Abstract
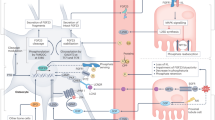
The discovery of fibroblast growth factor 23 (FGF-23) has expanded our understanding of phosphate and vitamin D homeostasis and provided new insights into the pathogenesis of hereditary hypophosphatemic and hyperphosphatemic disorders, as well as acquired disorders of phosphate metabolism, such as chronic kidney disease. FGF-23 is secreted by osteoblasts and osteocytes in bone and principally targets the kidney to regulate the reabsorption of phosphate, the production and catabolism of 1,25-dihydroxyvitamin D and the expression of α-Klotho, an anti-ageing hormone. Secreted FGF-23 plays a central role in complex endocrine networks involving local bone-derived factors that regulate mineralization of extracellular matrix and systemic hormones involved in mineral metabolism. Inactivating mutations of PHEX, DMP1 and ENPP1, which cause hereditary hypophosphatemic disorders and primary defects in bone mineralization, stimulate FGF23 gene transcription in osteoblasts and osteocytes, at least in part, through canonical and intracrine FGF receptor pathways. These FGF-23 regulatory pathways may enable systemic phosphate and vitamin D homeostasis to be coordinated with bone mineralization. FGF-23 also functions as a counter-regulatory hormone for 1,25-dihydroxyvitamin D in a bone–kidney endocrine loop. FGF-23, through regulation of additional genes in the kidney and extrarenal tissues, probably has broader physiological functions beyond regulation of mineral metabolism that account for the association between FGF-23 and increased mortality and morbidity in chronic kidney disease.
Key Points
-
FGF-23 is a hormone produced by osteoblasts and osteocytes in bone that causes phosphaturia and inhibits 1,25-dihydroxyvitamin D production through its binding to FGFR–α-Klotho complexes in kidney tubules
-
Primary elevations of circulating FGF-23 concentrations cause hereditary hypophosphatemic disorders and acquired hypophosphatemic disorders, and reductions in circulating FGF-23 concentrations cause familial tumoral calcinosis
-
FGF-23 is regulated by local bone-derived factors through activation of FGFR-1 pathways and by systemic factors, including 1,25-dihydroxyvitamin D and parathyroid hormone (in a context-dependent fashion) in osteoblasts
-
FGF-23 participates in several physiologically relevant endocrine axes involving 1,25-dihydroxyvitamin D or parathyroid hormone, and possibly additional hormonal networks involving secreted α-Klotho and other kidney-derived factors
-
Elevations in serum FGF-23 is an early adaptive response in chronic kidney disease, leading to maintenance of phosphate balance and development of secondary hyperparathryroidism through reductions in 1,25-dihydroxyvitamin D levels
-
Elevations of circulating FGF-23 are an unexpectedly strong predictor of mortality in patients with renal failure, as a result of mechanisms that remain to be elucidated
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ferron, M. et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142, 296–308 (2010).
Fulzele, K. et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142, 309–319 (2010).
Pi, M., Wu, Y. & Quarles, L. D. GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J. Bone Miner. Res. 26, 1680–1683 (2011).
Quarles, L. D. Evidence for a bone-kidney axis regulating phosphate homeostasis. J. Clin. Invest. 112, 642–646 (2003).
Itoh, N. & Ornitz, D. M. Functional evolutionary history of the mouse Fgf gene family. Dev. Dyn. 237, 18–27 (2008).
Stachowiak, M. K. et al. Integrative nuclear FGFR1 signaling (INFS) as a part of a universal “feed-forward-and-gate” signaling module that controls cell growth and differentiation. J. Cell. Biochem. 90, 662–691 (2003).
Yamashita, T., Yoshioka, M. & Itoh, N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem. Biophys. Res. Commun. 277, 494–498 (2000).
Liu, S. et al. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J. Biol. Chem. 278, 37419–37426 (2003).
Liu, S. et al. Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 291, E38–E49 (2006).
Stubbs, J., Liu, S. & Quarles, L. D. Role of fibroblast growth factor 23 in phosphate homeostasis and pathogenesis of disordered mineral metabolism in chronic kidney disease. Semin. Dial. 20, 302–308 (2007).
Kurosu, H. et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281, 6120–6123 (2006).
Urakawa, I. et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 (2006).
Yu, X. et al. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology 146, 4647–4656 (2005).
