Abstract
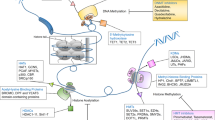
Global and gene-specific changes in the epigenome are hallmarks of most tumour types, including those of pituitary origin. In contrast to genetic mutations, epigenetic changes (aberrant DNA methylation and histone modifications) are potentially reversible. Drugs that specifically target or inhibit DNA methyltransferases (DNMTs) and histone deacetylases (HDACs) can be used to restore the expression of epigenetically silenced genes. These drugs can potentially increase the sensitivity of tumour cells to conventional treatment modalities, such as chemotherapy and radiotherapy. Drug-induced reversal of transcriptional silencing can also be used to restore dopamine-D2-receptor-negative, hormone-refractory tumours to their previous receptor-positive, hormone-responsive status. Synergy between HDAC and DNMT inhibitors makes these pharmacological agents more therapeutically effective when administered in combination than when used alone. Studies in pituitary tumour cell lines show that drug-induced re-expression of the epigenetically silenced dopamine D2 receptor leads to an increase in apoptosis mediated by a receptor agonist. Collectively, the use of drugs to directly or indirectly reverse gene-specific epigenetic changes, in combination with conventional therapeutic interventions, has potential for the clinical management of multiple tumour types—including those of pituitary origin. Furthermore, these drugs can be used to identify epigenetically regulated genes that could be novel, tumour-specific therapeutic targets.
Key Points
-
Global and gene-specific changes to the epigenome are hallmarks of most cancer types, including pituitary tumours
-
Changes to the epigenome, such as aberrant DNA methylation and histone modifications, frequently lead to inappropriate expression or silencing of genes
-
Unlike genetic mutations, epigenetic changes are potentially reversible
-
Drug-mediated reversal of epigenetic transcriptional silencing can increase tumour sensitivity to conventional treatments
-
Proof-of-principle studies show that epigenome-targeting drugs can induce re-expression of the epigenetically silenced dopamine D2 receptor in pituitary tumour cells, and augment apoptosis through a receptor-agonist-mediated pathway
-
Novel strategies for either direct or indirect reversal of aberrant epigenetic changes might provide new therapeutic options for the clinical management of pituitary tumours
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jaenisch, R. & Bird, A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33 (Suppl.), 245–254 (2003).
Jones, P. A. & Baylin, S. B. The epigenomics of cancer. Cell 128, 683–692 (2007).
Sharma, S., Kelly, T. K. & Jones, P. A. Epigenetics in cancer. Carcinogenesis 31, 27–36 (2010).
Rodriguez-Paredes, M. & Esteller, M. Cancer epigenetics reaches mainstream oncology. Nat. Med. 17, 330–339 (2011).
Fuks, F., Burgers, W. A., Brehm, A., Hughes-Davies, L. & Kouzarides, T. DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nat. Genet. 24, 88–91 (2000).
Fuks, F. et al. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J. Biol. Chem. 278, 4035–4040 (2003).
Dudley, K. J., Revill, K., Clayton, R. N. & Farrell, W. E. Pituitary tumours: all silent on the epigenetics front. J. Mol. Endocrinol. 42, 461–468 (2009).
Farrell, W. E. Pituitary tumours: findings from whole genome analyses. Endocr. Relat. Cancer 13, 707–716 (2006).
Zhu, X. et al. Deoxyribonucleic acid methyltransferase 3B promotes epigenetic silencing through histone 3 chromatin modifications in pituitary cells. J. Clin. Endocrinol. Metab. 93, 3610–3617 (2008).
Bird, A. P. CpG-rich islands and the function of DNA methylation. Nature 321, 209–213 (1986).
Craig, J. M. & Bickmore, W. A. The distribution of CpG islands in mammalian chromosomes. Nat. Genet. 7, 376–382 (1994).
Eden, A., Gaudet, F., Waghmare, A. & Jaenisch, R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science 300, 455 (2003).
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007).
Hebbes, T. R., Thorne, A. W. & Crane-Robinson, C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 7, 1395–1402 (1988).
Jenuwein, T. & Allis, C. D. Translating the histone code. Science 293, 1074–1080 (2001).
Al-Azzawi, H. et al. Reversal of endogenous dopamine receptor silencing in pituitary cells augments receptor-mediated apoptosis. Endocrinology 152, 364–373 (2011).
