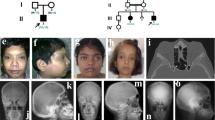
Abstract
Osteopetrosis is a genetic condition of increased bone mass, which is caused by defects in osteoclast formation and function. Both autosomal recessive and autosomal dominant forms exist, but this Review focuses on autosomal recessive osteopetrosis (ARO), also known as malignant infantile osteopetrosis. The genetic basis of this disease is now largely uncovered: mutations in TCIRG1, CLCN7, OSTM1, SNX10 and PLEKHM1 lead to osteoclast-rich ARO (in which osteoclasts are abundant but have severely impaired resorptive function), whereas mutations in TNFSF11 and TNFRSF11A lead to osteoclast-poor ARO. In osteoclast-rich ARO, impaired endosomal and lysosomal vesicle trafficking results in defective osteoclast ruffled-border formation and, hence, the inability to resorb bone and mineralized cartilage. ARO presents soon after birth and can be fatal if left untreated. However, the disease is heterogeneous in clinical presentation and often misdiagnosed. This article describes the genetics of ARO and discusses the diagnostic role of next-generation sequencing methods. The management of affected patients, including guidelines for the indication of haematopoietic stem cell transplantation (which can provide a cure for many types of ARO), are outlined. Finally, novel treatments, including preclinical data on in utero stem cell treatment, RANKL replacement therapy and denosumab therapy for hypercalcaemia are also discussed.
Key Points
-
Autosomal recessive osteopetrosis (ARO) is a genetically and phenotypically heterogeneous disease; most forms result from late endosomal trafficking defects that prevent osteoclast ruffled-border formation
-
Early molecular diagnosis is essential to guide management decisions, and novel genetic tools should enable identification of causative mutations in all patients with ARO in the near future
-
Haematopoietic stem cell transplantation (HSCT) can cure ARO if given in early life to patients with osteoclast-intrinsic disease without neurodegenerative complications
-
New treatments that target RANKL/RANK signalling offer promise in ARO subtypes that currently cannot be cured by HSCT and to prevent hypercalcaemia after HSCT
-
Study of osteopetrosis-causing mutations continues to identify essential genes for osteoclast function, to increase understanding of osteoclast biology and to reveal novel targets for therapeutic intervention
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stark, Z. & Savarirayan, R. Osteopetrosis. Orphanet. J. Rare Dis. 4, 5 (2009).
Del Fattore, A., Cappariello, A. & Teti, A. Genetics, pathogenesis and complications of osteopetrosis. Bone 42, 19–29 (2008).
de Vernejoul, M. C. & Kornak, U. Heritable sclerosing bone disorders: presentation and new molecular mechanisms. Ann. NY Acad. Sci. 1192, 269–277 (2010).
Perdu, B. & Van Hul, W. Sclerosing bone disorders: too much of a good thing. Crit. Rev. Eukaryot. Gene Expr. 20, 195–212 (2010).
Villa, A., Guerrini, M. M., Cassani, B., Pangrazio, A. & Sobacchi, C. Infantile malignant, autosomal recessive osteopetrosis: the rich and the poor. Calcif. Tissue Int. 84, 1–12 (2009).
Sly, W. S., Hewett-Emmett, D., Whyte, M. P., Yu, Y. S. & Tashian, R. E. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc. Natl Acad. Sci. USA 80, 2752–2756 (1983).
Marks, S. C. Jr. Osteoclast biology: lessons from mammalian mutations. Am. J. Med. Genet. 34, 43–54 (1989).
Doffinger, R. et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NFκB signaling. Nat. Genet. 27, 277–285 (2001).
Villa, A., Vezzoni, P. & Frattini, A. Osteopetroses and immunodeficiencies in humans. Curr. Opin. Allergy Clin. Immunol. 6, 421–427 (2006).
Gelb, B. D., Shi, G. P., Chapman, H. A. & Desnick, R. J. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 273, 1236–1238 (1996).
Mory, A. et al. Kindlin-3: a new gene involved in the pathogenesis of LAD-III. Blood 112, 2591 (2008).
