therapy (2013)
-
The p53–PUMA axis suppresses iPSC generation
Nature Communications (2013)