Li, H., Martin, A. C., David, V. & Quarles, L. D. Compound deletion of Fgfr3 and Fgfr4 partially rescues the Hyp mouse phenotype. Am. J. Physiol. Endocrinol. Metab. 300, E508–E517 (2010).
Shimada, T. et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am. J. Physiol. Renal Physiol. 289, F1088–F1095 (2005).
Shimada, T. et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl Acad. Sci. USA 98, 6500–6505 (2001).
Bai, X., Miao, D., Li, J., Goltzman, D. & Karaplis, A. C. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology 145, 5269–5279 (2004).
Larsson, T. et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the α1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 145, 3087–3094 (2004).
Fukumoto, S. & Yamashita, T. Fibroblast growth factor-23 is the phosphaturic factor in tumor-induced osteomalacia and may be phosphatonin. Curr. Opin. Nephrol. Hypertens. 11, 385–389 (2002).
Shimada, T. et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 113, 561–568 (2004).
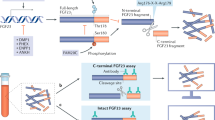
White, K. E. et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 60, 2079–2086 (2001).
Tomiyama, K. et al. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc. Natl Acad. Sci. USA 107, 1666–1671 (2010).
Beckman, M. J. et al. Human 25-hydroxyvitamin D3-24-hydroxylase, a multicatalytic enzyme. Biochemistry 35, 8465–8472 (1996).
Hoenderop, J. G. et al. Regulation of gene expression by dietary Ca2+ in kidneys of 25-hydroxyvitamin D3–1 α-hydroxylase knockout mice. Kidney Int. 65, 531–539 (2004).
Meyer, M. H., Dulde, E. & Meyer, R. A. Jr . The genomic response of the mouse kidney to low-phosphate diet is altered in X-linked hypophosphatemia. Physiol. Genomics 18, 4–11 (2004).
Marsell, R. et al. Gene expression analysis of kidneys from transgenic mice expressing fibroblast growth factor-23. Nephrol. Dial. Transplant. 23, 827–833 (2008).
Quarles, L. D. Endocrine functions of bone in mineral metabolism regulation. J. Clin. Invest. 118, 3820–3828 (2008).
Benet-Pagès, A., Orlik, P., Strom, T. M. & Lorenz-Depiereux, B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum. Mol. Genet. 14, 385–390 (2005).
Larsson, T. et al. Fibroblast growth factor-23 mutants causing familial tumoral calcinosis are differentially processed. Endocrinology 146, 3883–3891 (2005).
Sitara, D. et al. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. Am. J. Pathol. 169, 2161–2170 (2006).
Sitara, D. et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 23, 421–432 (2004).
Kato, K. et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem. 281, 18370–18377 (2006).
Liu, S. & Quarles, L. D. How fibroblast growth factor 23 works. J. Am. Soc. Nephrol. 18, 1637–1647 (2007).
Brown, J. R. & Auger, K. R. Phylogenomics of phosphoinositide lipid kinases: perspectives on the evolution of second messenger signaling and drug discovery. BMC Evol. Biol. 11, 4 (2010).
Sprecher, E. Familial tumoral calcinosis: from characterization of a rare phenotype to the pathogenesis of ectopic calcification. J. Invest. Dermatol. 130, 652–660 (2010).
Zeger, M. D. et al. Hypophosphatemic rickets in opsismodysplasia. J. Pediatr. Endocrinol. Metab. 20, 79–86 (2007).
Moe, S. et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 69, 1945–1953 (2006).
Liu, S., Tang, W., Zhou, J., Vierthaler, L. & Quarles, L. D. Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 293, E1636–E1644 (2007).
Yuan, B. et al. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J. Clin. Invest. 118, 722–734 (2008).
Bowe, A. E. et al. FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem. Biophys. Res. Commun. 284, 977–981 (2001).
Benet-Pagès, A. et al. FGF23 is processed by proprotein convertases but not by PHEX. Bone 35, 455–462 (2004).
Guo, R., Liu, S., Spurney, R. F. & Quarles, L. D. Analysis of recombinant Phex: an endopeptidase in search of a substrate. Am. J. Physiol. Endocrinol. Metab. 281, E837–E847 (2001).
Quarles, L. D. & Drezner, M. K. Pathophysiology of X-linked hypophosphatemia, tumor-induced osteomalacia, and autosomal dominant hypophosphatemia: a perPHEXing problem. J. Clin. Endocrinol. Metab. 86, 494–496 (2001).