Malinin, N. L. et al. A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat. Med. 15, 313–318 (2009).
Harris, E. S., Weyrich, A. S. & Zimmerman, G. A. Lessons from rare maladies: leukocyte adhesion deficiency syndromes. Curr. Opin. Hematol. 20, 16–25 (2013).
Frattini, A. et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat. Genet. 25, 343–346 (2000).
Sobacchi, C. et al. The mutational spectrum of human malignant autosomal recessive osteopetrosis. Hum. Mol. Genet. 10, 1767–1773 (2001).
Bliznetz, E. A. et al. Genetic analysis of autosomal recessive osteopetrosis in Chuvashiya: the unique splice site mutation in TCIRG1 gene spread by the founder effect. Eur. J. Hum. Genet. 17, 664–672 (2009).
Fasth, A. Osteopetrosis—more than only a disease of the bone. Am. J. Hematol. 84, 469–470 (2009).
Souraty, N. et al. Molecular study of six families originating from the Middle-East and presenting with autosomal recessive osteopetrosis. Eur. J. Med. Genet. 50, 188–199 (2007).
Ihde, L. L. et al. Sclerosing bone dysplasias: review and differentiation from other causes of osteosclerosis. Radiographics 31, 1865–1882 (2011).
Dlouhy, B. J. & Menezes, A. H. Osteopetrosis with Chiari I malformation: presentation and surgical management. J. Neurosurg. Pediatr. 7, 369–374 (2011).
Steward, C. G. Neurological aspects of osteopetrosis. Neuropathol. Appl. Neurobiol. 29, 87–97 (2003).
Stocks, R. M., Wang, W. C., Thompson, J. W., Stocks, M. C. & Horwitz, E. M. Malignant infantile osteopetrosis: otolaryngological complications and management. Arch. Otolaryngol. Head Neck Surg. 124, 689–694 (1998).
Gerritsen, E. J. et al. Autosomal recessive osteopetrosis: variability of findings at diagnosis and during the natural course. Pediatrics 93, 247–253 (1994).
Landa, J., Margolis, N. & Di, C. P. Orthopaedic management of the patient with osteopetrosis. J. Am. Acad. Orthop. Surg. 15, 654–662 (2007).
da Silva Santos, P. S., Esperidiao, A. P. & de Freitas, R. R. Maxillofacial aspects in malignant osteopetrosis. Cleft Palate Craniofac. J. 46, 388–390 (2009).
Sekerci, A. E., Sisman, Y., Ertas, E. T., Sahman, H. & Aydinbelge, M. Infantile malignant osteopetrosis: report of 2 cases with osteomyelitis of the jaws. J. Dent. Child. (Chic.) 79, 93–99 (2012).
Mazzolari, E. et al. A single-center experience in 20 patients with infantile malignant osteopetrosis. Am. J. Hematol. 84, 473–479 (2009).
Gonen, K. A., Yazici, Z., Gokalp, G. & Ucar, A. K. Infantile osteopetrosis with superimposed rickets. Pediatr. Radiol. 43, 189–195 (2012).
Guerrini, M. M. et al. Human osteoclast-poor osteopetrosis with hypogammaglobulinemia due to TNFRSF11A (RANK) mutations. Am. J. Hum. Genet. 83, 64–76 (2008).
Pangrazio, A. et al. RANK-dependent autosomal recessive osteopetrosis: characterization of five new cases with novel mutations. J. Bone Miner. Res. 27, 342–351 (2012).
Frattini, A. et al. Chloride channel CLCN7 mutations are responsible for severe recessive, dominant, and intermediate osteopetrosis. J. Bone Miner. Res. 18, 1740–1747 (2003).
Pangrazio, A. et al. Mutations in OSTM1 (grey lethal) define a particularly severe form of autosomal recessive osteopetrosis with neural involvement. J. Bone Miner. Res. 21, 1098–1105 (2006).
Castellano, C. D. et al. Neuroimaging findings in malignant infantile osteopetrosis due to OSTM1 mutations. Neuropediatrics 38, 154–156 (2007).