Ezzat, S., Zhu, X., Loeper, S., Fischer, S. & Asa, S. L. Tumor-derived Ikaros 6 acetylates the Bcl-XL promoter to up-regulate a survival signal in pituitary cells. Mol. Endocrinol. 20, 2976–2986 (2006).
Bilodeau, S. et al. Role of Brg1 and HDAC2 in GR trans-repression of the pituitary POMC gene and misexpression in Cushing disease. Genes Dev. 20, 2871–2886 (2006).
Liang, G. et al. Distinct localization of histone H3 acetylation and H3-K4 methylation to the transcription start sites in the human genome. Proc. Natl Acad. Sci. USA 101, 7357–7362 (2004).
Kondo, Y. et al. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat. Genet. 40, 741–750 (2008).
Berdasco, M. & Esteller, M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev. Cell. 19, 698–711 (2010).
Fraga, M. F. & Esteller, M. Towards the human cancer epigenome: a first draft of histone modifications. Cell Cycle 4, 1377–1381 (2005).
Egger, G., Liang, G., Aparicio, A. & Jones, P. A. Epigenetics in human disease and prospects for epigenetic therapy. Nature 429, 457–463 (2004).
Jones, P. A. & Baylin, S. B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 3, 415–428 (2002).
Woloschak, M., Yu, A. & Post, K. D. Frequent inactivation of the p16 gene in human pituitary tumors by gene methylation. Mol. Carcinog. 19, 221–224 (1997).
Simpson, D. J., Bicknell, J. E., McNicol, A. M., Clayton, R. N. & Farrell, W. E. Hypermethylation of the p16/CDKN2A/MTSI gene and loss of protein expression is associated with nonfunctional pituitary adenomas but not somatotrophinomas. Genes Chromosomes Cancer 24, 328–336 (1999).
Simpson, D. J., Hibberts, N. A., McNicol, A. M., Clayton, R. N. & Farrell, W. E. Loss of pRb expression in pituitary adenomas is associated with methylation of the RB1 CpG island. Cancer Res. 60, 1211–1216 (2000).
Simpson, D. J., Clayton, R. N. & Farrell, W. E. Preferential loss of death associated protein kinase expression in invasive pituitary tumours is associated with either CpG island methylation or homozygous deletion. Oncogene 21, 1217–1224 (2002).
Frost, S. J., Simpson, D. J., Clayton, R. N. & Farrell, W. E. Transfection of an inducible p16/CDKN2A construct mediates reversible growth inhibition and G1 arrest in the AtT20 pituitary tumor cell line. Mol. Endocrinol. 13, 1801–1810 (1999).
Zhao, J., Dahle, D., Zhou, Y., Zhang, X. & Klibanski, A. Hypermethylation of the promoter region is associated with the loss of MEG3 gene expression in human pituitary tumors. J. Clin. Endocrinol. Metab. 90, 2179–2186 (2005).
Zhang, X. et al. Loss of expression of GADD45γ, a growth inhibitory gene, in human pituitary adenomas: implications for tumorigenesis. J. Clin. Endocrinol. Metab. 87, 1262–1267 (2002).
Bahar, A., Bicknell, J. E., Simpson, D. J., Clayton, R. N. & Farrell, W. E. Loss of expression of the growth inhibitory gene GADD45γ, in human pituitary adenomas, is associated with CpG island methylation. Oncogene 23, 936–944 (2004).
Dudley, K. J., Revill, K., Whitby, P., Clayton, R. N. & Farrell, W. E. Genome-wide analysis in a murine Dnmt1 knockdown model identifies epigenetically silenced genes in primary human pituitary tumors. Mol. Cancer. Res. 6, 1567–1574 (2008).
Revill, K., Dudley, K. J., Clayton, R. N., McNicol, A. M. & Farrell, W. E. Loss of neuronatin expression is associated with promoter hypermethylation in pituitary adenoma. Endocr. Relat. Cancer 16, 537–548 (2009).
Zhou, Y. et al. Activation of p53 by MEG3 non-coding RNA. J. Biol. Chem. 282, 24731–24742 (2007).
Hayward, B. E. et al. Imprinting of the Gsα gene GNAS1 in the pathogenesis of acromegaly. J. Clin. Invest. 107, R31–R36 (2001).
Bahar, A. et al. Isolation and characterization of a novel pituitary tumor apoptosis gene. Mol. Endocrinol. 18, 1827–1839 (2004).