Feng, J. Q. et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 38, 1310–1315 (2006).
Liu, S. et al. Pathogenic role of Fgf23 in Dmp1-null mice. Am. J. Physiol. Endocrinol. Metab. 295, E254–E261 (2008).
Wu, H et al. Dentin matrix protein 1 (DMP1) signals via cell surface integrin. J. Biol. Chem. 286, 29462–29469 (2011).
Steiglitz, B. M., Ayala, M., Narayanan, K., George, A. & Greenspan, D. S. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J. Biol. Chem. 279, 980–986 (2004).
Chaussain, C. et al. MMP2-cleavage of DMP1 generates a bioactive peptide promoting differentiation of dental pulp stem/progenitor cell. Eur. Cell. Mater. 18, 84–95 (2009).
Ogbureke, K. U. & Fisher, L. W. Expression of SIBLINGs and their partner MMPs in salivary glands. J. Dent. Res. 83, 664–670 (2004).
David, V. & Quarles, L. D. ASARM mineralization hypothesis: a bridge too far? J. Bone Miner. Res. 25, 692–694 (2010).
Bai, X. et al. Partial rescue of the Hyp phenotype by osteoblast-targeted PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) expression. Mol. Endocrinol. 16, 2913–2925 (2002).
Erben, R. G. et al. Overexpression of human PHEX under the human b-actin promoter does not fully rescue the Hyp mouse phenotype. J. Bone Miner. Res. 20, 1149–1160 (2005).
Liu, S., Guo, R., Tu, Q. & Quarles, L. D. Overexpression of Phex in osteoblasts fails to rescue the Hyp mouse phenotype. J. Biol. Chem. 277, 3686–3697 (2002).
Lorenz-Depiereux, B., Schnabel, D., Tiosano, D., Häusler, G. & Strom, T. M. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am. J. Hum. Genet. 86, 267–272 (2010).
Levy-Litan, V. et al. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am. J. Hum. Genet. 86, 273–278 (2010).
Chen, I. P., Wang, L., Jiang, X., Aguila, H. L. & Reichenberger, E. J. A Phe377del mutation in ANK leads to impaired osteoblastogenesis and osteoclastogenesis in a mouse model for craniometaphyseal dysplasia (CMD). Hum. Mol. Genet. 20, 948–961 (2011).
Liu, S. et al. Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol. Endocrinol. 23, 1505–1518 (2009).
Jean, G. et al. Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol. Dial. Transplant. 24, 948–955 (2009).
White, K. E. et al. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am. J. Hum. Genet. 76, 361–367 (2005).
Stevens, D. A. et al. Thyroid hormone activates fibroblast growth factor receptor-1 in bone. Mol. Endocrinol. 17, 1751–1766 (2003).
Martin, A. et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 25, 2551–2562 (2011).
Wohrle, S. et al. FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF23 signaling and regulating FGF23 expression in bone. J. Bone Miner. Res. 26, 2486–2497 (2011).
Xiao, L. et al. Nuclear isoforms of fibroblast growth factor 2 are novel inducers of hypophosphatemia via modulation of FGF23 and KLOTHO. J. Biol. Chem. 285, 2834–2846 (2010).
Nauman, E. A., Sakata, T., Keaveny, T. M., Halloran, B. P. & Bikle, D. D. bFGF administration lowers the phosphate threshold for mineralization in bone marrow stromal cells. Calcif. Tissue Int. 73, 147–152 (2003).
Liang, H., Pun, S. & Wronski, T. J. Bone anabolic effects of basic fibroblast growth factor in ovariectomized rats. Endocrinology 140, 5780–5788 (1999).
Uzum, A. K. et al. Effects of vitamin D replacement therapy on serum FGF23 concentrations in vitamin D-deficient women in short term. Eur. J. Endocrinol. 163, 825–831 (2010).
Samadfam, R., Richard, C., Nguyen-Yamamoto, L., Bolivar, I. & Goltzman, D. Bone formation regulates circulating concentrations of fibroblast growth factor 23. Endocrinology 150, 4835–4845 (2009).
Tsuji, K., Maeda, T., Kawane, T., Matsunuma, A. & Horiuchi, N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1α,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J. Bone Miner. Res. 25, 1711–1723 (2010).
Brown, W. W. et al. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen's metaphyseal chondrodysplasia. J. Clin. Endocrinol. Metab. 94, 17–20 (2009).