Pangrazio, A. et al. Molecular and clinical heterogeneity in CLCN7-dependent osteopetrosis: report of 20 novel mutations. Hum. Mutat. 31, E1071–E1080 (2010).
Schinke, T. et al. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat. Med. 15, 674–681 (2009).
Rees, H., Ang, L. C., Casey, R. & George, D. H. Association of infantile neuroaxonal dystrophy and osteopetrosis: a rare autosomal recessive disorder. Pediatr. Neurosurg. 22, 321–327 (1995).
Ben, H. H. et al. Association of severe autosomal recessive osteopetrosis and Dandy–Walker syndrome with agenesis of the corpus callosum. Acta Orthop. Belg. 67, 528–532 (2001).
Abinun, M. & Pieniazek, P. Successful haematopoietic stem cell transplantation for osteopetrosis due to TCRIG1 mutation. Arch. Dis. Child. 95, 984 (2010).
Stark, Z., Pangrazio, A., McGillivray, G. & Fink, A. M. Association of severe autosomal recessive osteopetrosis and structural brain abnormalities: A case report and review of the literature. Eur. J. Med. Genet. 56, 36–38 (2013).
Yarali, N., Fisgin, T., Duru, F. & Kara, A. Osteopetrosis and Glanzmann's thrombasthenia in a child. Ann. Hematol. 82, 254–256 (2003).
Buchbinder, D. et al. Successful cord blood transplantation in a patient with malignant infantile osteopetrosis and hemophilia. Pediatr. Transplant. 17, E20–E24 (2013).
Pangrazio, A. et al. A homozygous contiguous gene deletion in chromosome 16p13.3 leads to autosomal recessive osteopetrosis in a Jordanian patient. Calcif. Tissue Int. 91, 250–254 (2012).
Askmyr, M. K., Fasth, A. & Richter, J. Towards a better understanding and new therapeutics of osteopetrosis. Br. J. Haematol. 140, 597–609 (2008).
Mellis, D. J., Itzstein, C., Helfrich, M. H. & Crockett, J. C. The skeleton: a multi-functional complex organ. The role of key signalling pathways in osteoclast differentiation and in bone resorption. J. Endocrinol. 211, 131–143 (2011).
Leisle, L., Ludwig, C. F., Wagner, F. A., Jentsch, T. J. & Stauber, T. ClC-7 is a slowly voltage-gated 2Cl−/1H+-exchanger and requires Ostm1 for transport activity. EMBO J. 30, 2140–2152 (2011).
Tabata, K. et al. Rubicon and PLEKHM1 negatively regulate the endocytic/autophagic pathway via a novel Rab7-binding domain. Mol. Biol. Cell 21, 4162–4172 (2010).
Ye, S. et al. LIS1 regulates osteoclast formation and function through its interactions with dynein/dynactin and Plekhm1. PLoS ONE 6, e27285 (2011).
Aker, M. et al. An SNX10 mutation causes malignant osteopetrosis of infancy. J. Med. Genet. 49, 221–226 (2012).
Megarbane, A. et al. Homozygous stop mutation in the SNX10 gene in a consanguineous Iraqi boy with osteopetrosis and corpus callosum hypoplasia. Eur. J. Med. Genet. 56, 32–35 (2013).
Worby, C. A. & Dixon, J. E. Sorting out the cellular functions of sorting nexins. Nat. Rev. Mol. Cell Biol. 3, 919–931 (2002).
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Nakagawa, N. et al. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem. Biophys. Res. Commun. 253, 395–400 (1998).
Takayanagi, H. New developments in osteoimmunology. Nat. Rev. Rheumatol. 8, 684–689 (2012).
Susani, L. et al. TCIRG1-dependent recessive osteopetrosis: mutation analysis, functional identification of the splicing defects, and in vitro rescue by U1 snRNA. Hum. Mutat. 24, 225–235 (2004).
Pangrazio, A. et al. Autosomal recessive osteopetrosis: report of 41 novel mutations in the TCIRG1 gene and diagnostic implications. Osteoporos. Int. 23, 2713–2718 (2012).