Abbass, S. A., Asa, S. L. & Ezzat, S. Altered expression of fibroblast growth factor receptors in human pituitary adenomas. J. Clin. Endocrinol. Metab. 82, 1160–1166 (1997).
Zhu, X., Lee, K., Asa, S. L. & Ezzat, S. Epigenetic silencing through DNA and histone methylation of fibroblast growth factor receptor 2 in neoplastic pituitary cells. Am. J. Pathol. 170, 1618–1628 (2007).
Kondo, T. et al. Epigenetically controlled fibroblast growth factor receptor 2 signaling imposes on the RAS/BRAF/mitogen-activated protein kinase pathway to modulate thyroid cancer progression. Cancer Res. 67, 546–5470 (2007).
Kondo, T., Zhu, X., Asa, S. L. & Ezzat, S. The cancer/testis antigen melanoma-associated antigen-A3/A6 is a novel target of fibroblast growth factor receptor 2-IIIb through histone H3 modifications in thyroid cancer. Clin. Cancer Res. 13, 4713–4720 (2007).
Zhu, X., Asa, S. L. & Ezzat, S. Fibroblast growth factor 2 and estrogen control the balance of histone 3 modifications targeting MAGE-A3 in pituitary neoplasia. Clin. Cancer Res. 14, 1984–1996 (2008).
Asa, S. L. & Ezzat, S. The pathogenesis of pituitary tumours. Nat. Rev. Cancer 2, 836–849 (2002).
Fedele, M. et al. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell 9, 459–471 (2006).
Fedele, M., Pierantoni, G. M., Visone, R. & Fusco, A. E2F1 activation is responsible for pituitary adenomas induced by HMGA2 gene overexpression. Cell Div. 1, 17 (2006).
Fedele, M., Palmier, D. & Fusco A. HMGA2: a pituitary tumour subtype-specific oncogene? Mol. Cell. Endocrinol. 326, 19–24 (2010).
Zhu, X. et al. Deoxyribonucleic acid methyltransferase 3B promotes epigenetic silencing through histone 3 chromatin modifications in pituitary cells. J. Clin. Endocrinol. Metab. 93, 3610–3617 (2008).
Zhu, X., Asa, S. L. & Ezzat, S. Ikaros is regulated through multiple histone modifications and deoxyribonucleic acid methylation in the pituitary. Mol. Endocrinol. 21, 1205–1215 (2007).
Wade, P. A. Methyl CpG binding proteins: coupling chromatin architecture to gene regulation. Oncogene 20, 3166–3173 (2001).
Robertson, K. D. et al. DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat. Genet. 25, 338–342 (2000).
Rountree, M. R., Bachman, K. E. & Baylin, S. B. DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nat. Genet. 25, 269–277 (2000).
Belinsky, S. A. et al. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res. 63, 7089–7093 (2003).
Melmed, S. Pathogenesis of pituitary tumors. Nat. Rev. Endocrinol. 7, 257–266 (2011).
Hofland, L. J., Feelders, R. A., de Herder, W. W. & Lamberts, S. W. Pituitary tumours: the sst/D2 receptors as molecular targets. Mol. Cell Endocrinol. 326, 89–98 (2010).
Melmed, S. et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96, 273–288 (2011).
Sherlock, M., Woods, C. & Sheppard, M. C. Medical therapy in acromegaly. Nat. Rev. Endocrinol. 7, 291–300 (2011).
Pivonello, R. et al. Dopamine receptor expression and function in corticotroph ectopic tumors. J. Clin. Endocrinol. Metab. 92, 65–69 (2007).
Ben-Jonathan, N. & Hnasko, R. Dopamine as a prolactin (PRL) inhibitor. Endocr. Rev. 22, 724–763 (2001).
Ferone, D. et al. Preclinical and clinical experiences with the role of dopamine receptors in the treatment of pituitary adenomas. Eur. J. Endocrinol. 156 (Suppl. 1), S37–S43 (2007).
Colao, A., di Sarno, A., Pivonello, R., di Somma, C. & Lombardi, G. Dopamine receptor agonists for treating prolactinomas. Expert Opin. Investig. Drugs 11, 787–800 (2002).
Molitch, M. E. Pharmacologic resistance in prolactinoma patients. Pituitary 8, 43–52 (2005).
Pellegrini, I., Costa, R., Grisoli, F. & Jaquet, P. Abnormal dopamine sensitivity in some human prolactinomas. Horm. Res. 31, 19–23 (1989).