Rhee, Y. et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 49, 636–643 (2011).
Riminucci, M. et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J. Clin. Invest. 112, 683–692 (2003).
Kobayashi, K. et al. Expression of FGF23 is correlated with serum phosphate level in isolated fibrous dysplasia. Life Sci. 78, 2295–2301 (2006).
Liu, S. et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 17, 1305–1315 (2006).
Kolek, O. I. et al. 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G1036–G1042 (2005).
Mirams, M., Robinson, B. G., Mason, R. S. & Nelson, A. E. Bone as a source of FGF23: regulation by phosphate? Bone 35, 1192–1199 (2004).
Nishida, Y. et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 70, 2141–2147 (2006).
Larsson, T., Nisbeth, U., Ljunggren, O., Jüppner, H. & Jonsson, K. B. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 64, 2272–2279 (2003).
Moe, S. M. et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 257–264 (2011).
Ferrari, S. L., Bonjour, J. P. & Rizzoli, R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J. Clin. Endocrinol. Metab. 90, 1519–1524 (2005).
Burnett, S. A. et al. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J. Bone Miner. Res. 21, 1187–1196 (2006).
Vervloet, M. G. et al. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin. J. Am. Soc. Nephrol. 6, 383–389 (2011).
Antoniucci, D. M., Yamashita, T. & Portale, A. A. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J. Clin. Endocrinol. Metab. 91, 3144–3149 (2006).
Perwad, F. et al. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146, 5358–5364 (2005).
Ben-Dov, I. Z. et al. The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 117, 4003–4008 (2007).
Kawata, T. et al. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J. Am. Soc. Nephrol. 18, 2683–2688 (2007).
Lavi-Moshayoff, V., Wasserman, G., Meir, T., Silver, J. & Naveh-Many, T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am. J. Physiol. Renal Physiol. 299, F882–F889 (2010).
Sato, T. et al. Total parathyroidectomy reduces elevated circulating fibroblast growth factor 23 in advanced secondary hyperparathyroidism. Am. J. Kidney Dis. 44, 481–487 (2004).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Li, S. A. et al. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct. Funct. 29, 91–99 (2004).
Rhee, Y. et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. J. Bone Miner. Res. 26, 1035–1046 (2011).
Galitzer, H., Ben-Dov, I. Z., Silver, J. & Naveh-Many, T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 77, 211–218 (2010).
Komaba, H. & Fukagawa, M. FGF23-parathyroid interaction: implications in chronic kidney disease. Kidney Int. 77, 292–298 (2010).
Saji, F. et al. Fibroblast growth factor 23 production in bone is directly regulated by 1α,25-dihydroxyvitamin D, but not PTH. Am. J. Physiol. Renal Physiol. 299, F1212–F1217 (2010).
Tebben, P. J., Singh, R. J., Clarke, B. L. & Kumar, R. Fibroblast growth factor 23, parathyroid hormone, and 1α,25-dihydroxyvitamin D in surgically treated primary hyperparathyroidism. Mayo Clin. Proc. 79, 1508–1513 (2004).
Saji, F. et al. Regulation of fibroblast growth factor 23 production in bone in uremic rats. Nephron Physiol. 111, 59–66 (2009).
Helvig, C. F. et al. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int. 78, 463–472 (2010).
Clemens, T. L. & Karsenty, G. The osteoblast: an insulin target cell controlling glucose homeostasis. J. Bone Miner. Res. 26, 677–680 (2011).
Bhandaru, M. et al. Decreased bone density and increased phosphaturia in gene-targeted mice lacking functional serum- and glucocorticoid-inducible kinase 3. Kidney Int. 80, 61–67 (2011).
Tsujikawa, H., Kurotaki, Y., Fujimori, T., Fukuda, K. & Nabeshima, Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 17, 2393–2403 (2003).
Shiraki-Iida, T. et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 424, 6–10 (1998).
Kuro-o, M. Klotho. Pflugers Arch. 459, 333–343 (2010).
Weber, T. J., Liu, S., Indridason, O. S. & Quarles, L. D. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J. Bone Miner. Res. 18, 1227–1234 (2003).
Isakova, T. et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol. Dial. Transplant. 26, 584–591 (2011).
Oliveira, R. B. et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin. J. Am. Soc. Nephrol. 5, 286–291 (2010).