Pangrazio, A. et al. Characterization of a novel Alu–Alu recombination-mediated genomic deletion in the TCIRG1 gene in five osteopetrotic patients. J. Bone Miner. Res. 24, 162–167 (2009).
Ott, C. E. et al. Severe neuronopathic autosomal recessive osteopetrosis due to homozygous deletions affecting OSTM1. Bone 55, 292–297 (2013).
Pangrazio, A. et al. SNX10 mutations define a subgroup of human Autosomal Recessive Osteopetrosis with variable clinical severity. J. Bone Miner. Res. 28, 1041–1049 (2012).
Hecht, J. et al. Detection of novel skeletogenesis target genes by comprehensive analysis of a Runx2−/− mouse model. Gene Expr. Patterns 7, 102–112 (2007).
Zhu, C. H., Morse, L. R. & Battaglino, R. A. SNX10 is required for osteoclast formation and resorption activity. J. Cell Biochem. 113, 1608–1615 (2012).
Choi, M. et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc. Natl Acad. Sci. USA 106, 19096–19101 (2009).
Glazov, E. A. et al. Whole-exome re-sequencing in a family quartet identifies POP1 mutations as the cause of a novel skeletal dysplasia. PLoS Genet. 7, e1002027 (2011).
Simpson, M. A. et al. Mutations in NOTCH2 cause Hajdu–Cheney syndrome, a disorder of severe and progressive bone loss. Nat. Genet. 43, 303–305 (2011).
Sirmaci, A. et al. Mutations in ANKRD11 cause KBG syndrome, characterized by intellectual disability, skeletal malformations, and macrodontia. Am. J. Hum. Genet. 89, 289–294 (2011).
Sarig, O. et al. Short stature, onychodysplasia, facial dysmorphism, and hypotrichosis syndrome is caused by a POC1A mutation. Am. J. Hum. Genet. 91, 337–342 (2012).
Yamaguchi, T. et al. Exome resequencing combined with linkage analysis identifies novel PTH1R variants in primary failure of tooth eruption in Japanese. J. Bone Miner. Res. 26, 1655–1661 (2011).
Zankl, A. et al. Multicentric carpotarsal osteolysis is caused by mutations clustering in the amino-terminal transcriptional activation domain of MAFB. Am. J. Hum. Genet. 90, 494–501 (2012).
Campeau, P. M. et al. Whole-exome sequencing identifies mutations in the nucleoside transporter gene SLC29A3 in dysosteosclerosis, a form of osteopetrosis. Hum. Mol. Genet. 21, 4904–4909 (2012).
Molho-Pessach, V. et al. The H syndrome is caused by mutations in the nucleoside transporter hENT3. Am. J. Hum. Genet. 83, 529–534 (2008).
Cliffe, S. T. et al. SLC29A3 gene is mutated in pigmented hypertrichosis with insulin-dependent diabetes mellitus syndrome and interacts with the insulin signaling pathway. Hum. Mol. Genet. 18, 2257–2265 (2009).
Bruzzaniti, A. & Baron, R. Molecular regulation of osteoclast activity. Rev. Endocr. Metab. Disord. 7, 123–139 (2006).
Teitelbaum, S. L. & Ross, F. P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649 (2003).
Van Wesenbeeck, L. & Van Hul, W. Lessons from osteopetrotic mutations in animals: impact on our current understanding of osteoclast biology. Crit. Rev. Eukaryot. Gene Expr. 15, 133–162 (2005).
Helfrich, M. H., Crockett, J. C., Hocking, L. J. & Coxon, F. P. The pathogenesis of osteoclast diseases: some knowns, but still many unknowns. BoneKEy–Osteovision 4, 61–77 (2007).
Coxon, F. P. & Taylor, A. Vesicular trafficking in osteoclasts. Semin. Cell Dev. Biol. 19, 424–433 (2008).
Teitelbaum, S. L. The osteoclast and its unique cytoskeleton. Ann. NY Acad. Sci. 1240, 14–17 (2011).
Kornak, U. et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104, 205–215 (2001).
Blair, H. C. et al. In vitro differentiation of CD14 cells from osteopetrotic subjects: contrasting phenotypes with TCIRG1, CLCN7, and attachment defects. J. Bone Miner. Res. 19, 1329–1338 (2004).