Pellegrini, I. et al. Resistance to bromocriptine in prolactinomas. J. Clin. Endocrinol. Metab. 69, 500–509 (1989).
Filopanti, M. et al. Dopamine D2 receptor gene polymorphisms and response to cabergoline therapy in patients with prolactin-secreting pituitary adenomas. Pharmacogenomics J. 8, 357–363 (2008).
Kelly, T. K., De Carvalho, D. D. & Jones, P. A. Epigenetic modifications as therapeutic targets. Nat. Biotechnol. 28, 1069–1078 (2010).
Yang, X., Lay, F., Han, H. & Jones, P. A. Targeting DNA methylation for epigenetic therapy. Trends Pharmacol. Sci. 31, 536–546 (2010).
Flotho, C. et al. The DNA methyltransferase inhibitors azacitidine, decitabine and zebularine exert differential effects on cancer gene expression in acute myeloid leukemia cells. Leukemia 23, 1019–1028 (2009).
Gius, D. et al. Distinct effects on gene expression of chemical and genetic manipulation of the cancer epigenome revealed by a multimodality approach. Cancer Cell 6, 361–371 (2004).
Zhu, W. G. et al. Increased expression of unmethylated CDKN2D by 5-aza-2'-deoxycytidine in human lung cancer cells. Oncogene 20, 7787–7796 (2001).
Soengas, M. S. et al. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature 409, 207–211 (2001).
Komashko, V. M. & Farnham, P. J. 5-Azacytidine treatment reorganizes genomic histone modification patterns. Epigenetics 5, 229–240 (2010).
Cortez, C. C. & Jones, P. A. Chromatin, cancer and drug therapies. Mutat. Res. 647, 44–51 (2008).
Gore, S. D. et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 66, 6361–6369 (2006).
Karagiannis, T. C. & El-Osta, A. Clinical potential of histone deacetylase inhibitors as stand alone therapeutics and in combination with other chemotherapeutics or radiotherapy for cancer. Epigenetics 1, 121–126 (2006).
Yang, A. S., Estecio, M. R., Garcia-Manero, G., Kantarjian, H. M. & Issa, J. P. Comment on “Chromosomal instability and tumors promoted by DNA hypomethylation” and “Induction of tumors in mice by genomic hypomethylation” (author reply). Science 302, 1153 (2003).
Chesnokova, V. et al. Senescence mediates pituitary hypoplasia and restrains pituitary tumor growth. Cancer Res. 67, 10564–10572 (2007).
Chesnokova, V. et al. p21kip1 restrains pituitary tumor growth. Proc. Natl Acad. Sci. USA 105, 17498–17503 (2008).
Plimack, E. R., Kantarjian, H. M. & Issa, J. P. Decitabine and its role in the treatment of hematopoietic malignancies. Leuk. Lymphoma 48, 1472–1481 (2007).
Kantarjian, H. M. et al. Results of decitabine (5-aza-2'deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer 98, 522–528 (2003).
Cheng, J. C. et al. Preferential response of cancer cells to zebularine. Cancer Cell 6, 151–158 (2004).
Cheng, J. C. et al. Inhibition of DNA methylation and reactivation of silenced genes by zebularine. J. Natl Cancer Inst. 95, 399–409 (2003).
Kristensen, L. S., Nielsen, H. M. & Hansen, L. L. Epigenetics and cancer treatment. Eur. J. Pharmacol. 625, 131–142 (2009).
Yoshida, M., Hoshikawa, Y., Koseki, K., Mori, K. & Beppu, T. Structural specificity for biological activity of trichostatin A, a specific inhibitor of mammalian cell cycle with potent differentiation-inducing activity in Friend leukemia cells. J. Antibiot. (Tokyo) 43, 1101–1106 (1990).
Mai, A. & Altucci, L. Epi-drugs to fight cancer: from chemistry to cancer treatment, the road ahead. Int. J. Biochem. Cell Biol. 41, 199–213 (2009).
Chiba, T. et al. Identification of genes up-regulated by histone deacetylase inhibition with cDNA microarray and exploration of epigenetic alterations on hepatoma cells. J. Hepatol. 41, 436–445 (2004).
Dannenberg, L. O. & Edenberg, H. J. Epigenetics of gene expression in human hepatoma cells: expression profiling the response to inhibition of DNA methylation and histone deacetylation. BMC Genomics 7, 181 (2006).