Nagano, N. et al. Effect of manipulating serum phosphorus with phosphate binder on circulating PTH and FGF23 in renal failure rats. Kidney Int. 69, 531–537 (2006).
Saito, H. et al. Circulating FGF-23 is regulated by 1α,25-dihydroxyvitamin D3 and phosphorus in vivo. J. Biol. Chem. 280, 2543–2549 (2005).
Mori, S. et al. Direct binding of integrin αvβ3 to FGF1 plays a role in FGF1 signaling. J. Biol. Chem. 283, 18066–18075 (2008).
Guo, R. et al. Inhibition of MEPE cleavage by Phex. Biochem. Biophys. Res. Commun. 297, 38–45 (2002).
Rowe, P. S. et al. Surface plasmon resonance (SPR) confirms that MEPE binds to PHEX via the MEPE-ASARM motif: a model for impaired mineralization in X-linked rickets (HYP). Bone 36, 33–46 (2005).
Lu, Y. et al. Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev. Biol. 303, 191–201 (2007).
Rached, M. T. et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 11, 147–160 (2010).
Topaz, O. et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat. Genet. 36, 579–581 (2004).
Ichikawa, S. et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Musculoskelet. Neuronal Interact. 7, 318–319 (2007).
Wetmore, J. B. & Quarles, L. D. Calcimimetics or vitamin D analogs for suppressing parathyroid hormone in end-stage renal disease: time for a paradigm shift? Nat. Clin. Pract. Nephrol. 5, 24–33 (2009).
Tentori, F. et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 70, 1858–1865 (2006).
Young, E. W. et al. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 44 (Suppl.), 34–38 (2004).
Razzaque, M. S., St-Arnaud, R., Taguchi, T. & Lanske, B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol. Dial. Transplant. 20, 2032–2035 (2005).
Block, G. A. et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 15, 2208–2218 (2004).
Gutiérrez, O. M. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 359, 584–592 (2008).
Gutierrez, O. et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J. Am. Soc. Nephrol. 16, 2205–2215 (2005).
Hasegawa, H. et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 78, 975–980 (2010).
Imanishi, Y. et al. FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int. 65, 1943–1946 (2004).
Stubbs, J. R. & Quarles, L. D. Fibroblast growth factor 23: uremic toxin or innocent bystander in chronic kidney disease? Nephrol. News Issues 23, 33–34 (2009).
Stubbs, J. R., Idiculla, A., Slusser, J., Menard, R. & Quarles, L. D. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J. Am. Soc. Nephrol. 21, 353–361 (2010).
Quarles, L. D. The bone and beyond: 'Dem bones' are made for more than walking. Nat. Med. 17, 428–430 (2011).
Wetmore, J. B., Liu, S., Krebill, R., Menard, R. & Quarles, L. D. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin. J. Am. Soc. Nephrol. 5, 110–116 (2010).
Rosen, C. J. Clinical practice. Vitamin D insufficiency. N. Engl. J. Med. 364, 248–254 (2011).
Parker, B. D. et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann. Intern. Med. 152, 640–648 (2010).
Fliser, D. et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J. Am. Soc. Nephrol. 18, 2600–2608 (2007).
Mirza, M. A. et al. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler. Thromb. Vasc. Biol. 31, 219–227 (2011).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares associations with the following companies: Amgen (consultant, speakers bureau), KAI Pharmaceuticals (consultant).
Rights and permissions
About this article
Cite this article
Quarles, L. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat Rev Endocrinol 8, 276–286 (2012). https://doi.org/10.1038/nrendo.2011.218
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2011.218
This article is cited by
-
Oncostatin M is a regulator of fibroblast growth factor 23 (FGF23) in UMR106 osteoblast-like cells
Scientific Reports (2023)
-
A decrease in serum 1,25(OH)2D after elective hip replacement and during bone healing is associated with changes in serum iron and plasma FGF23
Journal of Endocrinological Investigation (2022)
-
High-Dose Intravenous Iron with Either Ferric Carboxymaltose or Ferric Derisomaltose: A Benefit-Risk Assessment
Drug Safety (2022)
-
Co-culture of ASCs/EPCs and dermal extracellular matrix hydrogel enhances the repair of full-thickness skin wound by promoting angiogenesis
Stem Cell Research & Therapy (2021)
-
Congenital Conditions of Hypophosphatemia Expressed in Adults
Calcified Tissue International (2021)