Del Fattore, A. et al. Clinical, genetic, and cellular analysis of 49 osteopetrotic patients: implications for diagnosis and treatment. J. Med. Genet. 43, 315–325 (2006).
Maranda, B. et al. Clinical and cellular manifestations of OSTM1-related infantile osteopetrosis. J. Bone Miner. Res. 23, 296–300 (2008).
Li, Y. P., Chen, W., Liang, Y., Li, E. & Stashenko, P. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat. Genet. 23, 447–451 (1999).
Scimeca, J. C. et al. The gene encoding the mouse homologue of the human osteoclast-specific 116-kDa V-ATPase subunit bears a deletion in osteosclerotic (oc/oc) mutants. Bone 26, 207–213 (2000).
Kasper, D. et al. Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 24, 1079–1091 (2005).
Chalhoub, N. et al. Grey-lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat. Med. 9, 399–406 (2003).
Qin, A. et al. V-ATPases in osteoclasts: structure, function and potential inhibitors of bone resorption. Int. J. Biochem. Cell Biol. 44, 1422–1435 (2012).
Hiesinger, P. R. et al. The V-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell 121, 607–620 (2005).
Sun-Wada, G. H. et al. The a3 isoform of V-ATPase regulates insulin secretion from pancreatic β-cells. J. Cell Sci. 119, 4531–4540 (2006).
Lee, B. S., Gluck, S. L. & Holliday, L. S. Interaction between vacuolar H+-ATPase and microfilaments during osteoclast activation. J. Biol. Chem. 274, 29164–29171 (1999).
Holliday, L. S. et al. The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site. J. Biol. Chem. 275, 32331–32337 (2000).
Zuo, J. et al. Actin binding activity of subunit B of vacuolar H+-ATPase is involved in its targeting to ruffled membranes of osteoclasts. J. Bone Miner. Res. 21, 714–721 (2006).
Nakamura, I. et al. Lack of vacuolar proton ATPase association with the cytoskeleton in osteoclasts of osteosclerotic (oc/oc) mice. FEBS Lett. 401, 207–212 (1997).
Nakamura, I. et al. Phosphatidylinositol-3 kinase is involved in ruffled border formation in osteoclasts. J. Cell Physiol. 172, 230–239 (1997).
Chen, S. H. et al. Vacuolar H+-ATPase binding to microfilaments: regulation in response to phosphatidylinositol 3-kinase activity and detailed characterization of the actin-binding site in subunit B. J. Biol. Chem. 279, 7988–7998 (2004).
Shinohara, M. et al. Class IA phosphatidylinositol 3-kinase regulates osteoclastic bone resorption through protein kinase B-mediated vesicle transport. J. Bone Miner. Res. 27, 2464–2475 (2012).
Ochotny, N. et al. The V-ATPase a3 subunit mutation R740S is dominant negative and results in osteopetrosis in mice. J. Bone Miner. Res. 26, 1484–1493 (2011).
Hurtado-Lorenzo, A. et al. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat. Cell Biol. 8, 124–136 (2006).
Jentsch, T. J. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit. Rev. Biochem. Mol. Biol. 43, 3–36 (2008).
Stauber, T. & Jentsch, T. J. Chloride in Vesicular Trafficking and Function. Annu. Rev. Physiol. 75, 453–477 (2012).
Graves, A. R., Curran, P. K., Smith, C. L. & Mindell, J. A. The Cl−/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 453, 788–792 (2008).
Lange, P. F., Wartosch, L., Jentsch, T. J. & Fuhrmann, J. C. ClC-7 requires Ostm1 as a β-subunit to support bone resorption and lysosomal function. Nature 440, 220–223 (2006).
Novarino, G., Weinert, S., Rickheit, G. & Jentsch, T. J. Endosomal chloride-proton exchange rather than chloride conductance is crucial for renal endocytosis. Science 328, 1398–1401 (2010).
Saito, M., Hanson, P. I. & Schlesinger, P. Luminal chloride-dependent activation of endosome calcium channels: patch clamp study of enlarged endosomes. J. Biol. Chem. 282, 27327–27333 (2007).