Cameron, E. E., Bachman, K. E., Myöhänen, S., Herman, J. G. & Baylin, S. B. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat. Genet. 21, 103–107 (1999).
Klisovic, D. D. et al. Depsipeptide (FR901228) inhibits proliferation and induces apoptosis in primary and metastatic human uveal melanoma cell lines. Invest. Ophthalmol. Vis. Sci. 44, 2390–2398 (2003).
Rowther, F. B., Richardson, A., Clayton, R. N. & Farrell, W. E. Bromocriptine and dopamine mediate independent and synergistic apoptotic pathways in pituitary cells. Neuroendocrinology 91, 256–267 (2010).
Scott, S. A. et al. Zebularine inhibits human acute myeloid leukemia cell growth in vitro in association with p15INK4B demethylation and re-expression. Exp. Hematol. 35, 263–273 (2007).
Cheng, J. C. et al. Continuous zebularine treatment effectively sustains demethylation in human bladder cancer cells. Mol. Cell. Biol. 24, 1270–1278 (2004).
Li, L. C., Yeh, C. C., Nojima, D. & Dahiya, R. Cloning and characterization of human estrogen receptor β promoter. Biochem. Biophys. Res. Commun. 275, 682–689 (2000).
Lapidus, R. G. et al. Methylation of estrogen and progesterone receptor gene 5' CpG islands correlates with lack of estrogen and progesterone receptor gene expression in breast tumors. Clin. Cancer Res. 2, 805–810 (1996).
Yang, X. et al. Transcriptional activation of estrogen receptor α in human breast cancer cells by histone deacetylase inhibition. Cancer Res. 60, 6890–6894 (2000).
Yang, X. et al. Synergistic activation of functional estrogen receptor (ER)-α by DNA methyltransferase and histone deacetylase inhibition in human ER-α-negative breast cancer cells. Cancer Res. 61, 7025–7029 (2001).
Walton, T. J. et al. DNA demethylation and histone deacetylation inhibition co-operate to re-express estrogen receptor β and induce apoptosis in prostate cancer cell-lines. Prostate 68, 210–222 (2008).
Fenaux, P. et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 10, 223–232 (2009).
Gore, S. D. & Hermes-DeSantis, E. R. Future directions in myelodysplastic syndrome: newer agents and the role of combination approaches. Cancer Control 15 (Suppl.), 40–49 (2008).
Kim, M. S. et al. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 63, 7291–7300 (2003).
Karagiannis, T. C. & El-Osta, A. Modulation of cellular radiation responses by histone deacetylase inhibitors. Oncogene 25, 3885–3893 (2006).
Dudley, K. J., Revill, K., Whitby, P., Clayton, R. N. & Farrell, W. E. Genome-wide analysis in a murine Dnmt1 knockdown model identifies epigenetically silenced genes in primary human pituitary tumors. Mol. Cancer. Res. 6, 1567–1574 (2008).
Revill, K., Dudley, K. J., Clayton, R. N., McNicol, A. M. & Farrell, W. E. Loss of neuronatin expression is associated with promoter hypermethylation in pituitary adenoma. Endocr. Relat. Cancer 16, 537–548 (2009).
Author information
Authors and Affiliations
Contributions
K. Yacqub-Usman, A. Richardson, C. V. Duong and W. E. Farrell researched data for the Review and all authors made substantial contributions to discussion of content. W. E. Farrell wrote and edited the article. A. Richardson and R. N. Clayton also edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yacqub-Usman, K., Richardson, A., Duong, C. et al. The pituitary tumour epigenome: aberrations and prospects for targeted therapy. Nat Rev Endocrinol 8, 486–494 (2012). https://doi.org/10.1038/nrendo.2012.54
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2012.54
This article is cited by
-
CircVPS13C promotes pituitary adenoma growth by decreasing the stability of IFITM1 mRNA via interacting with RRBP1
Oncogene (2022)
-
Potential biomarkers and lncRNA-mRNA regulatory networks in invasive growth hormone-secreting pituitary adenomas
Journal of Endocrinological Investigation (2021)
-
Genomic Alterations in Sporadic Pituitary Tumors
Current Neurology and Neuroscience Reports (2018)
-
Molecular Mechanisms Underlying Pituitary Pathogenesis
Biochemical Genetics (2016)
-
Histone modification as a drug resistance driver in brain tumors ⁎
Oncology and Translational Medicine (2016)