Luzio, J. P., Bright, N. A. & Pryor, P. R. The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem. Soc. Trans. 35, 1088–1091 (2007).
Weinert, S. et al. Lysosomal pathology and osteopetrosis upon loss of H+-driven lysosomal Cl− accumulation. Science 328, 1401–1403 (2010).
Cigic, B. & Pain, R. H. Location of the binding site for chloride ion activation of cathepsin C. Eur. J. Biochem. 264, 944–951 (1999).
Wartosch, L., Fuhrmann, J. C., Schweizer, M., Stauber, T. & Jentsch, T. J. Lysosomal degradation of endocytosed proteins depends on the chloride transport protein ClC–7. FASEB J. 23, 4056–4068 (2009).
Van Wesenbeeck, L. et al. Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans. J. Clin. Invest. 117, 919–930 (2007).
Sun, Q., Westphal, W., Wong, K. N., Tan, I. & Zhong, Q. Rubicon controls endosome maturation as a Rab7 effector. Proc. Natl Acad. Sci. USA 107, 19338–19343 (2010).
Tsai, N. P., Tsui, Y. C. & Wei, L. N. Dynein motor contributes to stress granule dynamics in primary neurons. Neuroscience 159, 647–656 (2009).
Ng, P. Y. et al. Disruption of the dynein–dynactin complex unveils motor-specific functions in osteoclast formation and bone resorption. J. Bone Miner. Res. 28, 119–134 (2013).
Taranta, A. et al. Genotype–phenotype relationship in human ATP6i-dependent autosomal recessive osteopetrosis. Am. J. Pathol. 162, 57–68 (2003).
Shroff, R., Beringer, O., Rao, K., Hofbauer, L. C. & Schulz, A. Denosumab for post-transplantation hypercalcemia in osteopetrosis. N. Engl. J. Med. 367, 1766–1767 (2012).
Chen, Y. et al. A SNX10/V-ATPase pathway regulates ciliogenesis in vitro and in vivo. Cell Res. 22, 333–345 (2012).
Crockett, J. C., Mellis, D. J., Scott, D. I. & Helfrich, M. H. New knowledge on critical osteoclast formation and activation pathways from study of rare genetic diseases of osteoclasts: focus on the RANK/RANKL axis. Osteoporos. Int. 22, 1–20 (2010).
Liu, C. et al. Structural and functional insights of RANKL-RANK interaction and signaling. J. Immunol. 184, 6910–6919 (2010).
Ta, H. M. et al. Structure-based development of a receptor activator of nuclear factor-κB ligand (RANKL) inhibitor peptide and molecular basis for osteopetrosis. Proc. Natl Acad. Sci. USA 107, 20281–20286 (2010).
Douni, E. et al. A RANKL G278R mutation causing osteopetrosis identifies a functional amino acid essential for trimer assembly in RANKL and TNF. Hum. Mol. Genet. 21, 784–798 (2012).
Henriksen, K. et al. Dissociation of bone resorption and bone formation in adult mice with a non-functional V-ATPase in osteoclasts leads to increased bone strength. PLoS ONE 6, e27482 (2011).
Oliveira, G., Boechat, M. I., Amaral, S. M. & Young, L. W. Osteopetrosis and rickets: an intriguing association. Am. J. Dis. Child. 140, 377–378 (1986).
Marks, S. C. Jr, Seifert, M. F. & Lane, P. W. Osteosclerosis, a recessive skeletal mutation on chromosome 19 in the mouse. J. Hered. 76, 171–176 (1985).
Wise, G. E. & King, G. J. Mechanisms of tooth eruption and orthodontic tooth movement. J. Dent. Res. 87, 414–434 (2008).
Wise, G. E., He, H., Gutierrez, D. L., Ring, S. & Yao, S. Requirement of alveolar bone formation for eruption of rat molars. Eur. J. Oral Sci. 119, 333–338 (2011).
Dick, H. M. & Simpson, W. J. Dental changes in osteopetrosis. Oral Surg. Oral Med. Oral Pathol. 34, 408–416 (1972).
Helfrich, M. H. Osteoclast diseases and dental abnormalities. Arch. Oral Biol. 50, 115–122 (2005).
Jälevik, B., Fasth, A. & Dahllof, G. Dental development after successful treatment of infantile osteopetrosis with bone marrow transplantation. Bone Marrow Transplant. 29, 537–540 (2002).
Koehne, T. et al. Osteopetrosis, osteopetrorickets and hypophosphatemic rickets differentially affect dentin and enamel mineralization. Bone 53, 25–33 (2012).
Xue, Y., Wang, W., Mao, T. & Duan, X. Report of two Chinese patients suffering from CLCN7-related osteopetrosis and root dysplasia. J. Craniomaxillofac. Surg. 40, 416–420 (2012).
McLeod, N. M., Brennan, P. A. & Ruggiero, S. L. Bisphosphonate osteonecrosis of the jaw: a historical and contemporary review. Surgeon 10, 36–42 (2012).
Qi, W. X., Tang, L. N., He, A. N., Yao, Y. & Shen, Z. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: a meta-analysis of seven randomized controlled trials. Int. J. Clin. Oncol. http://dx.doi.org/10.1007/s10147-013-0561-6.
Coccia, P. F. et al. Successful bone-marrow transplantation for infantile malignant osteopetrosis. N. Engl. J. Med. 302, 701–708 (1980).
Schulz, A. S. et al. Osteopetrosis: a heterogeneous group of dieases requiring individualized therapeutic strategies [online], (2013).
Steward, C. G. et al. High peripheral blood progenitor cell counts enable autologous backup before stem cell transplantation for malignant infantile osteopetrosis. Biol. Blood Marrow Transplant. 11, 115–121 (2005).
Johansson, M. K. et al. Hematopoietic stem cell-targeted neonatal gene therapy reverses lethally progressive osteopetrosis in oc/oc mice. Blood 109, 5178–5185 (2007).
Naldini, L. Ex vivo gene transfer and correction for cell-based therapies. Nat. Rev. Genet. 12, 301–315 (2011).
Frattini, A. et al. Rescue of ATPa3-deficient murine malignant osteopetrosis by hematopoietic stem cell transplantation in utero. Proc. Natl Acad. Sci. USA 102, 14629–14634 (2005).
Tondelli, B. et al. Fetal liver cells transplanted in utero rescue the osteopetrotic phenotype in the oc/oc mouse. Am. J. Pathol. 174, 727–735 (2009).
Sobacchi, C. et al. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat. Genet. 39, 960–962 (2007).
Hofbauer, L. C. et al. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone Miner. Res. 15, 2–12 (2000).
Lloyd, S. A. et al. Soluble RANKL induces high bone turnover and decreases bone volume, density, and strength in mice. Calcif. Tissue Int. 82, 361–372 (2008).
Tomimori, Y. et al. Evaluation of pharmaceuticals with a novel 50-hour animal model of bone loss. J. Bone Miner. Res. 24, 1194–1205 (2009).
Campbell, G. M., Ominsky, M. S. & Boyd, S. K. Bone quality is partially recovered after the discontinuation of RANKL administration in rats by increased bone mass on existing trabeculae: an in vivo micro-CT study. Osteoporos. Int. 22, 931–942 (2011).
Mundy, G. R. & Edwards, J. R. The osteoclast—not always guilty. Cell Metab. 6, 157–159 (2007).
Kim, N., Odgren, P. R., Kim, D. K., Marks, S. C. Jr & Choi, Y. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc. Natl Acad. Sci. USA 97, 10905–10910 (2000).
Lo Iacono, N. et al. Osteopetrosis rescue upon RANKL administration to Rankl−/− mice: a new therapy for human RANKL-dependent ARO. J. Bone Miner. Res. 27, 2501–2510 (2012).
Lo Iacono, N. et al. RANKL Cytokine: From pioneer of the osteoimmunology era to cure for a rare disease. Clin. Dev. Immunol. http://dx.doi.org/10.1155/2013/412768.
Gerritsen, E. J. A. et al. Bone marrow transplantation for autosomal recessive osteopetrosis. A report from the Working Party on Inborn Errors of the European Bone Marrow Transplantation Group. J. Pediatr. 125, 896–902 (1994).
Driessen, G. J. et al. Long-term outcome of haematopoietic stem cell transplantation in autosomal recessive osteopetrosis: an EBMT report. Bone Marrow Transplant. 32, 657–663 (2003).
Grigoriadis, A. E. et al. Directed differentiation of hematopoietic precursors and functional osteoclasts from human ES and iPS cells. Blood 115, 2769–2776 (2010).
Helfrich, M., Perdu, B. & Coxon, F. Human recessive osteopetrosis. New understanding of osteoclast function through molecular and functional analysis of a rare genetic bone disease. Osteologie 18, 260–267 (2009).
Schulz, A. et al. Osteopetrosis–Consensus Guidelines of the ESID and the EBMT Working Party Inborn Errors [online], (2011).
Kong, Y. Y. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 (1999).
Li, J. et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc. Natl Acad. Sci. USA 97, 1566–1571 (2000).
Mansour, A. et al. Osteoclasts promote the formation of hematopoietic stem cell niches in bone marrow. J. Exp. Med. 209, 537–549 (2012).
Lymperi, S., Ersek, A., Ferraro, F., Dazzi, F. & Horwood, N. J. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood. 117, 1540–1549 (2011).
Miyamoto, K. et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J. Exp. Med. 208, 2175–2181 (2011).
Henriksen, K., Bollerslev, J., Everts, V. & Karsdal, M. A. Osteoclast activity and subtypes as a function of physiology and pathology—implications for future treatments of osteoporosis. Endocr. Rev. 32, 31–63 (2011).
Corbacioglu, S. et al. Stem cell transplantation in children with infantile osteopetrosis is associated with a high incidence of VOD, which could be prevented with defibrotide. Bone Marrow Transplant. 38, 547–553 (2006).
Corbacioglu, S. et al. Defibrotide for the treatment of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Expert Rev. Hematol. 5, 291–302 (2012).
Steward, C. G. et al. Severe pulmonary hypertension: a frequent complication of stem cell transplantation for malignant infantile osteopetrosis. Br. J. Haematol. 124, 63–71 (2004).
Acknowledgements
The authors acknowledge grant support from the following sources: Ministero della Salute grants GR-2008-1134,625 to C. Sobacchi and RF-2009-1499,542 to A. Villa; Telethon Foundation grant GGP12178 to C. Sobacchi; Research Projects of National Interest (PRIN) Project grant 20122M7T8X_003 by the National Research Program-National Research Council (PNR-CNR) Aging Program 2012–2014 to A. Villa; Arthritis Research UK grants 17285 and 19379 to F. P. Coxon and M. H. Helfrich, respectively. The authors wish to thank: D. Mellis and J. Crockett (University of Aberdeen) for contributing Figure 4 panels a and d, and discussion regarding RANK and RANKL mutants; M. Hoenig (University Medical Centre, Ulm) for critical comments on the manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sobacchi, C., Schulz, A., Coxon, F. et al. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol 9, 522–536 (2013). https://doi.org/10.1038/nrendo.2013.137
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2013.137
This article is cited by
-
Hem1 is essential for ruffled border formation in osteoclasts and efficient bone resorption
Scientific Reports (2024)
-
Estradiol increases cortical and trabecular bone accrual and bone strength in an adolescent male-to-female mouse model of gender-affirming hormone therapy
Bone Research (2024)
-
Molecular Heterogeneity of Osteopetrosis in India: Report of 17 Novel Variants
Indian Journal of Hematology and Blood Transfusion (2024)
-
Successful complete oral rehabilitation of a patient with osteopetrosis with extensive pre-treatments, bone grafts, dental implants and fixed bridges: a multidisciplinary case report
BMC Oral Health (2023)
-
Atp6i deficient mouse model uncovers transforming growth factor-β1 /Smad2/3 as a key signaling pathway regulating odontoblast differentiation and tooth root formation
International Journal of Oral Science (2023)